 About Authors:
About Authors:
Indraneel sinha*, Mr. Sanjay sahai, Mr. Sunil jajoo, Mr. Abhijeet bhatkar
Post graduate diploma in pharmaceutical management,
Indian institute of health management research,
jaipur
*indraneel.sinha.999@gmail.com
COMPANY PROFILE
Sun Pharmaceuticals was set up in 1983 and the company started off with only 5 products to cure psychiatric illness. Sun Pharma is best known worldwide as the manufacture of specialty Active Pharmaceuticals Ingredients (API) and formulations.
However, the company is also concerned with chronic treatments such as cardiology, psychiatry, neurology, gastroenterology, diabetology and respiratory ailments. Active Pharmaceuticals Ingredients (API) includes peptides, steroids, hormones, and anti?cancer drugs and their quality is internationally approved. Mr. Dilip S. Shanghvi is the Executive Chairman and Managing Director of Sun Pharma and Mr. Kamalesh H. Shah is the secretary.
[adsense:336x280:8701650588]
In 1983, when Sun Pharma was set up, it only dealt with two states in India – West Bengal and Bihar. In 1985, it started trading nationally and by 2000, Sun Pharmaceuticals made its way to the international market. Products used in cardiology were manufactured in 1987 and at that time, Monotrate was one of the first products that was launched and went on to become a best-seller. In 1993, Sun Pharmaceuticals Industries set up their own research institute and named it SPARC (Sun Pharmaceutical Advanced Research Centre). In 1994, Sun Pharma enrolled itself in the main stock exchanges in India. Subsequently in 1995, the first API manufacturing plant was established at Panoli to capture the international market.
Sun Pharma shifted its headquarters in Mumbai as it is the center of Indian commercial trade. Sun Pharma's speedy activity is one of its best attributes that has made it gain an international status across the world. Quality remains the prime concern and is maintained by the team.
In the US, which is the company`s largest market, Sun Pharma has built a strong pipeline of generics, directly and through its subsidiaries Caraco and Sun Pharmaceutical Inc. Taro adds strong dermatology range to this portfolio.
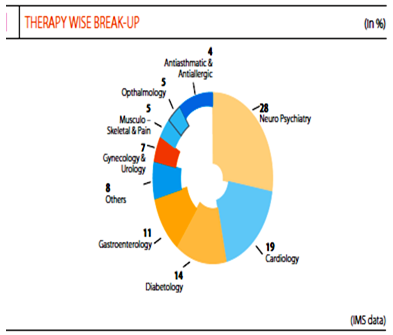
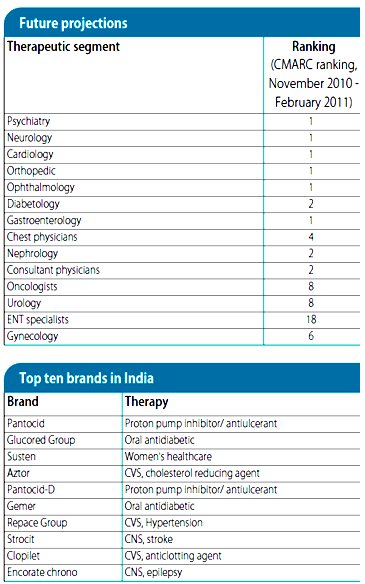
Sun holds no. 1 with the specialties as per CMARC which is at the same position as last year, and gained market share in psychiatry, neurology, gastroenterology, diabetology, ophthalmology and orthopedics. Sun pharma now holds 4.3% market share in highly competitive Indian market.
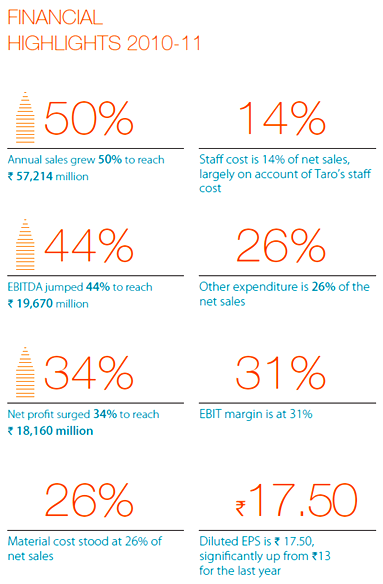
2010-11 was a good year for Sun Pharma, as were the preceding years. Sun’s financial performance was strong, they completed a significant acquisition, enriched the portfolio of products they offer in the US, strengthened their speciality rankings in India and rest of world markets, added to their intellectual capital, and yet again reaffirmed their commitment to high standards of corporate governance and stakeholder transparency.
Today SPIL, is the largest Indian company in the US generics space,& the largest pharma company in India in chronic therapies, and an emerging force in the rest of the world markets.
Reference Id: PHARMATUTOR-ART-1409
SunPharma's product portfolio consists of 4 maincategoriesof products:
1.IndiaBrandedGenerics
2.USGenerics
3.InternationalBrandedGenerics
4.ActivePharmaceuticalIngredients(API)
The company is engaged in manufacturing of product in the following therapy areas:
· CNS disorders
· Cardiology
· Diabetes and Metabolic disorders
· Gastroenterology
· Ophthalmology
· Oncology
· Pain
· Allergy, Asthma and Inflammation
· Gynaecology
|
Speciality |
Marketing Division |
|
Psychiatry |
Synergy, Symbiosis and Sirius |
|
Neurology |
Synergy, Symbiosis and Sirius |
|
Diabetology |
Arian, Azura Life Sciences and Avior |
|
Cardiology |
Arian, Azura Life Sciences and Avior |
|
Ophthalmology |
Avesta and Milmet |
|
Gastroenterology and Others |
Sun and Solares |
|
Asthma (Chest) and COPD |
Radiant |
|
Orthopedics |
Sun and Ortus |
|
Gynecology |
Spectra and Inca Life Sciences |
|
Fertility |
Inca Life Sciences |
|
Urology |
Inca Life Sciences |
|
Dermatology |
Ortus |
|
Oncology |
Sun Oncology A and B |
|
Interventional Cardiology |
Azura Critical Care |
|
Anesthesia and ICU |
Sun Speciality Care |
Other group companies:
Caraco Pharmaceutical Laboratories-
Based in Detroit, Michigan, Caraco develops, manufactures, market and distributes generic and private label pharmaceuticals and markets them throughout the United States.
