1. A technique of using very small metal particles coated with desired DNA in the gene transfer is called:-
(a) Microinjection (b) Biolistic (c) Liposome mediated (d) Electroporation
2. Arrange the following steps in sequence of their order for production of recombinant Insulin:-
A. Fusion of A and B chains for disulphide bond.
B. Cynogen bromide treatment to remove methi onine and â galactosidase.
C. Introduction of A and B chain in the plasmid containing â galactosidase g ene.
D. Synthesis of A and B chain in E coli.
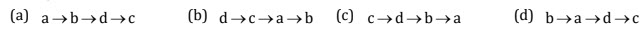
3. Motif is represented by:-
(a) Commas repeated on the lattice (b) 3D translational periodic arrangement of points
(c) Geometric shapes of lattice (d) Centre of symmetry in lattice
4. Statement 1 : Vortex formation can be minimized by push pull mechanism.
Statement 2 : Vortex formation reduces the mixing intensity by increasing the velocity of impeller.
(a) True, False (b) True, True (c) False, False (d) False, True
5. Which of the following fluid can be considered as an ideal fluid?
(a) Viscous fluid (b) Non-viscous fluid (c) Compressible fluid (d) All of these
6. Which of the following agencies is not classified as an ‘executive agency’ for administration of the act under the provision of Drugs and Cosmetics Act 1940?
(a) Licensing authority
(b) Drug inspectors
(c) Drugs Consultative Committee
(d) Customs collectors
7. As per Factories Act 1948, in CHAPTER VI dealing with working hours of adults, no adult worker shall be required or allowed to work in a factory for more than _______________ hours in a week.
(a) 30 (b) 40 (c) 48 (d) 56
8. Henri Fayol’s principle “Espirit de corps” means:-
(a) Corporate objective (b) Group objective (c) Team activity (d) Team spirit
9. How customer’s bias about the product will influence the marketing communication?
(a) Positive effect (b) Negative effect (c) No effect (d) Both positive and Negative
10. Which of the following is not patentable in India as per The Patents Act 1970?
(a) New product
(b) New process
(c) New use of existing drug
(d) New process for existing drug
11. Match the following enzymes in Column I with their respective functions under Column II
Column I & Column II
i. DNA ligase (p) Synthe size a DNA copy of RNA
ii. Alkaline phosphatase (q) Forms a bond between 3’ –OH and 5’-PO4
iii. Reverse transcriptase (r) Removes terminal PO4 from 3’ or 5’end of DNA
iv. Polynucleotide kinase (s) Adds phosphate to 5’ –OH end
(a) i-r, ii-s, iii-p, iv-q (b) i-p, ii-q, iii-r, iv-s (c) i-q, ii-r, iii-p, iv-s (d) i-s, ii-p, iii-q, iv-r
12. Which of the following replacement of amino acid in a protein may produce greatest change in its conformation?
(a) Ser ------->Thr (b) Glu ------->Val (c) Gln ------->Tyr (d) Phe -------> Ile
13. The hexose monophosphate pathway produces distinctively two useful products. Identify these products with the ratio in which they are produced.
(a) One NADPH to two ribose-6-phosphate (b) Two NADPH to one ribose-5-phosphate
(c) Two NADPH to one ribulose-5-phosphate (d) Two NADPH to one fructose-6-phosphate
14. The correct statement about Vitamin D is:-
(a) The oral administration of 1, 25-dihydoxycholecalciferol is required in chronic renal failure
(b) 25-Hydroxycholecalciferol is the active form of the vitamin
(c) Vitamin D antagonizes the effects of parathyroid hormone
(d) A deficiency of vitamin D causes an increase in calcitonin secretion
15. All of the following enzymes are used in ELISA except:-
(a) Glucose oxidase (b) Alkaline phosphatase (c) Coagulase (d) β-galactosidase
Go to "Next Page" for more questions...
Subscribe to Pharmatutor Job Alerts by Email
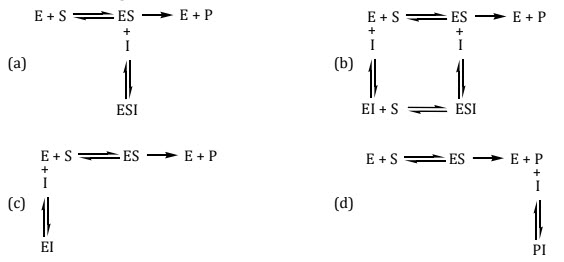
16. Which of the following equilibrium suggests noncompetitive inhibition of enzyme E for conversion of substrate S to product P with inhibitor I?
