{ DOWNLOAD AS PDF }
ABOUT AUTHORS
C.V.H.Hemavathy*, B. Raj kumar, R. Kiran kumar, G. Hema latha
Department of Pharmacy,
Kottam Institute of Pharmacy,
Erravally X Roads, Mahaboob Nagar
*hemarayudu19@gmail.com
ABSTRACT
Hepatoprotective activity of by inducing ccl4 hepatotoxicity. To study the hepatoprotective activity of against CCL4 induced hepatotoxcity. To evaluate the mechanism of hepatoprotection in terms of Liver antioxidant mechanism, Histopathological study. The animals were divided into Four groups of three animals each. Except the normal group all the other groups received ccl4 in at a dose of 0.1 ml/kg by intraperitoneally for 14 days. Normal groups received plain tween 80 orally. On the 14th day all the rats from all the groups were sacrificed, blood was collected from each animal for serum analysis and their livers were stored under freezing conditions for the estimation of endogenous anti oxidants and one sample from each group was stored in 10% formalin for histopathological studies. In bio-chemical studies- Serum analytical methods (AST), (ALT), (Alk.P), (Bil), (TP), (TC). The present findings observed in this study revealed that,glimepirideis natural antioxidant lignin possess significant antioxidant activity against ccl4 induced hepatotoxicity via antioxidant mechanism. However, further research is required to find out the other possible mechanism of hepatoprotection to conform that as glimepiridehepatoprotective molecule.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2424
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 4, Issue 8 Received On: 18/02/2016; Accepted On: 13/03/2016; Published On: 01/08/2016 How to cite this article: Hemavathy CVH, Kumar BR, Kumar RK, latha GH; Hepatoprotective activity of glimepiride by inducing CCl4 hepatotoxicity; PharmaTutor; 2016; 4(8); 42-48 |
INTRODUCTION
The liver is an important and largest organ. Hepato or hepatic is a medical term from the greek word hepar, which means liver. It has a wide range of functions, which are detoxification, protein synthesis, and production of biochemical necessary for digestion. The liver is necessary for survival; a human can only last up to 24 hours without liver function. Liver plays a major role in detoxification and excretion of many endogenous and exogenous compounds, any injury to it or impairment to its function may leads to complications on one’s health. Drug-induced liver injury (DILI) is a major health problem that challenges not only health care professionals but also the pharmaceutical industries and drug regulatory agencies. According to the United States, DILI accounts for more than 50% of acute liver failure, including hepatotoxicity caused by overdose of acetaminophen (APAP, 39%) and idiosyncratic liver injury triggered by other drugs (13%) (Ostapowicz et al., 2002). Other hepatotoxic drugs, such as risperidone, trovafloxacin, and nefazodone, have been assigned “black box” warnings (Lasser et al., 2002). Liver damage occurs by either due to direct damage to the liver cells or due to a secondary damage resulting from obstruction of the bile flow. Rarely, it can be due to obstruction to the blood flow either in the portal vein or in the hepatic vein.
AIM & OBJECTIVE
The main objective of the study was to evaluate the hepato protective activity of glimepirideagainst CCl4 induced hepatotoxicity. Evaluate the mechanism of hepatoprotection in terms of Liver antioxidant mechanism; Histopathological study.
REVIEW OF LITERATURE
Takashi ideet. Al. Effect of Sesamin, a sesame lignin, on the hepatic fatty acid metabolism was examined in the rat. Increase of the dietary level of Sesamin progressively increased the mitochondrial and peroxisome fatty acid oxidation rate. Mitochondrial activity almost doubled in rats fed a 0.5% Sesamin diet. Peroxisomal activity became more than 10 times higher in rats fed a 0.5% Sesamin diet, compared to those fed a Sesamin-free diet. Dietary Sesamin also markedly increased the hepatic activity and mRNA levels of various fatty acid oxidation enzymes. In contrast, dietary Sesamin decreased the hepatic activity and mRNA abundance of lipogenic enzymes. This was associated with the down-regulation of sterol regulatory element-binding protein-1, a transcriptional factor that regulates the lipogenic enzyme gene expression. Dietary Sesamin significantly decreased the triacylglycerol secretion accompanying the increase in ketone body production by the perfused rat liver. It is apparent that Sesamin affects the fatty acid metabolism and lipoprotein production in the liver, and hence lowers the serum lipid levels.
