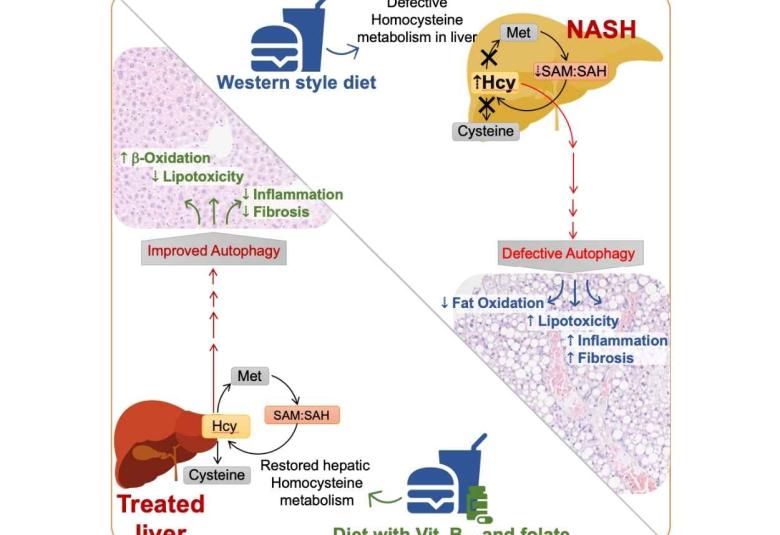
B vitamins can potentially be used to treat advanced non-alcoholic fatty liver disease : Scientists
Scientists at Duke-NUS Medical School in Singapore have uncovered a mechanism that leads to an advanced form of fatty liver disease—and it turns out that vitamin B12 and folic acid supplements could reverse this process.
These findings could help people with non-alcoholic fatty liver disease, an umbrella term for a range of liver conditions affecting people who drink little to no alcohol, which affects 25 per cent of all adults globally, and four in 10 adults in Singapore.