Sun Pharmaceutical Industries Inc. (SPI)-
Sun Pharmaceutical Industries Inc is a Michigan Corporation and a wholly owned subsidiary of Sun Pharmaceutical Industries Lt India. A plant spread over 35,000 sq ft, in Bryan, Ohio, manufactures liquids, creams, and ointments. This plant was purchased from Valeant Pharma.
Sun Pharmaceutical (Bangladesh)-
Sun Pharmaceutical (Bangladesh) is a private limited company incorporated in March 2001 under the Companies Act 1994. This company was formed jointly with Sun Pharma, City Overseas Ltd, a company incorporated in Bangladesh and Sun Pharma Global Inc, a company incorporated under the laws of the British Virgin Islands. The company began commercial operations in October 2004. The company owns and operates a pharmaceutical factory and makes pharmaceutical products that are sold in the local market.
Alkaloida Chemical Company Exclusive Group Ltd.-
ICN Hungary, purchased from Valeant Pharmaceuticals in 2005, is one of the few units worldwide, authorized to make controlled substances. ICN Hungary has now been renamed Alkaloida Chemical Company.
Solares division:
This division markets brands in the respiratory therapy area , products for gastroenterology as well as pain management. An indigenously synthesized Terlipressin, Terlyz and a novel delivery based pancreatic enzyme, Panlipase, have been launched recently.
Therapy area coverage:
Ø Gastroenterology
Ø Respiratory
Ø Pain Management Products
Top 5 brands:
Ø Sompraz
Ø Lesuride
Ø Betavert
Ø Predmet
Ø Febutaz
EXECUTIVE SUMMARY
OVERALL SAMPLE OF DOCTORS (SAMPLE SIZE = 165)
· The objective of the study was to: “Map the prevalence and treatment strategies used in effective Vertigo Management. Determining the usage & place in therapy of once a day preparation was also to be determined.”
· To accomplish the set objectives, 165 doctors were taken into the sample set. Out of which 40 were Neurologists, 65 Consulting Physicians, 35 E.N.T doctors, 20 Cardiologists and 10 Diabetologists.
· Amongst the sampled doctors, 38.79% observed 10-15% patients requiring anti-vertigo medications.
· The % of female patients who seek anti-vertigo medications were found to be on the higher side amongst sampled doctors.
· A 40:60 (Male:Female) ratio is observed most by 60% doctors in their practice.
· 40-50 years was the prevalent category of the patients amongst 61.54% sampled doctors.
· 65.45% of sampled doctors observed Vertigo patients with Secondary to pre-existing ailment.
· Diabetes Mellitus (51.24%) is the most common secondary to pre-existing ailment, followed by Hypertension & Cardiac problems (28.86%) amongst the Sampled doctors.
· BPPV is the most common cause of Vertigo, followed by Vestibular Neuronitis and Labyrinthitis amongst the sampled Sampled doctors.
· 53.12% Sampled doctors resort to Pharmacological therapy for treating patients of Vertigo.
· Betahistine is the most preferred drug of choice for treating vertigo amongst 80.5% sampled doctors.
· 40.61% of the Sampled doctors prefer to treat with 8mg TID dose of Betahistine for patients of vertigo.
· 1-2 weeks is the most preferred duration of therapy with Betahistine amongst 71.52% Sampled doctors.
· Dosage titration was found to be prominent amongst 90.3% of the sampled doctors.
· With the usual approach being tapering down the dosage in patients where symptoms are relieved and do not recur, and then putting it off. But in some recurrent cases of vertigo the dosage is increased.
· Betahistine O.D awareness was found amongst 95.15% doctors. Only 4.85% Sampled doctors were still unaware of Betahistine O.D formulation.
· Out of all the sampled doctors 54.55% did not use Betahistine O.D formulation, they reportedly used the conventional doses.
· Amongst all sampled doctors those who use Betahistine once a day preparation, 70.67% mostly prescribe 24mg C.R, while 29.33% use 48mg C.R formulation.
· The duration for which 24mg C.R is prescribed the most is 1 week by 54.71% Sampled doctors, followed by 2 weeks amongst 43.33% Sampled doctors.
· 60.61% of the sampled doctors prefer to use O.D formulation as a start up dose, while the rest (39.39%) preferred to use it as a maintenance dose only.
· O.D formulation was not found to be used specifically against any indication amongst 90.3% sampled doctors.
· Amongst the sampled doctors, 84.24% do not use two Anti-Vertigo molecules together.
· 42.42% of the sampled doctors have not tried this combination, but 44.85% said it to be a potent and viable combination.
· They were further aided by 7.27% of doctors who felt it maybe a fruitful combination.
Note: The figures in bracket are indicative of the percentage of responses given by doctors.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
INTRODUCTION TO SUBJECT
Dizziness accounts for an estimated 5 percent of primary care clinic visits. The patient history can generally classify dizziness into one of four categories: vertigo, disequilibrium, presyncope, or light-headedness. The main causes of vertigo are benign paroxysmal positional vertigo, Meniere’s disease, vestibular neuritis, and labyrinthitis.
More than two million people per year visit their doctor for vestibular / balance disorders. These disorders are the ninth most common complaint that leads patients to visit their family physicians. Furthermore, it is one of the most common reasons elderly people seek medical advice. Patients often describe balance problems in terms of vertigo, dizziness, imbalance, blackouts, light-headedness, and motion sickness. But, not all of these symptoms are always caused by disorders of the vestibular system. Moreover, although one person may describe a balance problem using one or more of these terms, another person may use a different combination of these terms to describe the very same condition. In fact, some people will even use the word dizziness to indicate that they simply do not feel well. It is hence quite a task for the clinician to decipher the patient’s complaints and put them in their proper perspective. A very thorough history taking and the clinical tests of balance function are hence very important to ensure that the clinician is on the right track and has not been deceived into unnecessarily prescribing anti-vertigo drugs in cases which are not exactly disorders of the balance system.
VERTIGO
Vertigo, a type of dizziness, is the illusion of motion, usually rotational motion. Associated symptoms include nausea, emesis and diaphoresis. Vertigo should be distinguished from other types of dizziness, such as imbalance (dysequilibrium) and light-headedness (presyncope). Most cases of vertigo can be diagnosed clinically and managed in the primary care setting. As patients age, vertigo becomes an increasingly common presenting complaint.
