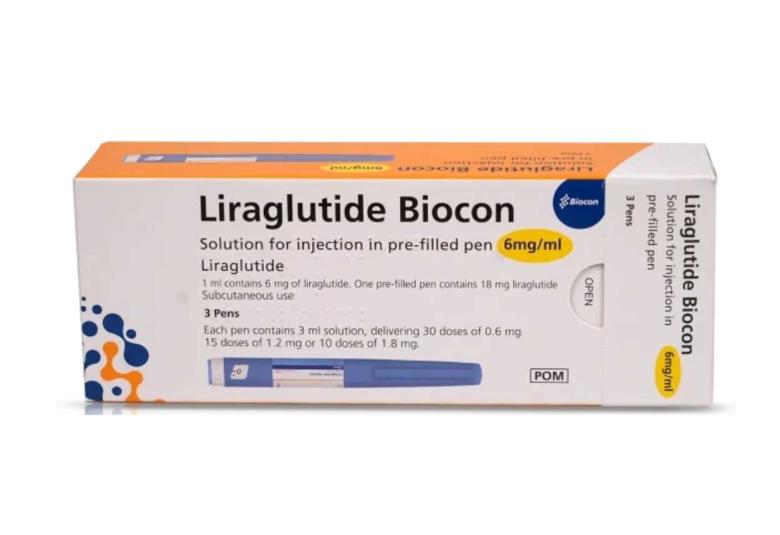
Biocon Limited gets approval for diabetes drug Liraglutide in India
Biocon Pharma Limited, has received approval for its Liraglutide drug product (6 mg/ml solution for injection in pre-filled pen and cartridge), from the Drugs Controller General of India, CDSCO (Central Drugs Standard Control Organisation).