 About Authors:
About Authors:
1Shashi Kant*, Satinder Kumar,
Research Scholar Department of Pharmacy.
2Dr. Bharat Prashar
HOD & Associate professor,
Department of Pharmaceutical Sciences,
Manav Bharti University, Solan (H.P)
* shashi_ranaute@yahoo.in
Abstract:
In this general overview, we discussed about dentifrices, types of dentifrices, formulation, evaluation and its recent trends. There have been many dentifrices produced over the years, many focusing on marketing strategies to sell products, such as offering whitening capabilities. The most essential dentifrice recommended by dentists is toothpaste which is used in conjunction with a toothbrush to help remove food debris and dental plaque.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1339
IMPORTANT IN THIS ARTICLE:
Introduction:
Dentifrices are preparations meant to clean the teeth and other parts of oral cavity (gums) using a finger or a toothbrush. They are available as tooth powder, toothpastes, gels, dental creams and even as dental foams. They are meant to enhance the personal appearance of the teeth (daily removal of pellicles) by maintaining cleaner teeth, reduction of bad odour (removal of putrifying food particles from spaces between teeth) and also make the gum healthy.[1][2][3]
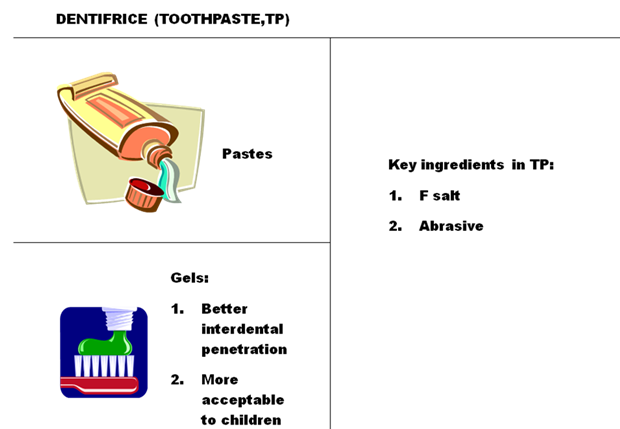
Types of Dentifrices:
1) Toothpaste:
Toothpaste is a dentifrice used in conjunction with a toothbrush to help maintain oral hygiene. The essential components are an abrasive, binder, surfactant and humectants. Other ingredients are also used. The scientific and dental community also recommends a fluoride containing toothpaste. The main purpose of the paste is to help remove debris and plaque with some marketed to serve accessory functions such as breath freshening and teeth whitening.
2) Toothpowder:
Tooth powder is an alternative to toothpaste. It comes in both a fluoride and non-fluoride version.
Tooth powder was generally used among the Romans, who used a variety of substances, such as the bones, hoofs, and horns of certain animals; crabs; egg-shells, and the shells of the oyster and the murex. They were reduced to a fine powder after having been previously burnt, and sometimes mixed with honey.
Ingredients that were sometimes added were ground myrrh, niter and hart shorn. Tooth powder was used to clean and whiten teeth and to fix them when loose, to strengthen the gums, and to assuage toothache.
3) Mouthwash:
Mouthwashes come in a variety of compositions, many claiming to kill bacteria that make up plaque or to freshen breath. In their basic form, they are usually recommended to be used after brushing but some manufacturers recommend pre-brush rinsing. Dental research has recommended that mouthwash should be used as an aid to brushing rather than a replacement, because the sticky resistant nature of plaque prevents it from being actively removed by chemicals alone, and physical detachment of the sticky proteins is required.
Scientific evidence suggests three main types of mouthwash:
Plaque inhibiting – this prevents dental disease
Ant gingivitis – this prevents gum disease
Fluoride – designed to strengthen enamel, preventing cavities or repairing existing ones to some degree[1][4]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
General Ingredients:
These ingredients are common for toothpowder and toothpaste
Abrasives/Polishing Agents
Foaming/Wetting/Cleaning/Surface active Agents
Sweetening Agents
Flavouring agents
Toothpastes also contain additional agents like
Binding/Gelling Agents
Water
Humectants
Preservatives
Therapeutic Agents
Miscellaneous Agents
Abrasives/Polishing Agents:
These are solid cleansing materials which primarily, act by removing the debris and residual stain from the teeth by providing friction and secondarily by polishing the surface of the enamel. They generally comprise of 20-50% of the total formulation. Examples are silica, sodium metaphosphate, magnesium trisilicate, precipitated chalk, tribasic calcium phosphate, hydrated alumina. Some attempts have been made to substitute mineral abrasives with softer organic substances etc, that will clean the surface of the teeth and the same time overcome the eroding action of mineral abrasives but they have been found very expensive.