Mehrdad Roghani et al In this study, the effect of chronic treatment with Sesamin on vascular permeability in rats with streptozotocin (STZ)-induced diabetes was investigated. Male diabetic rats received Sesamin at a dose of either 10 or 20 mg/kg for 7 weeks, beginning 1 week after diabetes induction. Vascular permeability was estimated by measuring Evans blue dye extravasation. Oxidative stress markers, including malondialdehyde (MDA) and superoxide dismutase (SOD) activity, were also measured in aortic tissue.
R. Kim et al Lignin’s constitute a group of phytochemicals, which are produced by oxidative dimerization of two phenylpropanoid units. Furfuran type lignin’s such as secoisolariciresinol, matairesinol, lariciresinol or pinoresinol are widely distributed in edible plants, and most of those dietary lignans are metabolized by the gut microflora to enterolactone and enterodiol, also known as enterolignans, traditionally classified as phytoestrogens. The rich sources of lignans are flaxseed, sesame seeds, cereal products, and Brassica vegetables. There is a growing interest in biological functions of lignans from edible plants, since a higher intake of edible plants containing lignans is known to reduce the incidence of certain chronic diseases. This review deals with the isolation and preparation of furfuran type lignans from edible plants, and their bioactivities such as anticancer, antioxidant, cardio vasculoprotective, neuro protective, and anti-inflammatory activities, so that recent informations about bioactive lignans from edible plants may be available for the development of potential functional food agents.
DRUG PROFILE
AMARYL is effective as initial drug therapy. In patients where monotherapy with AMARYL or metformin has not produced adequate glycemic control, the combination of AMARYL and metformin may have a synergistic effect, since both agents act to improve glucose tolerance by different primary mechanisms of action. This complementary effect has been observed with metformin and other sulfonylureas, in multiple studies.
The primary mechanism of action of glimepiride in lowering blood glucose appears to be dependent on stimulating the release of insulin from functioning pancreatic beta cells. In addition, extrapancreatic effects may also play a role in the activity of sulfonylureas such as glimepiride. This is supported by both preclinical and clinical studies demonstrating that glimepiride administration can lead to increased sensitivity of peripheral tissues to insulin. These findings are consistent with the results of a long-term, randomized, placebo-controlled trial in which AMARYL therapy improved postprandial insulin/C-peptide responses and overall glycemic control without producing clinically meaningful increases in fasting insulin/C-peptide levels. However, as with other sulfonylureas, the mechanism by which glimepiride lowers blood glucose during long-term administration has not been clearly established. In considering the use of AMARYL in asymptomatic patients, it should be recognized that blood glucose control in Type 2 diabetes has not definitely been established to be effective in preventing the long-term cardiovascular and neural complications of diabetes. However, the Diabetes Control and Complications Trial (DCCT) demonstrated that control of HbA1c and glucose was associated with a decrease in retinopathy, neuropathy, and nephropathy for insulin-dependent diabetic (IDDM)patients.
MATERIALS AND METHODS
wistar albino rats were used for the study. The animals were housed in groups of six and maintained under standard conditions (27±2ºC, relative humidity 44 - 56% and light and dark cycles of 10 and 14 hours respectively) and fed with standard rat diet and purified drinking water ad libitum for 1 week before and during the experiments.
All the experiments were performed in the morning according to current guidelines for the care of laboratory animals and the ethical guidelines for the investigation of experimental pain in conscious animals.All the studies conducted were approved by the Institutional Animal Ethical (IAEC)(ANCP/IAEC/1026 under the OCED guidelines of 465.