Vertigo is an extremely common condition. It is very often a self limiting disorder which can be managed by the family physician. Only in a very few cases, would a referral to a specialist be required. However, in actual practice when the patient with vertigo goes to the family physician- he is immediately referred to the neurologist, ENT specialist or neurotologist, which is very often not required and is an undue harassment for the patient.
A brief physiology of the balance system
The balance system is a multi-organ, multi-system mechanism. Most medical specialists e.g. a cardiologist, an ophthalmologist or a gastroenterologist usually deals with only one particular system of the body, but for the neurotologist or the medical specialist who specializes in the management of balance disorders / vertigo, there are several body systems which are working together. These include:
• The central nervous system
• The peripheral nervous system
• The musculo-skeletal system
• The visual system
• The vestibular system
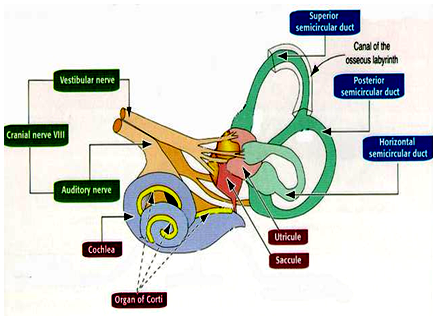
Over and above these main systems, other systems like the psychic system, the cardiovascular system and probably also the auditory system are also involved in the maintenance of balance and in disorders of any of these systems, the balance system gets screwed. Different systems of the body work in tandem to maintain the body’s balance. This is what makes the disorders of the balance system so complex and so very hard to diagnose and treat.
Maintenance of balance
How is the body’s balance maintained?
Nature has given us certain sensors which are spread in different parts of the body and these sensors inform the brain about the stability of the ground and the surroundings where the person is situated.
Once the brain comes to know about how stable the ground is or how stable are the surroundings, the brain evolves a motor output – thereby creating a contraction of different body muscles. As a result of the very precise and accurately timed contraction of the relevant muscles of the body, the body’s stability is restored thereby preventing a giddiness episode, instability or a fall. Starting from the sensors picking up the information about the stability of the ground and surroundings, its processing in the brain and the generation of the motor output, -transmission of the motor output through the peripheral nerves to the relevant muscles is quite a complex system involving many different organs of the body. The entire activity is like a reflex i.e a spontaneous action –very much like that of the rest of the body’s other reflex actions.
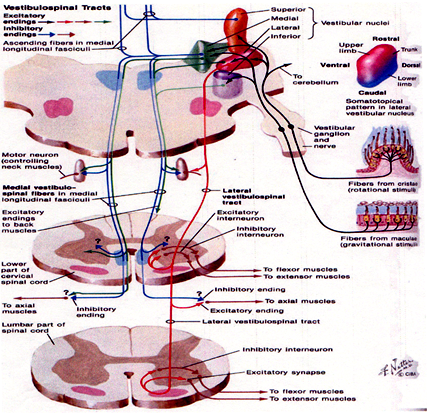
The figure represents the different reflex pathways by which the body’s balance is maintained. This picture is extremely important for us because when we discuss the diagnosis and management, these different aspects become very relevant. Like the other reflexes in the body, the maintenance of balance is also a reflex action with the involvement of a sensory organ, an afferent neuralpathway, a center of the reflex in the brain stem, an efferent neural pathway and an effector motor organ.
The afferent sensory organ such as the eyes, the vestibular organs in the ears and the proprioceptors (the sensors situated in different parts of the body such as the soles of the feet, or the neck) pick up information about the stability of the surroundings and that of the ground and pass it to the center of the reflex which is situated in the brain stem by an afferent neural pathway. For example the vestibular nerve is the afferent neural pathway for the information coming from the vestibular labyrinth. The center of the reflex is situated in the brain stem where the four vestibular nuclei are situated. The efferent neural pathway which comprises of neural tracts in the brain and spinal cord and the peripheral nerves carries the motor output to the different muscles of the body.
The three reflex pathways
The information from the three sources – the eyes, vestibular organs in the ears and the proprioceptors is integrated in the brain –giving the brain a perfect idea about the stability of the ground and the surroundings. The brain then evolves the motor output and this motor output will be directed to certain muscles of the body. It is directed basically to 2 groups of muscles. One group of muscles is the skeletal muscles of the limbs, the trunk and the neck and that works through the vestibulospinal pathway. This reflex system is known as the vestibulospinal reflex. The other group of muscles is the small muscles of the eyes and it works through the vestibuloocular reflex pathway.
Causes:
Vertigo results from acute unilateral vestibular lesions that can be peripheral (labyrinth or vestibular nerve) or central (brainstem or cerebellum). In contrast, tumors and ototoxic medications produce slowly progressive unilateral or bilateral lesions. Lesions that progress slowly or processes that affect both vestibular apparatuses equally usually do not result in vertigo. Most common causes of this condition are Benign Paroxysmal Positional Vertigo (BPPV), Acute vestibular neuronitis or Labyrinthitis, Meniere’s disease, Migraine and anxiety disorders. Less common causes include Vertebrobasilar ischemia and retrocochlear tumors.
