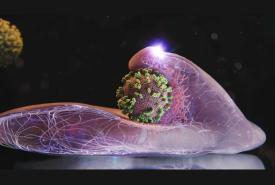
Fever, aching limbs and a runny nose – as winter returns, so too does the flu. The disease is triggered by influenza viruses, which enter our body through droplets and then infect cells.
Researchers from Switzerland and Japan have now investigated this virus in minute detail. Using a microscopy technique that they developed themselves, the scientists can zoom in on the surface of human cells in a Petri dish. For the first time, this has allowed them to observe live and in high resolution how influenza viruses enter a living cell.
 Harsh Bhardwaj1*, Sakshi Jain2, Raaz K Maheshwari3
Harsh Bhardwaj1*, Sakshi Jain2, Raaz K Maheshwari3


 Vinay Kumar Singh.
Vinay Kumar Singh.