PharmD professionals to join in the fight against COVID-19 in Karnataka
Pharm.D doctors and interns may join in the fight against COVID-19 (corona virus) in Karnataka reported by Karnataka State PharmD Doctors Association (KSPDA) to PharmaTutor
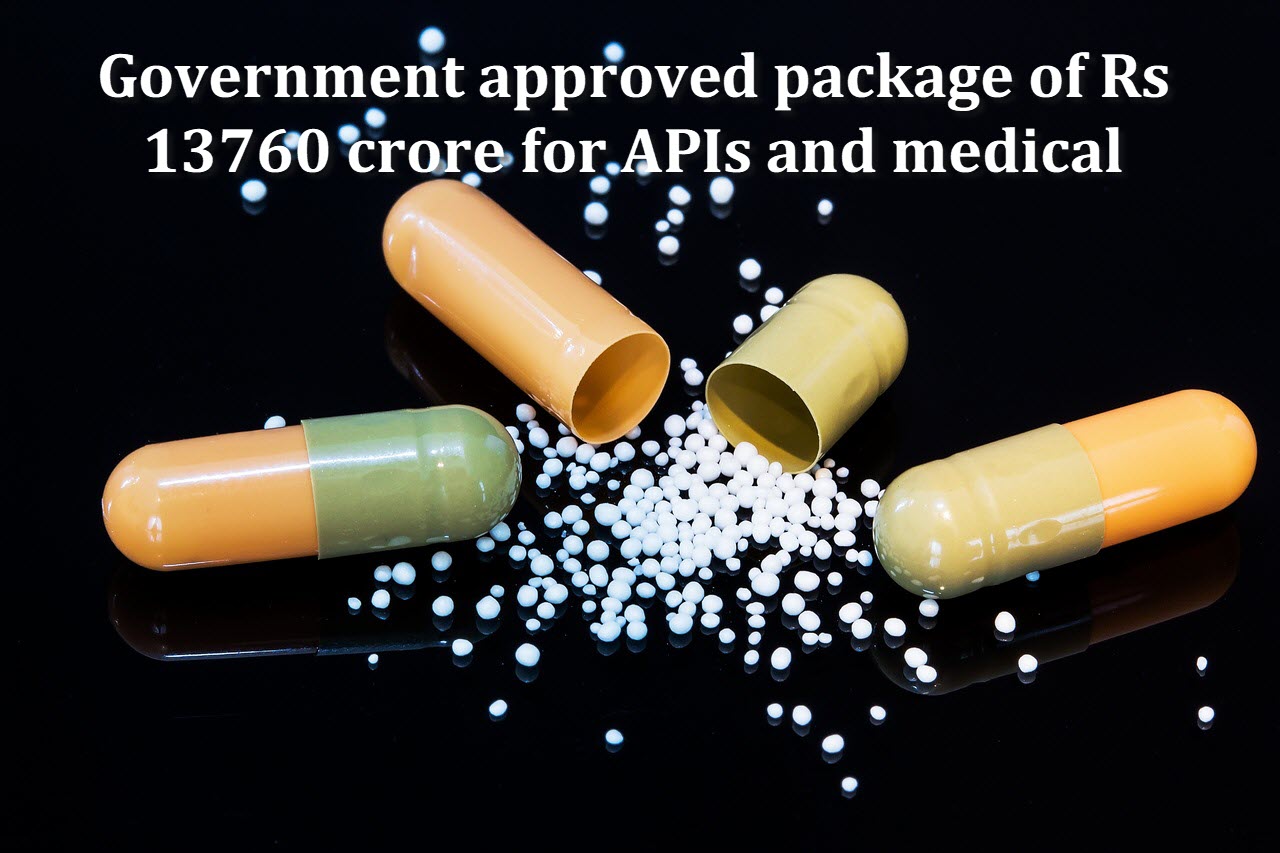
The cabinet has approved four schemes which totally include Rs 13,760 crore to promote the domestic production of active pharmaceutical ingredients (APIs) and medical devices in the country and their exports.
Dr Poonam Khetarpal Singh, regional director of the World Health Organisation (WHO) South-East Asia region, reported that there is no airborne case of the COVID-19 known yet.
Measure of public health prepardeness in respect of APIs / intermediate / KSMs which are closely monitor the production and availability of APIs and their formulations to prevent black marketing and hoarding.
Drug controller cum licensing authority, director of health services, Chandigarh are monitoring for availability of APIs and drugs in context of outbreak of COVID-19 in China. NPPA (National Pharmaceutical pricing authority) chairman had written a letter to Drug Controller, Chandigarh and all the other State's/ UTs Chief Secretaries for taking measures on price control due to COVID-19 outbreak in the country.
The Government has made amendments in the export policy and restricted export of specified APIs (Active Pharmaceutical Ingredients) and formulations made from 13 APIs for safety purpose due to COVID-19 outbreak in India. A notification issued by Directorate General of Foreign Trade, Commerce and Industry says that the restrictions will come into immediate effect and until further orders.

The Central Drugs Standard Control Organisation (CDSCO) has released the list of 139 drug formulations and their reference products permitted by DCGI to conduct bio-equivalence (BE) studies.

e-Pharmacies strictly do not accept orders for habit forming medicines, narcotics, or any other sensitive medicines. Additionally, given the importance of the e-Pharmacy sector globally, several large investors have recently reached out to the e-Pharmacy players, expressing concern about the general investment and regulatory climate in India

Pharmacovigilance Programme of India (PvPI) urges healthcare professionals to take require measures and do not prescribe Nimesulide for children under 12 years of age. PvPI urges to spread awareness among healthcare professionals to take all necessary precautions in order to avoid such mishappenings and to promote rational use of medicines.