EXPERIMENTAL DESIGN
The animals were divided into four groups of three animals in each group.
Group I was given with vehicle (10 ml/kg).
Group II was given with CCL4 50% v/v in tween 80 at a dose of 0.1 ml/kg on the 13th day only starting from 1st day of treatment.
Group III was given with glimepiride(10mg/kg) for 12 days starting from the first day of treatment, and on 13th day it was treated with CCl4 50% v/v in at a dose of 0.1 ml/kg.
Group IV was given with glimepiride(20 mg/kg) for 12 days starting from the first day of treatment, and on 13th day it was treated with CCl4 50% v/v in at a dose of 0.1 ml/kg.
On the 14th day all the rats from all the groups were sacrificed, blood was collected from each animal for serum analysis and their livers were stored under freezing conditions for the estimation of endogenous anti oxidants and one sample from each group was stored in 10% formalin for histopathological studies.
ESTIMATION OF ASPARTATE AMINO TRANSFERASE (AST) or SGOT
Method: Reitman and Frankel method.
Principle: SGOT catalyses the following reaction i.e. the transfer of an amino group from L-aspartate to α-ketoglutarate.α- Ketoglutarate + L- Aspartate ↔ L- Glutamate + Oxaloacetate. Oxaloacetate so formed is coupled with 2,4-Dinitrophenyl hydrazine (2,4-DNPH) to give the corresponding hydrazone which gives brown color in alkaline medium and this can be measured colorimetrically.
ESTIMATION OF ALANINE AMINO TRANSFERASE (ALT) or SGPT
Method: Reitman and Frankel method.
Principle: SGPT catalyses the following reaction i.e. the transfer of an amino group from L-alanine to α-ketoglutarate.
α- Ketoglutarate + L- Alanine ↔ L- Glutamate + Pyruvate
Pyruvate so formed is coupled with 2,4-Dinitrophenyl hydrazine(2,4-DNPH) to give the corresponding hydrazone which gives brown color in alkaline medium and this can be measured colorimetrically.
ESTIMATION OF ALKALINE PHOSPHATASE (Alk.P)
Method: Kind and King’s method.
Principle: Alkaline phosphatase from serum converts phenyl phosphate to inorganic phosphate and phenol at pH 10. Phenol so formed reacts with 4-Aminoantipyrine in alkaline medium in presence of oxidizing agent potassium ferricyanide and forms an orange-red complex, which can be measured colorimetrically. The color intensity is proportional to the enzyme activity.
Phenol + 4-Aminoantipyrine → orange-red complex
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
ESTIMATION OF TOTAL BILIRUBIN (TB) ESTIMATION OF TOTAL BILIRUBIN (TB)
Method: Modified Jendrassik and Grof’s method.
Principle: Bilirubin is formed from hemoglobin and transported to liver as bilirubin-albumin complex. In liver a major part of bilirubin is converted to bilirubin diglucuronde/conjugated bilirubin/direct bilirubin, which is water-soluble. Unconjugated bilirubin/free bilirubin/indirect bilirubin is not water-soluble.
Conjugated bilirubin is excreted into the bile by the liver and stored in the gall bladder or transferred directly to the small intestine. Bilirubin is further broken down by bacteria in the intestine to urobilins, which contribute to the color of faeces. A small percentage of these compounds are reabsorbed and eventually appear in the urine as urobilinogen. The abnormal retention of bilirubin usually results in jaundice, a condition characterized by increased bilirubin in blood and deposition of a brownish yellow pigment in the skin, sclera and mucous membrane.
Increased direct or total bilirubin may indicate erythroblastosis fetalis, sickle cell anemia or pernicious anemia. Increased direct bilirubin may indicate cirrhosis, hepatitis.
