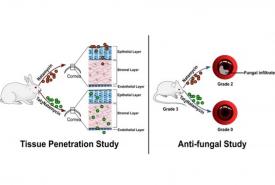
Connective Tissue Protein Fights Bacterial Infections While Restraining Immune Overreaction in Sepsis
A connective tissue protein known to support the framework of organs also encourages immune responses that fight bacterial infections, while restraining responses that can be deadly in the condition called sepsis, a new study finds.