PERIPHERAL VESTIBULAR DISORDERS
Peripheral vestibular disorders are limited to cranial nerve VIII and all distal structures. Patients with a peripheral disorder demonstrate nystagmus to the contralateral side which suppresses with visual fixation. Nystagmus improves with gaze towards the lesion and worsens with gaze opposite the lesion. Patients may also report a falling sensation. Vegetative symptoms are not uncommon, and one can expect nausea, vomiting, and possibly sweating and bradycardia. The rate of recovery typically decreases with age and severity, and with the use of vestibulosuppressive medications
MENIERE’S SYNDROME
The term Meniere’s syndrome is often used synonymously with the terms Meniere’s disease (MD) and endolymphatic hydrops, although they are different. Endolymphatic hydrops describes an increase in endolymphatic pressure resulting in inappropriate nerve excitation which gives rise to the symptom complex of vertigo, fluctuating hearing loss, and tinnitus. The exact mechanism by which this increase in pressure produces the symptoms of MD is greatly debated and beyond the scope of this paper. Numerous disease processes can result in endolymphatic hydrops; if there is a known etiology then it is termed Meniere’s syndrome. MD is a term used for endolymphatic hydrops of unknown etiology. The true incidence of MD is unclear due to difficulty in diagnosis. Caucasians are more often affected, and it is more prevalent in females than males. Typically, these patients complain of spontaneous episodic attacks of tinnitus, aural fullness, fluctuating hearing loss, and vertigo superimposed on a gradual decline in hearing. Symptoms are variable, however, and patients may have a predominance of either cochlear (tinnitus, hearing loss) or vestibular (vertigo) complaints. Attacks typically last minutes to hours; however, most commonly subside after 2 to 3 hours. Diagnosis is established with a thorough history detailing the aforementioned complaints, possibly accompanied by nausea, vomiting, and diaphoresis. Audiologic and vestibular testing is unreliable, but may show caloric weakness on electronystagmography (ENG) and sensorineural hearing loss on audiography. There is no cure for MD and the goal of treatment is symptomatic relief. Medical treatment is initiated prior to more invasive surgical intervention and consists of salt restriction, diuretics, vasodilators, anti-emetics, and anti-nausea medications. Those who fail medical treatment may consider surgical therapy. Surgical treatments can be classified as either hearing-conservative or non–hearing-conservative procedures and are appropriately chosen based on the patient’s audiometric results. For patients with serviceable hearing, endolymphatic sac decompression, vestibular neurectomy, and intratympanic aminoglycoside infusion are options. Labyrinthectomy is reserved for patients with no serviceable hearing
BENIGN PAROXYSMAL POSITIONAL VERTIGO
Benign paroxysmal positional vertigo (BPPV) is considered the most common peripheral vestibular disorder, affecting 64 of every 100,000 Americans.Women are more often affected and symptoms typically appear in the fourth and fifth decades of life. In 1980, Epley proposed that free-floating densities (canaliths) located in the semicircular canals deflect the cupula creating the sensation of vertigo.3 This is well documented in his Canalithiasis Theory. Although these canaliths are most commonly located in the posterior semicircular canal, the lateral and superior canal may also be involved. Patients with BPPV complain of vertigo with change in head position, rolling over, or getting out of bed, and the vertigo is often side specific. Vertigo occurs suddenly and lasts for less than 1 minute. Attacks are separated by remissions; however, patients may complain of constant light-headedness between episodes. Classic BPPV involving the posterior semicircular canal is characterized by the following: geotropic nystagmus with the problem ear down, predominantly rotary nystagmus toward the undermost ear, latency of a few seconds, duration limited to less than 20 seconds, reversal of nystagmus when the patient returns to an upright position, and a decline in response with repetitive provocation. Diagnosis is made primarily through history and also by eliciting typical physical findings during the Dix-Hallpike maneuver. The Dix-Hallpike maneuvre entails guiding a patient through a series of movements known to elicit nystagmus in a patient with BPPV. Electro-oculography and 2D videonystagmography are of limited use secondary to the inability of these tests to record torsional eye movement. Treatment is often supportive as a large percentage of patients will have spontaneous resolution of their symptoms. For those with persistent symptoms, the first line of treatment is canalith repositioning maneuvers. These maneuvers attempt to reposition the free-floating canalith particles from the semicircular canals to the utricle using gravity. These maneuvers are reported to be 91% effective. Patients with symptoms refractory to repositioning maneuvers may be candidates for singular neurectomy or posterior semicircular canal occlusion.
VESTIBULAR NEURONITIS
Vestibular neuronitis is the second most common peripheral cause of vestibular vertigo. Infection of the vestibular nerve results in nerve degeneration and may present bilaterally. Infection is most often thought to be of viral origin, usually from the herpes virus family. It may also result from bacterial invasion (e.g. Borrelia). It is believed that the superior vestibular nerve is more commonly involved secondary to its course throughout a long and narrower bony canal, making it more susceptible to compressive edema. The reported incidence of an upper respiratory infection prior to the development of vestibular symptoms varies from 23% to 100%.
Patients present with complaints of sudden vertigo, lasting up to several days, often with vegetative symptoms. As this process affects only the vestibular portion of the vestibulocochlear apparatus, there is an absence of cochlear symptoms. Vertiginous complaints gradually improve over days to weeks; however, imbalance may persist for months after resolution of acute disease. Recurrence is not uncommon and may occur several times per year. Physical examination is limited and should consist of audiometric evaluation and ENG. Patients may demonstrate nystagmus and caloric weakness on the affected side. Treatment is primarily supportive with the use of anti-emetics and anti-nausea medications. Vestibular suppressants should be used judiciously in the first few days of an acute attack. Prolonged use of these medications can delay recovery by inhibiting central compensation.
Furthermore, early ambulation is paramount in the central nervous system’s ability to compensate and is therefore recommended as soon as tolerable. High-dose methylprednisone has been shown to hasten recovery; however, prospective, randomized, double-blinded studies have failed to demonstrate added benefit from the use of antivirals (i.e. valacyclovir).
LABYRINTHITIS
Labyrinthitis is an inflammatory disorder of the membranous labyrinth, affecting both the vestibular and cochlear end organs. It may present unilaterally or bilaterally, and similar to vestibular neuronitis, it is often preceded by an upper respiratory infection. This disorder occurs when infectious microorganisms or inflammatory mediators invade the membranous labyrinth, damaging the vestibular and auditory end organs. Potential etiologies include viral pathogens, bacterial invasion, bacterial toxins, and systemic disease. Viral labyrinthitis usually occurs in adults in their fourth to seventh decades of life. Bacterial Labyrinthitis may result from both otogenic and meningitic infection, progressing to involve the labyrinth. Labyrinthitis of otogenic origin can be observed in any age group and may result from cholesteatoma or otitis media. Meningitic labyrinthitis is more common in children less than 2 years of age, who are more susceptible to developing meningitis. Otogenic infections typically cause unilateral symptoms while meningitic infections cause bilateral symptoms. Unlike vestibular neuronitis, patients with Labyrinthitis present with complaints indicative of both vestibular and cochlear damage. Vertigo presents suddenly and is accompanied by hearing loss. ENG may reveal nystagmus, and audiometry will reveal a sensorineural hearing loss or mixed hearing loss if middle ear effusion is present. Depending on the source of infection, patients may also present with findings consistent with otitis media, mastoiditis, or meningitis. Treatment is aimed primarily at eradication of the underlying infection and supportive care. Middle ear effusions and mastoiditis should be drained and treated with antibiotics. Meningitis should be treated with culture-directed antibiotics with central nervous system penetration and appropriate consultation. Anti-emetics and anti-nausea medications are helpful during the acute phase.