17. Which method is used for the Limit test for arsenic?
(a) Gutzeit method
(b) Oswald method
(c) Arrhenius method
(d) Karl-Fischer method
18. The agent used to prevent the dental carries is:-
(a) Sodium fluoride (b) Strontium chloride (c) Zinc chloride (d) Dicalcium phosphate
19. Which of the following definitions of an asymmetric reaction is the most accurate?
(a) A reaction that creates a new chiral centre in the product
(b) A reaction that involves a chiral reagent
(c) A reaction which creates a new chiral centre with selectivity for one enantiomer/
diasatereoisomer over another
(d) A reaction that is carried out on an asymmetric starting material
20. What software programme is used to determine the Verloop steric parameter in QSAR?
(a) Alchemy (b) Chem3D (c) Sterimol (d) Chem-Draw
21. The oral oligosaccharide hypoglycemic agent, which is administered at the start of the meal is:-
(a) Pioglitazone (b) Miglitol (c) Acarbose (d) Glimepride
22. Which functional group is crucial for anti-malarial activity of artemisinin?
(a) Aldehydic functional group
(b) Ethylene bridge
(c) Ketonic functional group
(d) Peroxide bridge
23. Select the drug which exhibits dual alpha and beta adrenergic receptor agonists activity.
(a) Terbutaline (b) Clonidine (c) Metaproterenol (d) Dobutamine
24. Appropriate hybridization schemes for the C atoms in molecule CH3CO2H are:-
(a) sp3 and sp (b) sp3 and sp2 (c) sp2 and sp (d) sp3 and sp3
25. In Universal indicators, a pH of 7 is shown with:-
(a) Yellow color (b) Green color (c) Blue color (d) Pink color
26. Which statement regarding Hückel’s rule is FALSE?
(a) There must be (4n + 2) pi (π) electrons
(b) The molecule must be planar
(c) The molecule must be cyclic
(d) Each of the pi ( π) electrons must be associated with a conjugated double bon
27. Anthracene is isomeric with:-
(a) Phenanthrene (b) Naphthalene (c) Benzene (d) Azulene
28. The molecular formula of phenanthrene is:-
(a) C14 H10 (b) C12 H10 (c) C14 H14 (d) C14 H8
29. In electrophilic substitution of pyridine, reaction of pyridine with H2O2 in acetic acid leads to
formation of:-
(a) 1,4-Dihydropyridine (b) 2-Hydroxypyridine (c) 2-Pyridone (d) Pyridine-N-oxide
30. Which compound is most basic?

Go to "Next Page" for more questions...
Subscribe to Pharmatutor Job Alerts by Email
31. Correct Nomenclature for the following bridged bicyclic ring system is:-
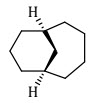
(a) bicyclo[4.4.0]decane
(b) bicyclo[4.3.0]decane
(c) bicyclo[4.3.1]decane
(d) bicyclo[4.4.1]decane
32. Which among the following correctly defines Diastereomer?
(a) These have same magnitude but different signs of optical rotation
(b) Nonsuperimposable object mirror relationship
(c) These differ in all physical properties
(d) Separation is very difficult
33. Galactose and Glucose are:-
(a) Epimers (b) Anomers (c) Isomers (d) Ketose-Aldose isomers
34. Which among the following is a non-essential amino acid?
(a) Lysine (b) Threonine (c) Serine (d) Histidine
35. Which of the following is a 3,3-sigmatropic reaction which converts a 1,5-diene to an isomeric 1,5 diene?
(a) Cope rearrangement
(b) Claisen rearrangement
(c) Photochemical [2+2] reaction
(d) Diels-Alder reaction
36. What quantity of an indicator solution shall be added when quantity is not mentioned in an assay
or test?
(a) 0.1 ml (b) 0.05 ml (c) 0.2 ml (d) 0.5 ml
37. In Kjeldahl method, sample containing nitrogen is digested with ______.
(a) Concentrated sodium hydroxide
(b) Fuming nitric acid
(c) Concentrated sulphuric acid
(d) Strong ammonia solution
38. What is the concentration of paracetamol in a 0.1 N sodium hydroxide solution, whose absorption in a 1 cm cell at its ʎmax, 257 nm, was found to be 0.825? The A (1%, 1 cm) in the IP monograph of paracetamol is given as 715 at 257 nm