Here conjugated bilirubin present in the serum reacts with diazotised sulphanilic acid to yield azobilirubin which absorbs at 546 nm. Total bilirubin present in serum reacts with diazotised sulphanilic acid in presence of activator to azobilirubin, which absorbs at 546 nm.
Bilirubin + Diazotised sulphanilic Acid → Azobilirubin compound
ESTIMATION OF TOTAL PROTEIN (TP)
Method: Biuret method (Doumas, 1972).
Principle: Among the various methods available for the quantitative estimation of proteins such as salt fractionation, electrophoresis, ultra centrifugation, etcKjeldahl’s method is the considered as the reference method. However this method is time consuming and cumbersome. Proteins bind with copper ions in the alkaline media of Biuret reagent to produce a purple colored complex whose absorbance is proportional to the protein concentration.
Histopathology:
Liver was collected after the rats were sacrificed in 10% formalin solution and utilized for the histopathological studies.
Liver was separated from all the groups and blotted free of blood and tissue fluids. They were fixed in bovine’s fluid (picric acid:Formalin:Aceticacid in the ratio of 75:52:5). After 24 hours the tissues were washed thoroughly in 70% alcohol and then dehydrated in ascending grades of alcohol (70,100%). Dehydration in absolute alcohol was followed by treatment of tissue with toluene: xylin (50:50) successively by 10%, 50%, 70%, 90% paraffin wax in toluene and finally to 100 % paraffin wax, at 60-62º C followed by embedding of tissue in wax.
5-15 micro-meter thick sections were serially cut in leitz microtome in horizontal plane and mounted on glass slides with the help of egg albumin in glycerin solution (50%v/v). The sections were deparaffinated in xylene and downgraded through 100, 90, 50 and 30% alcohol and then finally in water. They were then stained with 105 hematoxylin for 3-5 minutes and staining was intensified by running water. The hematoxylin stained section was stained with 10% eosin for two minutes and were then quickly passed through ascending grades of alcohol and finally treated with xylene followed by mounting in DPX.The sections were observed and desired area was photographed in anOlympus microscope. The sections were observed under 40X magnifications.
RESULTS: SERUM BIOCHEMICAL PARAMETARS.
Level of SGPT, SGOT, ALP, total bilirubin and total protein
|
Groups |
SGPT U/L |
SGOT U/L |
ALP U/L |
TOTAL BILIRUBIN mg/dL |
TOTAL PROTEIN mg/dL |
|
G1 |
29.67±0.84 |
66.3±3.7 |
81.5±1.1 |
1.3±0.06 |
8.3±0.46 |
|
G2 |
177.3±3.9# |
290.5±28.4# |
338.0±15.1# |
2.7±0.16# |
5.1±0.37# |
|
G3 |
38.03±0.29*** |
73.6±4.5*** |
107.7±6.2*** |
1.3±0.12*** |
7.5±0.13*** |
|
G4 |
66.0±6.8*** |
63.6±4.9*** |
71.5±6.0*** |
1.8±0.21*** |
7.4±0.02*** |
|
G5 |
48.5±4.5*** |
64.8±9.9*** |
68.0±6.6*** |
1.5±0.11*** |
7.7±0.06*** |
All values expressed as mean ± SEM; OnewayAnova followed by Newman-Keuls Multiple Comparison Test. #P<0.0001 vs G1; *** P<0.0001 vs G2
G1 – normal control rats were treated with vehicle (distilled water 10 ml/kg bwp.o.)
G2 – toxic control rats were treated with CCl4 at a single dose 0.1 ml/100 g body weight i.p.
G3 – rats were treated with glimepiride10 mg/kg body weight p.o.
G4 – rats were treated withglimepiride20 mg/kg body weight p.o.
G5 – rats were treated with silymarin 2 mg/100 g body weight p.o.