TREATMENT OF VERTIGO
Treatment of acute vertigo consists of bed rest (1-2 days maximum) and vestibular suppressant drugs such as Antihistaminics (Meclizine, Dimenhydrinate), molecules with GABA-ergic effects (Clonazepam, Diazepam), Phenothiazines (Prochlorperazine) etc. If vertigo persists beyond a few days, most authorities advise ambulation in an attempt to induce central compensatory mechanisms, despite the short term discomfort to the patient. Chronic vertigo of labyrinthine origin may be treated with a systematized vestibular rehabilitation program to facilitate central compensation. BPPV is often self-limited but, when persistent, may respond dramatically to specific repositioning exercise programs (Epley maneuvre) designed to empty particulate debris from posterior semi circular canals.
Medical management or pharmacotherapy of vertigo:--
The objectives are :
* Provide symptomatic relief —palliative therapy to reduce the vertigo.
* Increase cerebral and inner ear blood flow —one of the many causes of vestibular damage is hypoxia. Hypoxia of the brain and the inner ear induces a degenerative change which has to be prevented.
* Increase active transport through blood brain barrier —to maintain inner ear homeostasis. The ionic concentration of the different ions in the cells and the volume of fluid in the intracellular and extra cellular compartment have to be kept at an optimum and specified fixed level.
* Improve neural metabolism —supply nutrients to the neuronal tissues to protect the inner ear cells and the neural cells and prevent further damage.
Ideal anti vertigo drug is one that will:-
• Control the vertigo and or instability as well as nausea and vomiting
• Enhance the cerebral and inner ear blood flow
• Be reasonably safe and free from side effects
• Not depress the vestibular compensatory mechanism.
Symptomatic relief of vertigo is best brought about by:-
• Prochlorperazine
• Dimenhydrinate
• Meclizine
• Cinnarizine
• Betahistine
Control of nausea/vomiting by:-
• Trifluopromazine
• Domperidone
• Metoclopramide
• Promethazine
• Ondensetron
Mechanism of action:--
The control of vertigo is brought by the anticholinergic drugs and the nausea/vomiting is controlled by antidopaminergic drugs. The anticholinergic drugs control the vertigo by blocking the muscurinic receptors in the higher vestibular pathways. The transmission of neural impulses in the vestibular system is done by cholinergic pathways where these muscurinic receptors are located. If these muscurinic receptors are blocked, then the neural impulses cannot pass through these neural pathways. Blocking of the dopamine receptors in CTZ (chemoreceptor trigger zone) controls nausea and vomiting. The problem of giving anticholinergic drugs is that the cholinergic transmission through muscarinic receptors is present in many CNS pathways and other body tissues. There is no known anticholinergic / antimuscarinic drug that acts solely and exclusively in the vestibular pathways only. If you give anticholinergic therapy, it will block the transmission in all the cholinergic pathways, so the patient gets many other symptoms also. That is the main problem of giving symptomatic treatment to the patient. But when the patient gets acute vertigo, we need to give symptomatic relief also. So we have to find out how much to give and how much not to give.
Let us discuss the pros and cons of some of the commonly used drugs in vertigo
Commonly used drugs in vertigo:--
1. Dimenhydrinate
2. Diazepam
3. Prochlorperazine
4. Cinnarizine
5. Betahistine
6. Meclizine
Dimenhydrinate This is best for a patient who is having acute vertigo with vegetative symptoms like nausea/vomiting/sweating. This is an anti cholinergic drug like Prochlorperazine; its efficacy for giving symptomatic relief is less than that of Prochlorperazine, yet it provides reasonably good relief. It has been found to act best in the pathway from the vestibular nucleus to the reticular formation in the brain stem. The vegetative centers are there in the reticular formation. Dimenhydrinate partially helps to inhibit the projection of neural impulses from the vestibular nuclei to the reticular formation where the vegetative centers are situated. Thus if a patient is having severe vertigo with nausea and vomiting, which you are not able to control adequately with Prochlorperazine, then you can add a drug like Dimenhydrinate (marketed in the name of Gravol) 50mg thrice daily. Thus it is the drug of choice in acute vertigo with pronounced vegetative symptoms. It is however a CNS depressant, and is hence best discontinued as soon as acute symptoms subside. Its anti-emetic effect is not associated with extra-pyramidal symptoms.
Diazepam is also not very uncommonly used in the management of acute vertigo as it gives very good relief from vertigo. In those rare cases where acute vertigo is not being controlled by Prochlorperazine, Diazepam can be tried; but the problem of Diazepam is that it is a CNS depressant which will completely jeopardize the vestibular compensatory mechanism. So it can be used only for one or two doses at the most just to tide over the very acute phase and Prochlorperazine can then be continued for about 7 days. Another advantage of diazepam is its anxiolytic effect; moreover it not only controls the vertigo but partially also the accompanying nausea & vomiting.
Prochlorperazine (Stemetil 5mg tds): ---This is very effective in fact the most efficient drug for symptomatic relief. It acts on the muscurinic receptors and blocks the dopamine receptors in the brain and hence not only controls the vertigo but also the nausea and vomiting. It is but a CNS depressant like all the other anticholiinergic drugs; hence it is likely to inhibit the vestibular compensatory mechanism. This is true if you are using the drug for very prolonged periods because there are clinicians who use it for 3, 4 and 5 months or even more. Prochlorperazine is basically a drug which gives symptomatic relief; and once symptomatic relief has been obtained the other things will naturally fall into place. Controlling the symptoms for 5 or 7 days with this drug is ideal as it provides very good symptomatic relief. After the symptoms have been adequately controlled the patient should essentially be put on the vestibular exercises for the natural compensation to take place . The patient may be asked to keep two Stemetil MD(prochlorperazine mouth dissolving) tablets with him so that when he feels the vertigo he may suck a tablet or two. It gives instant relief.
Cinnarizine 25-75 mgs tds has a labyrinthine sedative effect. It has an antivasoconstrictive effect also. It increases the cerebral and inner ear blood supply; stabilizes the vascular endothelium and is a reasonablly safe drug. But it does not give the same symptomatic relief as Prochlorperazine. If you are having a very old aged patient who is expected to have hypoxia in the brain gets vertigoon and off, then you can think of putting the patient on Cinnarizine for some time, as it will increase the blood supply to the brain and inner ear and at the same time provide some symptomatic relief. But it does not make sense to give Cinnarizine to a young or middle aged patient with sudden onset of severe vertigo as here we are only wanting symptomatic relief in acute vertigo for which prochlorperazine is a better drug. Our objective is just symptomatic relief and not increase of blood supply as in such cases hypoxia of the brain/inner ear is not the expected pathology. Acute onset of severe vertigo is usually due to unilateral vestibular lesion and here our main objective is providing symptomatic relief for a few days as the condition is usually self-limiting and compensation is expected to take place naturally by itself.