Commonly used abrasive are listed below together their advantages and disadvantages.
Chalk or precipitated calcium carbonate: It is prepared by the double decomposition of calcium chloride and sodium carbonate in an aqueous solution. These are of low cost and are easily available in number of density grades, ranging from light to extra dense. However, the popularity has seen some set backs impurities present and variation in the abrasivity in different lots of the same grade.
Calcium Phosphate: There are a variety of insoluble calcium phosphates that are extremely popular and effective in dentifrices formulation.
Dicalcium phosphate, dehydrate is excellent and relatively low in abrasion but is incompatible with most fluorides. For dentifrices use it should contain a stabilizer to prevent grittiness, caking or hardening of the paste of ageing. For this purpose magnesium phosphate, magnesium stearate, magnesium sulphate, tetrasodium pyrophosphates are used.
Dicalcium phosphate, anhydrous is very abrasives and generally used in low concentrations to increase the total abrasivity of the paste. It is also incompatible with most fluorides.
Insoluble sodium metaphosphate: is moderately abrasives and compatible with fluorides but relatively costly.
Silica’s: Hydrate silica’s are becoming increasingly popular choices as dental abrasives. They are off two types.
-Abrasive silica
-Thickening silica
Abrasive silica: are dense, relatively non absorbent, odorless and tasteless powders. Commercial grade silica “xerogels” are manufactured under specific manufacturing conditions and are conspicuous by having such structures which are free void or air spaces.
Thickening silica: also referred to as “aerogels “commercially, are extremely small size particles with very large surface areas and have the capability of swelling and of thickening the resulting pastes.
Foaming/Wetting/Cleaning/Surface active Agents:
These are either a surface active agent or a soap which is used to aid the action of abrasives by reducing the surface tension and wetting, the surface of the teeth. They penetrate and loosen surface deposits, emulsify and suspend the debris, which the dentifrices remove from tooth surface. Surface active agents are foaming agents employed at levels of 0.5-2% to provide necessary foaming action.
The most popular is: sodium lauryl sulphate, other surfactants that may be used are sodium lauryl sacrosinate, sodium lauryl sulfoacetate and dioctyl sodium sulfosuccinate. Soap is generally used for lather making and cleansing action in dentifrices. The soap should be completely saponified, should contain 2% moisture, not more than 0.3% free alkali, calculated as sodium carbonate.
Sweetening Agents:
These are added to mask the bitter tastes of ingredients specially foaming agent and flavour oils. Nutritive sweeteners like carbohydrates cannot be used hence synthetic compounds like saccharine, aspartame, cyclamates or potassium acesulfane can be used in concentrations between 0.05-0.25percent.
Flavouring agents:
Dentifrices flavours belong to a class which not only satisfy the requirements of the formula but also satisfy the psychology of the consumer who is looking forward to fresh breath after brushing. Therefore they should help prepare a product which have a pleasant long lasting effect and which preferably has a medicinal or freshening impact. Examples are spearmint oil, peppermint oil, oil of winter green, clove oil, eucalyptus oil, anise oil, sassafras oil etc. The flavours are generally used at level between 0.2-2percent.
Binding/Gelling Agents:
Binders are natural or synthetic gums used in dentifrices formulations to hold the liquid and solid constituents in the form of a smooth paste. They increase the body and viscosity of the liquid phase as well as the final formulation, preventing liquid bleeding from the paste. Binders are generally used in concentrations between 0.9-2.0percent of the formulations. Natural synthetic gums, resins and other hydrocolloids may be employed. The most popular binder is “carboxy methyl cellulose”.
Water:
Deionised water should be used to formulate toothpastes, water is present in most dentifrices formula as both solvent for soluble ingredients as well as for supporting the binding agents, which swell after imbibing a lot of water.
Humectants:
These are one of the liquid components of a toothpaste. They are incorporated to prevent moisture loss and drying out of dentifrices so that the viscosity of the product is maintained. In opaque paste they are generally employed in concentrations of between 20%-40%. Clear gels are formulated with as much as 80%. Most frequently used are sorbitol, glycerol and propyl glycol.
Preservatives:
Formulations of toothpastes require the incorporation of preservatives to maintain the quality and stability of the product. Some preservative action is obtained by the flavouring oils and chloroform present. A mixture of 0.15% methyl paraben is effective as a preservative. Some flavouring (volatile) oils, chloroform, methyl hydroxyl benzoate, propyl hydroxyl benzoate are the common preservative used in toothpastes.
