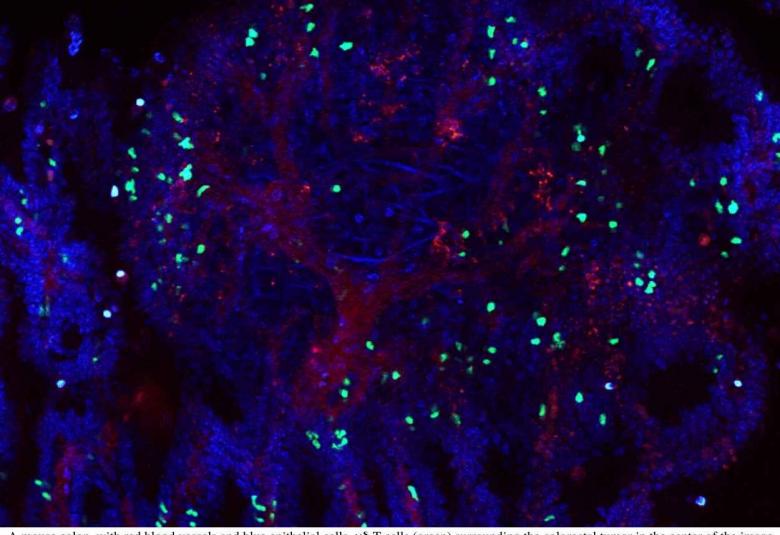
Colorectal cancer tumors both helped and hindered by T cells
Colorectal tumors are swarming with white blood cells, but whether these cells help or hinder the cancer is hotly debated. While some studies have shown that white blood cells heroically restrict tumor growth and combat colorectal cancer, equally compelling evidence casts the white blood cells as malignant co-conspirators bolstering the tumor and helping it spread.