Betahistine 8-16 mg tds 24mg bd: -- Like cinnarazine, betahistine also offers some symptomatic relief which of course is lesser than prochlorperazine (if betahistine is prescribed at the usual dose of 48 mg daily) and it also increases the blood supply to the brain and inner ear. The advantage of betahistine is that it is the only non-sedating anti-vertigo drug and is not a CNS depressant and so does not jeopardize the compensatory mechanism. It is ideal for a patient of uncompensated persistent subacute vertigo in a vasculopathic subject ( with suspected cerebral / inner ear hypoxia) where the primary aim is vestibular compensation. It is recommended in Meniere’s disease.
Meclizine:- This is not used much in our country. It is sold in the name of Diligan. The exact mechanism of this drug is not known. Like other antihistamines of H1 antagonistic group, anticholinergic activity and monoaminergic enhancing effect, believed to diminish excitability of neurons in vestibular nucleus. Meclizine is unfortunately a strong CNS depressant. It does not increase the cerebral and inner ear blood flow. It is more suited for motion sickness than for acute vertigo.
Getting to know the molecule: BETAHISTINE
This drug (brand name Serc, chemical name betahistine), is advocated as a vestibular suppressant mainly for Meniere's disease. Curiously, Serc was approved by the US FDA about 40 years ago for roughly 5 years, but later approval was withdrawn because lack of evidence for efficacy and because the major report of effectiveness contained deficiencies and misrepresentations (Sampson, 2003). The withdrawal was upheld by a US court of appeals in 1968. Subsequently, four double-blind studies have been done reporting reduction of vertigo attacks with betahistine (Frew and Menon, 1976: Wilmot and Menon; 1976; Meyer, 1985; Mira et al, 2003). Nevertheless, these studies may have been flawed and a recent review suggested that it is presently still unclear if betahistine has any effect in Meniere's disease (James and Burton, 2001).
Serc was again reviewed by the FDA in June of 1999 (click herefor details). Essentially, the conclusion was that there is no evidence that it is harmful, but also little evidence that it has any therapeutic effect. It thus is similar in official status to an inert substance. Serc has been reviewed by the "Cochrane database", who concluded in 2009 that "There is insufficient evidence to say whether betahistine has any effect on Ménière's disease"
Availability
At the time of this update (2/2010), we generally just send patient to Walgreens. Betahistine can also be easily obtained through US compounding pharmacies, with a prescription. It is difficult to see why an inert substance must be prescribed, but nevertheless, this is the situation in the US. Insurance often covers betahistine too.
Rationale for use of Betahistine:
For those who want the quick answer, nobody has a reasonable rationale of why betahistine should work for dizziness. Following is the longer explanation.
Although betahistine does not closely resemble histamine (see above), in the body it is a histamine agonist. There are 3 histamine receptors - -H1, H2 and H3. The rationale for its use is somewhat difficult to understand as H1 blocking antihistamines (such as meclizine) are also used quite commonly to treat vertigo. Explanations commonly given are that the drug is a vasodilator, or that it acts on subreceptors of histamine. H1 and H2 are post-synaptic receptors and H3 is a pre-synaptic receptor. Betahistine is a potent H3 receptor antagonist. (Lacour and Sterkers, 2001). Stimulation of the H3 receptor reduces histamine release, so antagonism of H3 increases histamine release. H3 receptors control the synthesis and release of histamine via a Gs protein (de Waele et al, 1995).
According to de Waele et al (1995), there are several lines of evidence that suggest that histamine receptors modulate the function of central vestibular neurons. One influence is via axons from the posterior hypothalamus. This may be related to wakefulness as histamine controls wakefulness. Both H1 and H2 binding sites have been detected in vestibular nuclei. These authors note that there is data suggesting both that H1 and H2 actions are excitatory on the vestibular nucleus, and that common H2 blocker drugs such as cimetidine antagonize the effect. This suggests that H2 blockers (commonly used to reduce stomach acid), at least those that cross into the brain, may have a central vestibular suppressant effect. H3 antagonists appear to inhibit horizontal vestibular gain without affecting alertness. This line of reasoning does not explain at all why betahistine would be helpful -- one would even think that it would be harmful if it excites the vestibular nucleus. This is just one example of the large array of confusing literature about this drug.
Most antivertigo drugs are H1 blockers. Many newer allergy medications are selective peripheral H1 blockers, and many older allergy medications, including those used for dizziness such as dramamine, are peripheral and central H1 blockers. Many medications for stomach problems that block acid are H2 blockers, but these are not generally thought to affect vestibular function (see above however).
Betahistine is an H3 antagonist, according to Timmerman (1991). H3 antagonism is felt to increase H1 and H2, so the net effect of H3 antagonism is histamine (H1 and H2) agonism. Confusingly though, some authors suggest that betahistine is an H3 agonist. The neuropharmacological literature is also complex. Arrang et al (1985) found betahistine to be a partial agonist against cerebral H1 receptors, and has no effect on H3 receptors. In other words, some H3 agonists might block histamine release, but betahistine (betahistine) doesn't affect these receptors in guinea-pigs. This would suggest that either betahistine is a placebo, or that it's effects result from some mechanism other than H3.
As mentioned above, it seems contradictory that both H1 blockers (meclizine) and H1 agonists (betahistine) are advocated for vertigo. It, however, is possible that the antihistamine effects of meclizine are less important than its anticholinergic effects, so there may not be a contradiction between the H1 effects. The general thought at the present is that the effects of betahistine relate to it's effect on H3 receptors, although what exactly this effect is still rather mysterious. If betahistine is indeed an H1 agonist and an H3 antagonist, it might suppress vestibular function and enhance alertness, both valuable qualities. By this thinking, betahistine could be reasonably combined with a selective H1 blocker that does not cross into the brain (such as fexofenadine).
Is it reasonable to combine meclizine (a central H1 blocker) and betahistine (a central H1 agonist) ? Well, perhaps if you are hoping to use the central anticholinergic properties of meclizine. It would see a more logical, however, to use a pure anticholinergic agent such as scopolamine instead of meclizine. Similarly, it seems reasonable to combine betahistine with a contemporary non-sedating antihistamine, as these medications do not cross the blood brain barrier.