Therapeutic Agents:
All toothpaste doesn’t contain these agents. These are added in specially formulated medicated toothpaste has either bacterial, bacteriostatics, enzymes-inhibiting or acid neutralizing qualities. They thus reduce dental disease prevents mouth odour. Chlorophyll fluoride salt, urea, triclosan, dibasic ammonium phosphate penicillin, chlorhexidine, sodium dehydroacetae, neem extract are added for there therapeutics agents.
Miscellaneous Agents:
Titanium dioxide may be added as a whitening agent whenever desired. Buffers salts such as sodium phosphate may be used to maintain ph at the desired levels. Certified colors may be added.[5][6][7]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
1) TOOTHPASTE:-
Formulation:
Tooth pastes are prepared either by the dry gum technique or wet gum technique . in the first technique all the solid components, including the binding agent ( but excluding surfactants ) is first dry mixed and then the liquid components , that is humectants and water is gradually added while in the second technique the binding agent is first mixed in the liquid phase, a mucilage prepared an then the rest of the solid ingredients are added (excepting surfactants ) and mixed well to produce a homogenous paste mass . The homogenous paste obtained from either processed must then be mixed with both the surfactants and flavored under the vacuum .
Based on this technique a no. of acceptable procedure are in use.
Cold process:-
The humectants such as glycerin or sorbitol is added to the bowl of the mixer. The binder is sprinkled in under agitation, so that the particles are dispersed in the absence of water, preventing swelling at this point a separate liquid phase is prepared, which includes the available water, sweetener, preservatives and any therapeutics additives. This solution is than added to the humectants binder mixer. The mixer is placed under vacuum for 5 min. to de-aerate the thick gelatinous liquid phase the vacuum is open and the abrasive are added with mixing until they are thoroughly wet down. Vacuum is re applied and the paste is mixed for at least 30 min. under 28 inches or more of vacuum. In the mean time the surface active agents and flavour are dispersed in about 5% of the available humectants at the conclusion of the 30 min. time, vacuum is again opened, the flavour mixer is added. 5 min. of additional mixing under vacuum will usually produce a smooth air free paste .
Heated liquid phase process:-
In this method the abrasive, binder and preservatives are premixed as dried powders in the mixer a hot solution of the humectants, water and sweetener is than slowly added with mixing of the dried powders . the resulting mass is mixed under vacuum for 30 min. after which the solution of flavour and surfactant is added for a final 5 min. of vacuum mixing
Multiple Liquid– Phase Process:-
This method is particularly adaptable to formulation using a magnesium aluminum silicate- carboxy methyl cellulose binder system. Magnesium aluminum silicate is added to hot water in the mixing vessels followed by the sweetener a separate phase is prepared consisting of the bulk of the humectants, the binder, the flavour and the preservatives . this solution is added to the mixer, followed by the balance of the humectants. 5 min. of vacuum mixing should be perform to desecrate the liquid mixer, abrasives added and again mixes for 30 min. under vacuum after this the surfactant is added in dry form, followed by another 5 min. of vacuum mixing. [1][9]
A Formula For Toothpaste:
|
INGREDIENTS |
% PROPORTION |
|
Precipitated calcium carbonate |
39.5% |
|
Glycerol |
20% |
|
Sodium Lauryl Sulphate |
6.3% |
|
Gum Tragacanth |
0.4% |
|
Sodium Saccharine |
0.1% |
|
Peppermint oil |
1.2% |
|
Water |
32.5% |
A Formula For Therapeutic Toothpaste:
|
INGREDIENTS |
% PROPORTION |
|
Calcium Carbonate |
50.0% |
|
Sodium copper chlorophylline |
0.30% |
|
Tetrasodium pyrophosphate |
0.25% |
|
Peppermint oil |
1.00% |
|
Gum tragacanth |
1.00% |
|
Glycerol |
20.00% |
|
Sodium saccharine |
0.10% |
|
Water |
24.85% |
Some Commercial Brands of Toothpastes:-
* Pepsodent
* Close up
* Colgate
* Meswak
* Dabur
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
2) TOOTHPOWDER:
Formulation and Preparations:The main components of toothpowders are solid particles of very fine size and the end product is also a very dry powder. Since the main components like abrasives, surface active agent are solid powders, it is required that they all are in very fine particle size, comminuted, if desired, passed through a sieve and mixed in a mortar in the lab scale and in blenders on an industrial scale. The flavoring oils are added in the end either by spaying on the powder mixture or first blending with one of the components and then mixing this blend to the rest of the mixture by the method of dilution or geometric progression.
