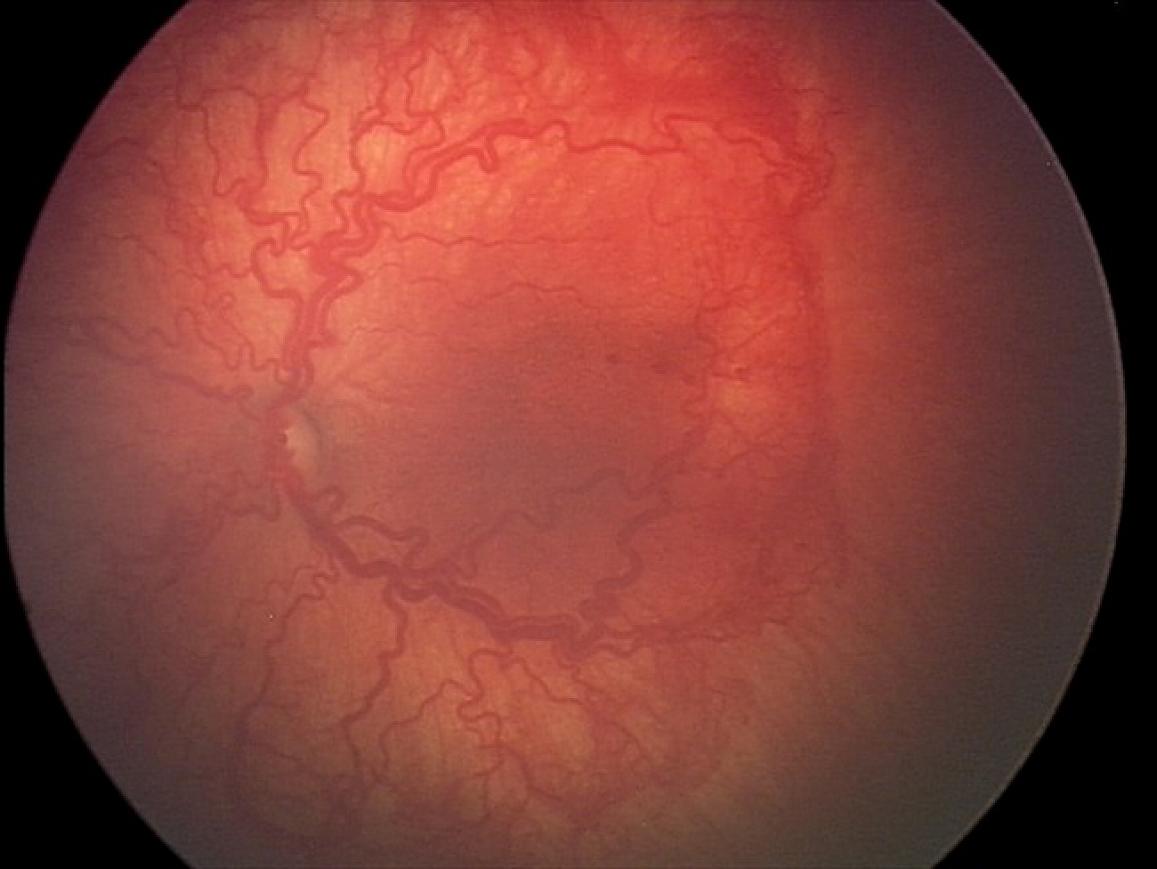
Results from a clinical trial published in 2011 confirmed the benefit of using Avastin over laser therapy for treating the most severe cases of ROP, which occur in a region of the retina known as posterior zone 1, as shown. David Wallace, M.D.
Babies born prematurely who require treatment to prevent blindness from retinopathy of prematurity (ROP) could be treated with a dose of Avastin (bevacizumab) that is a fraction of the dose commonly used for ROP currently. Results from the dose-finding study were published April 23 in JAMA Ophthalmology. The study was conducted by the Pediatric Eye Disease Investigator Group (PEDIG) and supported by the National Eye Institute (NEI), part of the National Institutes of Health.
Preterm babies are at high risk of abnormal blood vessel growth in the retina, the light-sensitive tissue in the back of the eye. These abnormal blood vessels are fragile and prone to leaking. If left untreated, vessel growth can lead to scarring and retinal detachment, the main cause of ROP-related vision loss. ROP is one of the leading causes of blindness in children.
Established ROP treatments include laser therapy and cryotherapy. Both interventions work by causing the abnormal blood vessels to stop growing before they can cause scarring and retinal detachment.
Avastin is one of several available drugs that inhibit abnormal blood vessel growth by suppressing the overproduction of a signal protein called vascular endothelial growth factor (VEGF).
The U.S. Food and Drug Administration approved Avastin in 2004 as a cancer therapy. Since then, ophthalmologists have used it off-label to inhibit abnormal blood vessel growth in ROP, as well as in other ocular disorders. Results from a clinical trial published in 2011 confirmed the benefit of using Avastin over laser therapy for treating the most severe cases of ROP, which occur in a region of the retina known as posterior zone 1.
They plan to follow children over time to compare the long-term effects of each strategy on vision and organ development. Previous studies suggest that babies treated with Avastin versus laser may be less likely to become myopic and require glasses for nearsightedness as they grow older.
The study involved 59 preterm infants with type 1 ROP, the most severe form. Each infant had one eye treated by a single injection containing 0.016 mg, 0.008 mg, 0.004 mg, or 0.002 mg of Avastin. If the other eye required treatment, it received twice the concentration (one dose level higher). By comparison, currently used doses of Avastin for ROP range from 0.25 mg to 0.625 mg.
Treatment was considered a short-term success if ROP improved by day four after therapy, and if there was no recurrence or need for additional treatment within four weeks. Such success was achieved in all eyes treated with the 0.016 mg and 0.008 mg doses, and in 9 of 10 eyes receiving 0.004 mg, but only in 17 of 23 eyes receiving 0.002 mg, resulting in the conclusion that 0.004 mg may be the lowest effective dose.
<< Back to Pharma News
Subscribe to PharmaTutor News Alerts by Email