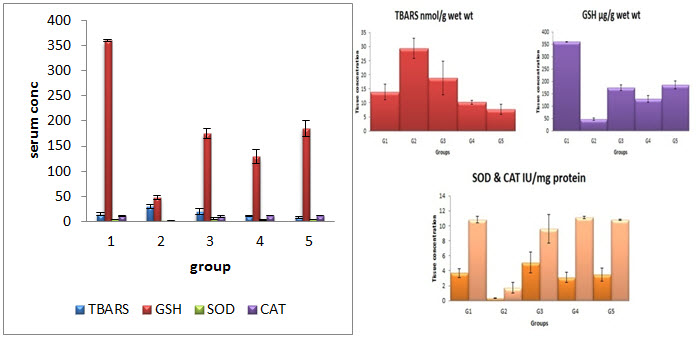
EFFECT ON TISSUE PARAMETERS
Level of TBARS , GSH, SOD and Catalase (CAT) in liver homogenate
|
Groups |
TBARS |
GSH |
SOD |
CAT |
|
G1 |
13.8±2.8 |
360.2±1.4 |
3.7±0.59 |
10.8±0.44 |
|
G2 |
29.4±3.6a |
47.1±4.4b |
0.34±0.01b |
1.7±0.71b |
|
G3 |
18.8±5.9* |
175.0±10.9*** |
5.1±1.4*** |
9.6±1.9*** |
|
G4 |
10.2±0.7** |
129.0±13.4*** |
3.1±0.68*** |
11.1±0.18*** |
|
G5 |
7.7±1.7** |
185.6±15.6*** |
3.5±0.86*** |
10.8±0.09*** |
All values expressed as mean ± SEM; OnewayAnova followed by Newman-Keuls Multiple Comparison Test. aP<0.01 vs G1; b P<0.0001 vs G1; * P<0.01 vs G2; ** P<0.001 vs G2; *** P<0.0001 vs G2.
G1 – normal control rats were treated with vehicle (distilled water 10 ml/kg bwp.o.)
G2 – toxic control rats were treated with CCl4 at a single dose 0.1 ml/100 g body weight i.p.
G3 – rats were treated withglimepiride10 mg/kg body weight p.o.
G4 – rats were treated with glimepiride20 mg/kg body weight p.o.
G5 – rats were treated with silymarin 2 mg/100 g body weight p.o.
EFFECT ON HISTOPATHOLOGY OF LIVER
Histopathology
Rats treated with vehicle (G1) shows normal architecture of hepatocytes and central vein. Rats Histopathology of liver. G1 – normal control rats were treated with vehicle (distilled water 10 ml/kg bwp.o.). G2 – toxic control rats were treated with CCl4 at a single dose 0.1 ml/100 g body weight i.p.G3 – rats were treated with glimepiride10 mg/kg body weight p.o.G4 – rats were treated withglimepiride 20 mg/kg body weight p.o.G5 – rats were treated with silymarin 2 mg/100 g body weight p.
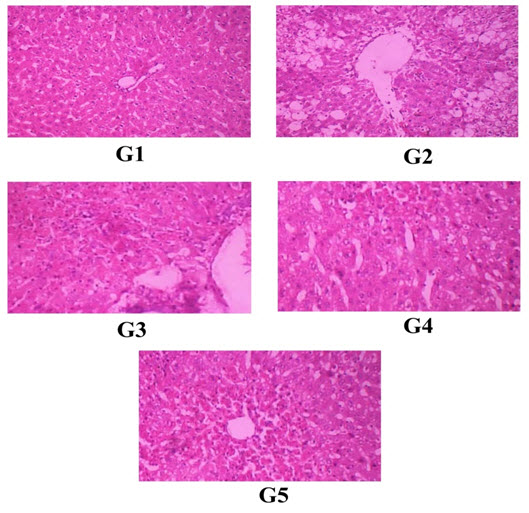
Histopathology of liver. G1 – normal control rats were treated with vehicle (distilled water 10 ml/kg bwp.o.). G2 – toxic control rats were treated with CCl4 at a single dose 0.1 ml/100 g body weight i.p.G3 – rats were treated with glimepiride10 mg/kg body weight p.o.G4 – rats were treated with glimepiride 20 mg/kg body weight p.o.G5 – rats were treated with silymarin 2 mg/100 g body weight p.o.