A Typical Toothpowder Formula [1]:
|
INGREDIENTS |
QUANTITY |
|
Hard soap (in fine powder) |
50 gm |
|
Precipitated calcium carbonate |
935gm |
|
Saccharine sodium |
2 gm |
|
Peppermint oil |
4 ml |
|
Cinnamon oil |
2 ml |
|
Methyl salicylate |
8 ml |
|
To make about |
1000 gm |
Some Commercial Brands of Toothpowders:-
* Colgate
* Dabur red
* Vico vajardanti
3) MOUTHWASH:
A mouth wash is an aqueous solution which is most often used for its deodorant, refreshing or antiseptic effect in the oral cavity. Water is the simplest mouthwash, and aqueous saline is the least complex type of mouthwash. A mouth wash may contain alcohol, glycerin, synthetic sweeteners and surface active, flavouring and colouring agents.
Types of Mouthwashes:-
1. Cosmetic mouthwashes.: contain water, flavour, alcohol, surface active agent.
2. Antiseptic mouthwashes: whose main purpose is to remove or destroy the bacteria normally found in the oral cavity in the large number.
3. Mouthwash concentrates: which are concentrated products labeled to be diluted before use.
4.Buffered mouthwashes: which primarily control the pH, within narrow ranges, in the oral cavity. E.g. alkaline buffered mouthwash.
5. Deodoring mouthwashes: which primarily serve to deodourize the oral cavity, by the antibacterial.
6. Therapeutic mouthwashes: which are specifically formulated for the purpose of relieving infection, preventing dental caries.
7. Liquid mouthwashes: ready to be used without any dilution.[9]
Formulation of Mouthwashes:
Most mouthwashes contain for basic ingredients:
-Alcohol
-Flavours
-Humectants
-Surfactants with water
A Formula For Mouthwash:[1][3]
|
INGREDIENTS |
% PROPORTION |
|
Boric acid |
1.5% |
|
Thymol |
0.1% |
|
Euclyptol |
0.5% |
|
Methyl salicylate |
0.5% |
|
Oil of thyme |
0.03% |
|
Menthol |
0.1% |
|
Alcohol |
30.00% |
|
Water |
67.67% |
Manufacture of Mouthwashes:
Manufacture of mouthwashes is extremely simple and in principle, it only requires one or more stainless steel tanks, efficient mixture and a storage tank. This is because all ingredients are soluble in water and the finished product has a viscosity more or less similar to that of water. Glass lined, non pitted mixing tanks are satisfactory for most of the products. Explosion roof equipment and a bonded storage area is required, and stringent safety precautions and very strict control on bonded ethanol is maintained.
Evaluation of Formulated Mouthwashes:
1.Soft Tissue Examination:- A complete oral tissue examination should be carried out to evaluate the condition of the oral mucosa of the volunteers.
2.Gingival index:- Can be determined employing Ram fjord teeth.
3.Odour Measurement:- organoleptic measurements can be made by odour judges based on the whole mouth expirates as well as odour assessment from the anterior and posterior of the tongue dorsum.
4.Oral Microbial Levels:- It is generally determined using the oral test, a technique which measures the rate of oxygen depletion in expectorated milk samples.[1][4][10]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
FILLING OF DENTRIFRICES IN TUBES:
The working process of a tube filling and closing machine can be divided into four main groups
-Handling of tubes
-Tube preparation
-Filling of Tubes
-Tubes closing and sealing
IN PROCESS EVALUTION AND STABILITY STUDIES:
While the formula is being validated, experimental paste should be evaluated to assure that they meet the pre established characteristics. Samples in tubes should be placed under stability testing at various temperatures, not only to ensure the stability of the formulation but also to assure compatibility with the tubes choose for marketing of the product.
Stability is the ability of toothpaste to retain its important characteristics essentially unchanged throughout its expected shelf life. test must access the physical stability of the paste as well as the chemical stability of its ingredients . in the case of therapeutic (medicated ) toothpaste , which are considered drugs , the stability of the active ingredients must be established and the reflected in the expiration data on the packages . stability evaluation must be conducted in conjunction with the packages development . final stability tests should always be performed on product packaged in its commercial container. Since stability studies should reflect the storage conditions that may be encountered during the expected lifetime of the product, there fore the evaluation should be carried out at temperatures ranging from 0 to 50 degree Celsius over the shelf life of the product. Insight into long term stability can often be attained through the use of accelerated stability studies during which increased temperatures may simulate the behavior of the product over along period in a relatively short studies a stability protocol may consist of the following schedule
-select the sample product
-perform the test for physical properties
-analyzed for chemical test
-weigh them store them at 5, 35, 45 degree centigrade and room temperature
-Samples are withdrawn at intervals and re-evaluated. Recommended evaluation intervals are 1 week, 1 month, 3 month and 6 months at elevated and reduced temperatures studies for 1, 2 and 3 years.