(a) 1.1 g/100 ml (b) 0.0011 mg/100 ml (c) 0.0011 g/100 ml (d) 0.0011 µg/100 ml
39. The unit for specific absorbance A (1%, 1cm) is:-
(a)µg/mL (b) mg/L (c) liter mole-1 cm-1 (d) dl g-1 cm-1
40. What is the nuclear magnetic resonance frequency of 1H in a 7.05 Tesla magnetic field strength?
(a) 300.0 MHz (b) 200.0 MHz (c) 60.0 MHz (d) 100 MHz
41. What is Hydrogen Deficiency Index (HDI) value for toluene?
(a) 1 (b) 2 (c) 3 (d) 4
42. In NMR, the aromatic proton resonate in a characteristic narrow range at:-
(a) δ 6.5 – δ 8.0 (b) δ 11.0 – δ 12.0 (c) δ 2.0 – δ 4.0 (d) δ 0.7 – δ 1.3
43. The difficulties of long elution time and poor resolution of complex mixtures are observed in elution analysis. These difficulties can be overcome by modification of elution analysis, known as:-
(a) Isocratic-elution analysis
(b) Gradient-elution analysis
(c) Displacement analysis
(d) Frontal analysis
44. Materials whose consistency depends on the duration of shear, as well as on the rate of shear, exhibit:-
(a) Rheopexy (b) Thixotropy (c) Viscoelasticity (d) Plasticity
45. Which of the following solutions are more likely to have the same osmotic pressure? Solutions of:
(a) Diluted nonelectrolytes with the same molal concentration
(b) Concentrated nonelectrolytes with the same molal concentration
(c) Diluted electrolytes with the same molal concentration
(d) Concentrated electrolytes with the same molal concentration
Go to "Next Page" for more questions...
Subscribe to Pharmatutor Job Alerts by Email
46. Which statements are correct for the micelle formation?
(P) Micelles are dynamic structures that are continually formed and broken down in solution.
(Q) The typical micelle diameter is about 2–3 µm and so they are visible under the light micro scope.
(R) Micelle formation is a spontaneous process.
(S) When the surfactant concentration is increased above the CMC, the number of micelles
increases and the free surfactant concentration decreases below CMC.
(a) P and Q (b) P and R (c) P and S (d) R and S
47. Which equation is used to predict the stability of a drug product at room temperature from experiments at accelerated temperature?
(a) Higuchi equation (b) The Arrhenius’ equation
(c) Hildebrand equation (d) The Hixson-Crowell equation
48. Which statement correctly describes Hess’s Law?
(a) The enthalpy of all reactants in their standard states is defined as zero
(b) Enthalpy changes can be calculated only if one or more of the reactants is/are element
(c) The enthalpy change of a reaction can be calculated only at 1 atm pressure and 25 °C
(d) The enthalpy change of a reaction is independent of the route of reaction
49. Identify the starting material A and B in the synthesis of Clomifene.
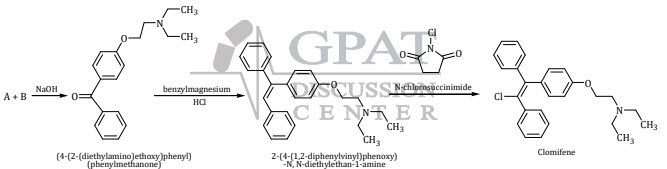
(a) Where A 4-hydroxy-benzophenone and B 2-diethylamino-ethyl chloride
(b) Where A 4-hydroxy benzaldehyde and B 4-methoxy aniline
(c) Where A 4-hydroxy-benzophenone and B 4-methoxy aniline
(d) Where A 4-hydroxy-benzophenone and B benzaldehyde
50. The role of glutathione in tissues includes all except:-
(a) Participate in decomposition of hydrogen peroxide
(b) Participate in activation of methionine
(c) Participate in detoxification reactions
(d) Biologically active in oxidized form
51. When Ke is constant and Ka is larger:-
(a) Cmax is more and tmax is longer
(b) Cmax is lesser and tmax is longer
(c) Cmax is lesser and tmax is short
(d) Cmax is more and tmax is short
52. When considering drug delivery to the brain which of the following is false?
(a) The cells in the blood vessels that supply the brain are tightly connected which restricts drug absorption
(b) Only relatively small lipophilic molecules readily, passively diffuse in to the brain
(c) Drugs with a low log P value show improved passive diffusion into the brain (P: oil / water
partition coefficient)