DISCUSSION
Several chemicals and drugs are involving in the development of toxicity. In the present research CCl4 was selected as chemical to induce hepatotoxicity in experimental animals. Carbon tetrachloride (CCl4) is effective hepatotoxin which is used in preclinical laboratory to induce liver damage in animals (Girish et al., 2009; Bagban et al., 2012). Cytochrome P450 an enzyme is act on CCl4 and converts CCl4 in to active trichloromethyl radical (CCl3.) which is further react with O2 to generate more free radicals. The generated free radical initiates the lipid peroxidation to cause cell necrosis and subsequent cell death (Manna et al., 2006; Shyamal et al., 2006).
In the present research administration of CCl4 significantly altered the serum marker enzymes of liver. Similar findings also observed by other investigators in this model (Rajesh and Latha, 2004; Bera et al., 2012; Mistry et al., 2013). During liver damage serum marker enzymes like SGPT, SGOT and ALP were released in to blood stream from hepatocytes (Shenoy et al., 2001). The elevated level of these enzymes along with total bilirubin and diminished level of total protein are indicative of cellular leakage and loss of functional integrity of cell membranes in liver (Rajesh and Latha, 2004; Fahmy et al., 2009). Increase in the normal upper limits in the measured serum transaminases of CCl4 administered group was a biochemical indication of liver injury. The biochemical parameters has been reverted significantly by the administration of glimipride in two different dose (10, 20 mg/kg).and the effect glimipride almost comparable to rats treated silymarin. So, the protective role glimipride of may be via stabilization of plasma membrane and protection of liver tissue from necrotic damage caused by CCl4.
Oxidative stress plays a major role in the development of hepatotoxicity. Generation of trichloromethyl radical from CCl4 during metabolism is an indication for generation of oxidative stress via lipid peroxidation (Jayakumar et al., 2006). The level of lipid peroxide is a measure ofmembrane damage and alteration in structure and functionof cellular membranes (Mistry et al., 2013). The elevated level of lipid peroxidation in the form of TBARS is marker enzyme for oxidative stress in liver homogenate of CCl4 treated animals is consistent with this hypothesis.
Endogenous antioxidants like GSH, SOD and CAT plays a major role to fight against free radicals. Depletion of GSH, SOD and CAT in CCl4 treated rats is confirmed the loss of antioxidant mechanism in animals against oxidative stress. Loss of antioxidant enzymes may cause accumulation of highly reactive free radicals leading to further damage. CCl4 leads to generation of peroxy and superoxide radicals which are associated with the inactivation of these antioxidant enzymes (Bera et al., 2012). Oral administration of in glimipride two different dose (10 and 20 mg/kg) and silymarin significantly decreased the TBARS and enhanced the levels of GSH, SOD and CAT is indicating the protective role of in glimipride against CCl4 induced oxidative stress. The protective mechanism of glimipride may be due to its putative antioxidant property.
The protective role further supported by histopathological study. CCl4 treated rat shows extensive necrosis on hepatocytes with enlarged central vein, massive fatty changes, ballooning degeneration and vacuolization. Treatment with is in glimipride reverted these histopathological changes to normal level indicating its hepatoprotective activity.
CONCLUSION
The present findings observed in this study revealed that, glimipride is lignin possess significant antioxidant activity against CCl4 induced hepatotoxicity via antioxidant mechanism. However, further research is required to find out the other possible mechanism of hepatoprotection to confirm the as glimipride hepatoprotective molecule.
REFERENCES
1. Hukkeri. VL, KusomsAkki. M, Sureban. RR, Gopala Krishna. B, Byahatti. VV andRajendra. S.V; Hepatoprotective activity of the leaves of Nyctanthes arbortristis Linn; IJPS; 2006; 68(4); 542-543.