-In addition, samples should pass three cycles of freezer, oven without separation or major changes in specifications. Initial expiration dates for drugs containing toothpastes may be based on accelerated test data, but should be modified as real time data becomes available. Paste that still remain their original specifications after three months at 45 degree Celsius may carry an estimated expiration date of 2 year after manufacture.
Stability of Gels
The formulation and manufacture of a gel system is not complete without an evaluation of the stability of that system. The chemical integrity of dispersed active ingredients must be assured over the shelf –life of the product.
Types of unstable gels may vary from gels that “set up” during storage and can no longer be expressed from a tube.
-“gels that undergo a separation of phases, either of the liquid(as in syneresis) or of the solid (as in particle sedimentation)
-gels” that suffer a progressive loss of viscosity o consistency, changing from semisolid to viscous liquid.
Indian standard comprise of specification for tooth powder, this standard prescribes the requirements and the method of sampling and test for toothpowders. As per this standard, toothpowder shall be smooth, uniform, free flowing fine powders, free from hard abrasive materials. The other requirement as per this standard are determination of fineness, moisture and volatile matter, pH of 10% aqueous suspension, foaming power, presence of lead, arsenic and hard and sharp edged abrasive particles.[1][7][8][9][10]
RECENT TRENDS:
Some years ago semisolid pastes (with viscosity) were sold in inverted polyethylene bottles. These had limited success and were followed by pressurized aerosols containers producing products called “Dental foams” dispensing paste through a dip tube and foam-style valve. These pastes were pressurized with nitrogen. But they failed in the market owing to several reasons. One of it was that as the paste was dispensed the remaining paste in the container tended to cavitate, eventually discharging the nitrogen, inactivating the container and wasting the balance of product. Accidental misuse caused loss of the nitrogen propellant and was responsible for many failures.[11]
CONCLUSION:
The snapshot of global oral health outlined in this article represents a ‘good news/bad news’ scenario. Overall, many adults are keeping their teeth longer, but retaining ones’ dentition longer translates to greater oral health challenges, such as plaque, calculus, and stain control. The development of new advanced all-in-one dentifrices, such as this advanced stannous-containing sodium fluoride formulation, represent a welcome supplement to patient oral care routines. A dentifrice serves as a logical delivery vehicle for therapeutic antimicrobials, plaque-removing surfactants, caries-fighting fluoride, and esthetic-enhancing ingredients, all in a single, accessible medium.
References:
1. Nanda, A. and Khar, R.K, “A book of Cosmetic Technology”, Page No. 442-471
2. Remington “The Science and Practice of Pharmacy”, Editor-Alfonso R. Gennaro, 19th edition vol. 1 and 2, Mack Publishing Company, Pennsylvania,1995
3. Cooper and Gun ’Dispensing for Pharmaceutical Students”,12th edition, 1987 edited by SJ Caeter, CBS Publishers and Distributors, Delhi
4. Adams C, Slack-Smith LM, Larson A,O’Grady M. Edentulism and associated factors in people 60 years and over from urban, rural and remote Western Australia. Aust Dent J 2003;48:10-14.
5. American DentalAssociation Description of Toothpaste"Toothpaste. 2010-04-15.
6. NIDCR/CDC Dental, Oral and CraniofacialData Resource Center, Oral Health, U.S. 2002 Annual Report. Executive Summary. Available at: drc.hhs.gov/report/summary.htm (Accessed 10/25/2009).
7. "Other ingredients in toothpaste". Archived from the original on October 18, 2007. Retrieved 2007-12-23
8. Toothwear and sensitivity: Clinical advances in restorative dentistry. London: Martin Dunitz; 2000:239-242
9. Addy M. Dentine hypersensitivity: New perspectives on an old problem. Int Dent J 2002;52:367-375
10. Nevitt GA, Witter DH, Bowman WD (September 1958). "Topical applications of sodium fluoride and stannous fluoride". Public Health Rep 73 (9): 847–50
11. K. Horton. Advance research study on dentrifrices,Universitat Rovira I Virgili, 2003. 543-549
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