(d) Polar molecules can be taken up into the brain through active transport
53. IVIVC utilizes the principles of statistical moment analysis:-
(a) Level A (b) Level B (c) Level C (d) Level D
54. The systems that follows, Weibull Mathematical Model used to describe drug release kinetics are:-
(a) Swellable polymeric devices
(b) Diffusion matrix formulation
(c) Erodible matrix formulation
(d) Transdermal system
55. Which method is used by pharmacists for complete blending of potent powders with large quantities of diluents?
(a) Spatulation (b) Levigation (c) Trituration (d) Geometric dilution
56. Substance used to reduce friction during tablet compression and facilitate ejection of tablets from the die cavity is called as:-
(a) Lubricant (b) Glidant (c) Anti-adherent (d) Humectant
57. What quantities of 95% v/v and 45% v/v alcohols are to be mixed to make 800 mL of 65% v/v alcohol?
(a) 480 mL of 95% and 320 mL of 45% alcohol
(b) 320 mL of 95% and 480 mL of 45% alcohol
(c) 440 mL of 95% and 360 mL of 45% alcohol
(d) 360 mL of 95% and 440 mL of 45% alcohol
58. The proportion of NaCl liquid to give 1.5% solution of drug isotonic with blood plasma is:- (The freezing point of 1% w/v solution of drug is -0.122 and NaCl is -0.576 °C)
(a) 0.79% (b) 0.585% (c) 0.9% (d) 0.5%
59. Which of the following statement is NOT TRUE about prokaryotes?
(a) Nucleus is not bounded by nuclear membrane
(b) Cell wall contains peptidoglycan
(c) 80S ribosomes are distributed in cytoplasm
(d) It is Haploid in nature
60. Match the following diseases under column I with the respective causative organisms under
Column II.
Column I Column II
i. Creutzfeldt-Jacob disease p. Yersinia pestis
ii. Typhus q. Prions
iii. Syphilis r. Rickettsia prowazekii
iv. Plague s. Treponema palladium
(a) i-r, ii-s, iii-p, iv-q (b) i-p, ii-q, iii-r, iv-s (c) i-q, ii-r, iii-s, iv-p (d) i-s, ii-p, iii-q, iv-r
Go to "Next Page" for more questions...
Subscribe to Pharmatutor Job Alerts by Email
61. As the dielectric constant values increases, the polarity of the solvents ________.
(a) Decreases
(b) Increases
(c) Remains constant
(d) Decreases and then remains constant
62. The angle of repose is calculated by __________.
(a) tan α = Radius/Height
(b) tan α = 1+ Radius/Height
(c) tan α = 1- Radius/Height
(d) tan α = Height/Radius
63. Spray drying / spray congealing method is generally used to prepare ________.
(a) Tablets (b) Microcapsules (c) Capsules (d) Ointments
64. HLB value of tragacanth is:-
(a) 4.7 (b) 8.7 (c) 13.2 (d) 14.3
65. Vials and bottles are regularly not subjected to following test:-
(a) Sterility test (b) Clarity test (c) Leaker (chamber) test (d) Pyrogen test
66. As per USP, test limit for treated soda lime glass with container size of 200 ml is:-
(a) 0.70ml of 0.02N Acid
(b) 1.0ml of 0.2N Acid
(c) 0.20ml of 0.02N Acid
(d) 0.70ml of 0.2N Acid
67. In plasma, phenobarbital is present as ionized and unionized forms in equal amount because:-
(a) It is weakly acidic drug
(b) It is weakly basic drug
(c) pH of plasma is 6.8
(d) pKa of the phenobarbital is 7.4
68. A material which is insoluble and inert and used in matrix tablet formulation is:-
(a) Polyethylene (b) Stearyl alcohol (c) Polyethylene glycol (d) Triglycerides
69. Which test is done for USP Type-I glass containers for injections?
(a) Water attack test
(b) Powdered glass test
(c) Powdered glass followed by water attack test
(d) Water attack followed powdered glass test
70. Isoelectric point of Type A gelatin is ________.
(a) pH 7.0 (b) pH 4.7 (c) pH 9.0 (d) pH 7.4
71. What is the effective ratio of methyl paraben and propyl paraben for anti-microbial activity?
(a) 1:1 (b) 5:1 (c) 2.5:1 (d) 10:1
72. Which of the following formula is used to determine shelf life as per first order reaction?
(a) t90 0.693/k (b) t90 0.104/k (c) t1/2 0.693/k (d) t1/2 0.105/k
73. Following are endogenous carriers use for targeted drug delivery except:-
(a) Lipoprotein (b) Serum Albumin (c) Erythrocyte (d) Microparticulates
74. The friability issue of the tablet can be solved by different ways except:-
(a) Increasing the upper punch pressure of tablet machine
(b) Addition of more tablet binder to granules
(c) Increasing the moisture content of granules
(d) Adjusting the lower punch pressure of tablet machine
75. What are the specific surface per unit volume Sv of spherical particles with density of 3 gm/cm3 and volume surface diameter, dvs of 2.57µm?
(a) 7.78 x 103 cm2/cm3
(b) 2.33 x 103 cm2/cm3
(c) 1.55 x 103 cm2/cm3
(d) 1.00 x 103 cm2/cm3