Does it work ?
The author of this review has had moderate success with giving betahistine to patients who have intractable Meniere's disease, and it part of his usual regimen for intractable Meniere's (often in combination with verapamil). It comes in doses of 8, 16 and 24 mg.The usual dose is 16 mg 3-4 times per day although greater effect is obtained for doses as high as 48 mg at a time (Strupp,et al. 2008). Most people that report a positive effect can decide this with a few days -- it doesn't seem to take 1 month to figure out whether betahistine is helping or not.
The author also uses Betahistine to treat severe motion intolerance (e.g. Matsnev and Sigaleva, 2007). Betahistine can be used in children (Gryczynska, Drobik-Wasiewicz et al. 2007). While claims have been made that Betahistine is associated with weight loss, these appear to be unfounded (Barak, Greenway et al. 2008).
There are numerous puzzling aspects to betahistine and the jury is still out regarding whether or not it is an effective medication. Some studies report that betahistine cannot be distinguished from placebo. There are also some troublesome reports comparing betahistine to other medications that are almost certainly placebos, that can be interpreted in the same way. Klein et al (1998) reported that a homeopathic medication was equivalent in efficacy to betahistine. As homeopathic medications are generally felt to be placebos because of the extremely high dilutions with which they are administered, this suggests that either the Klein study was flawed, or that betahistine is also a placebo. On the other hand, Fujino et al (1994) reported betahistine to have a positive effect when combined with rehabilitation.
Side Effects
Side-effects are generally minimal. The author of this review has encountered stomach upset in several, worsening asthma, headache (Barak et al, 2007), and chest tightness as side-effects. Of course, these symptoms could be found in a placebo.
Post-marketing studies conducted by the manufacturer suggest only a very small number of adverse effects (Jeck-Thole et al, 2006). In theory, stomach upset might be related to H2 agonism, as H2 blockers are used to treat gastric acidity. Against this idea is that betahistine is not a H2 agonist, but the increased release of histamine associated with h3 antagonism might stimulate H2. As mentioned above, it seems reasonable to combine betahistine with a selective H2 blocker that does not cross the blood brain barrier, or a proton pump inhibitor used for gastric acidity. Headache might also be a direct effect of histamine. On the other hand, some studies report reduction of headache by betahistine (Amelin et al, 2003).
In it's favor, betahistine does not cause drowsiness and has no effect on driving, reaction time or visual acuity (Betts et al, 1991)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESEARCH OBJECTIVE
Primary objective:
To determine the prevalence and treatment choice most effective in Vertigo management. Also, to develop an understanding of once a day preparation usage and place in therapy.
Secondary Objectives:
· To map the prevalence of vertigo at various specialities (Neurology, Cardiology, E.N.T, Medicine)
· To analyse the cause/s of vertigo under these specialties
· To understand the molecule management by doctors to counter Vertigo
· To understand usage of Betahistine in Vertigo management
· To develop understanding regarding dosage, frequency & duration of Betahistine usage for Vertigo treatment
· To map the effective position of Betahistine OD in therapy in comparison with other treatment choices
RESEARCH METHODOLOGY
1. SECONDARY RESEARCH:
Secondary data was collected before designing the questionnaire relevant to the project to have brief insights about the research work.
Information was collected through various sources which include medical journals, Textbooks and websites. Market study was done through ORG-IMS and C-MARC.
Following information was collected:-
1. Pathophysiology of Vertigo.
2. Management of Vertigo medically.
3. Market share and prescription pattern of various brands from ORG IMS and CMARC.
- After a proper understanding, a questionnaire was designed.
2. PRIMARY RESEARCH:
|
S.No. |
PARAMETER |
DESCRIPTION |
|
Research type |
Qualitative and Quantitative Research |
|
|
Data collection |
Primary |
|
|
Tool of data collection |
A market Research Questionnaire with open-ended as well as close-ended questions was used for data collection. |
|
|
Type of Questionnaire |
Semi-structured |
|
|
Mode of data collection |
One-to-one interview |
|
|
Interview technique |
In depth interview was conducted by using probing method for each question. |
|
|
Sample selection |
Area and speciality wise |
|
|
Sample size |
165 |
|
|
Target respondents |
Neurologists (40), Consultant physicians (60), E.N.T (35), Cardiologists (20), Diabetologists (10) |
|
|
Sampling Technique |
Convenience sampling based on geography |
|
|
Analysis of Data |
Was performed in Microsoft Excel and Coding method was used for the purpose of data entry |
Primary Data: Field work done in different areas of Mumbai (zone wise)
|
Zone |
Areas |
|
Western |
Mahim, Santacruz, Andheri, Jogeshwari, Goregaon, Kandivali, Borivali, Khar, Malad, Ville Parle, Bhyander |
|
Central |
Bandra, Dadar, Ghatkopar, Chembur, Byculla, Parel |
|
South |
Churchgate, Marine Lines, Grant road |
LIMITATIONS:
· The interviewer bias was a major limitation in the research. There could have been difference between the information generated and the information wanted due to the way in which the respondent must have interpreted the question.
· The research was carried out in parts of Mumbai and its surrounding areas. The primary data collected may not be a representative of other geographical areas.
· Sample size of 165 may not reflect the same results in actual size.
· Some subjects were less cooperative and showed unfriendly attitude.
NEUROLOGISTS
NEUROLOGISTS (SAMPLE SIZE = 40)
· The incidence of 10-15% patients was found to be a majority amongst 52.5% Neurologists, who have administered anti-vertigo medications.
· The % of female patients who seek anti-vertigo medications were found to be on the higher side amongst Neurologists.
· Out of all the Neurologists sampled, 62.5% felt that a 40:60 (Male:Female) ratio is most observed in their practice.
· 70% Neurologists reported that 40-50 years was the prevalent category of the patients.
· It was revealed through the study that 72.5% of the Neurologists, observed patients with Primary Vertigo.
· Diabetes Mellitus is the most common secondary to pre-existing ailment amongst 44% Neurologists, followed by Spondylosis (40%).
· BPPV is the most common cause of Vertigo, followed by Vestibular Neuronitis and Spondylosis amongst the sampled Neurologists.
· Pharmacological and Manoeuvres is the most preferred line of treatment amongst 70.58% Neurologists for effective Vertigo management.