2. Zimmerman HJ; Drug-induced liver disease; The Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd ed. Philadelphia, PA: Lippincott Williams &Wilkins; 1999; 427-456.
3. Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH; Timing of new black box warning and withdrawals for prescription medications; JAMA; 2002; 287(17); 2215-2220.
4. Temple RJ, Himmel MH; Safety of newly approved drugs: implications for prescribing; JAMA; 2002; 287(17); 2273-2275.
5. Michael P. Holt and Cynthia Ju; Mechanisms of Drug Induced Liver Injury; AAPS Journal;2006; 8(1); 48-54.
6. Gerard J. Tortora, Sundra Reynolds Grabowski; Priniciple of anatomy and physilogy; 7th Ed.; 1993; 790-795.
7. Kamal E. H. El-Tahir, Othman A, Gubara and AbdulaRahma M. Ageel ; Conventional drugs and herbal medicines induced hepatotoxilcity; Saudi Pharmaceutical Journal;2005; 13(4); 129-138.
8. Sherwood and Lauralee; Human physiology: from cells to systems; 3rd ed. Boston:Wadsworth; 1997; 790-795.
9. Tzanakakis; Liver assist Devices; Annual review of Biomedical Engineering 2; 607-632. (2000) 542-543.
10. Jafri. M.A, JalisSubhani. M, Kalim Javed, Surender Singh; Hepatoprotective activity of leaves of Cassia occidentalis against paracetamol and ethyl alcohol intoxication in rats; Journal of Ethnopharmacology; 1999; 66(3); 355–361.
11. Kumar. G, Sharmila Banu. G, Vanitha Pappa. P, Sundararajan. M, Rajasekara Pandian. M; Hepatoprotective activity of Trianthema portulacastrum L. against paracetmol and thioacetamide intoxication in albino rats; Journal of Ethanopharmacology; 2004; 92(1); 37-40.
12. Shanumugasundaram and Venkataraman; Hepatoprotective and antioxidant effects of Hygrophila auriculata (K. Schum) Heine Acanthaceae root extract; Journal of Ethnopharmacology; 2006; 104(1-2); 124–128.
13. Yong Woo Choi, Seung Oh Rew, OuKyoung Kwon and Se Ung Chon; Changes of SGOT and SGPT after halothane, Enflurane and Thalamonal Anesthesia; The Journal of Korean Society of Anesthesiologists; 1984; 17(1); 12-16.
14. Elise Andrews, Ann K. Daly; Flucloxacillin induced liver injury; Toxicology; 2008; 254(3); 158-163.
15. Friedman, Scott E, Grendell, James H, Mc Quaid, Kenneth R; Current diagnosis and treatment in gastroenterology; N New Yark. Lang Medical Books, McGrwHill; 2003; 664-679,
16. Ganesh Rajaraman, Jie Chen, Thomas K.H. Chang (2006) Ginkgolide; 1997; 790-795.
17. Nilesh Mehta, Lisa Oxick, Emmanuel Gbadeham (2008) ; Drug induced hepatotoxicity; emedicine. 1993; 790-795.
18. Kamal E. H. El-Tahir, Othman A, Gubara and AbdulaRahma M. Ageel; Conventional drugs and herbal medicines induced hepatotoxilcity; Saudi Pharmaceutical Journal; 2005; 13(4); 129-38.
19. Jussi J. Saukkonen, David L. Cohn, Robert M. Jasmer, Steven Schenker, John A. Jereb, Charles M. Nolan,Charles A. Peloquin, Fred M. Gordin, David Nunes, Dorothy B. Strader, John Bernardo, Raman Venkataramanan, and Timothy R. Sterling; An official ATS statement: Hepatotoxicity of antituberculosis therapy; Am JRespir. Crit. Care. Med.; 2006; 174(8); 935–952.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