· Betahistine is the most preferred drug of choice for treating vertigo amongst 86.96% Neurologists.
· 42.5% of the Neurologists prefer to treat with 8mg TID dose of Betahistine for patients with vertigo.
· 1-2 weeks is the most preferred duration of therapy with Betahistine amongst 75% Neurologists.
· 90% of the Neurologists were found to titrate the dosage in Vertigo treatment.
· With the usual approach being tapering down the dosage in patients where symptoms are relieved and do not recur, and then putting it off. But in some recurrent cases of vertigo the dosage is increased.
· 95% of the Neurologists were found to be aware of Betahistine once a day preparation. Only 5% Neurologists were still unaware of Betahistine O.D formulation.
· 55% of the sampled Neurologists, were found to use Betahistine O.D formulation.
· Amongst all Neurologists those who use Betahistine once a day preparation, 77.27% mostly prescribe 24mg C.R, while 22.73% use 48mg C.R formulation.
· The duration for which 24mg C.R is prescribed the most is 1 week by 50% Neurologists, followed by 2 weeks amongst 22.73% Neurologists.
· 67.5% of the Neurologists prefer to use O.D formulation as a start up dose, while the rest (32.5%) preferred to use it as a maintenance dose only.
· 82.5% of the Neurologists do not use O.D formulation particularly specific to any indication
· Amongst the Neurologists sampled, 90% do not use two Anti-Vertigo molecules together.
· 40% of the Neurologists have not tried this combination, but 37.5% said it to be a potent and viable combination. They were further aided by 12.5% of doctors who felt it maybe a fruitful combination.
1) In your practice what is the incidence of patients who need anti-vertigo medication?
Total Respondents = 40
|
Category |
Responses |
Percentage of responses |
|
5-10% |
8 |
20 |
|
10-15% |
21 |
52.5 |
|
15-25% |
8 |
20 |
|
More than 25% |
3 |
7.5 |
Table 1: Responses for incidence of patients requiring Anti-Vertigo Medication per week
Interpretation:
· The incidence of 10-15% patients was found to be a majority amongst 52.5% Neurologists, who have administered anti-vertigo medications.
· The incidence of 5-10% & 15-25% patients were also reported amongst 20% Neurologists.
· Only 7.5% of the Neurologists reported of more than 25% patients requiring anti-vertigo medication.
2) May I know the approximate bifurcation of patients gender wise (in %)?
Total Respondents = 40
|
% of Patients |
Approx Ratio |
Responses |
% of Responses |
|
Male |
40 |
25 |
62.5 |
|
Female |
60 |
||
|
Male |
50 |
6 |
15 |
|
Female |
50 |
||
|
Male |
30 |
9 |
22.5 |
|
Female |
70 |
||
|
Male |
20 |
0 |
0 |
|
Female |
80 |
||
Table 2: Responses for gender bifurcation of patients requiring Anti-Vertigo Medication per week
Interpretation:
· The % of female patients who seek anti-vertigo medications were found to be on the higher side amongst Neurologists.
· 62.5% of the Neurologists felt that a 40:60 (Male:Female) ratio is most observed in their practice.
· 30:70 (Male:Female) ratio was observed amongst 22.5% Neurologists.
· 15% Neurologists observed an equal ratio of 50:50 (Male:Female) in their practice.
3) Is it more predominant in any particular age group?
Total Respondents = 40
|
Age of Patients |
Responses |
% of Responses |
|
Below 20 years |
0 |
0 |
|
20-40 years |
7 |
17.5 |
|
40-50 years |
28 |
70 |
|
Above 50 years |
5 |
12.5 |
Table 3: Responses for prevalent age group of patients requiring Anti-Vertigo Medication per week
Interpretation:
· 70% Neurologists reported that 40-50 years was the prevalent category of the patients.
· 17.5% Neurologists found 20-40 years category to be more prevalent in their practice.
· Patients above 50 years age was reported with 12.5% Neurologists, while there were no patients reported below 20 years.
4) Of these patients reported, do you get most patients as primary vertigo/dizziness patients or it is secondary to some pre-existing ailment?
Total Respondents = 40
|
Type |
Responses |
% of Responses |
|
Primary |
29 |
72.5 |
|
Secondary |
11 |
27.5 |
Table 4: Responses for category of patient having secondary to pre-existing ailment
Interpretation:
· It was revealed through the study that 72.5% of the Neurologists, observed patients with Primary Vertigo.
· Only 27.5% of Neurologists observed Vertigo patients with Secondary to pre-existing ailment.
5) If it is secondary to pre-existing ailment, what is the most common pre-existing disease/disorder you see?
Total Respondents = 11 multiple responses (out of 40 Neurologists)
|
Disorder |
Responses |
% of Responses |
|
Diabetes Mellitus |
11 |
44 |
|
Hypertension & Cardiac problems |
2 |
8 |
|
ENT problems |
2 |
8 |
|
Spondylosis |
10 |
40 |
Table 5: Responses for patents having most common secondary to pre-existing ailment
Interpretation:
· Diabetes Mellitus is the most common secondary to pre-existing ailment amongst 44% Neurologists, followed by Spondylosis (40%).
· Hypertension & Cardiac problems along with ENT problems were also reported in 8% Neurologists respectively.
6) What are the most common causes of Vertigo that you see in your practice?
Total Respondents = 40
|
Cause |
Frequency of Ranks |
|||||
|
Rank 1 |
Rank 2 |
Rank 3 |
Rank 4 |
Rank 5 |
Rank 6 |
|
|
BPPV |
39 |
1 |
|
|
|
|
|
Vestibular Neuronitis |
1 |
19 |
10 |
2 |
|
|
|
Spondylosis |
|
17 |
10 |
2 |
1 |
|
|
Labyrinthitis |
|
1 |
6 |
3 |
|
|
|
Meniere’s Disease |
|
|
1 |
1 |
3 |
|
|
Vertebral Ischemia |
|
|
2 |
|
|
|
|
Migraine |
|
|
|
3 |
|
|
Table 6: Table depicting the most common cause of Vertigo as ranked
Interpretation:
· BPPV is the most common cause of Vertigo, followed by Vestibular Neuronitis and Spondylosis amongst the sampled Neurologists.
· Labyrinthitis, Meniere’s Disease and Vertebral Ischemia were the other causes reported by the Neurologists (in decreasing order of occurrence).
· Migraine is the least significant cause of Vertigo.
7) What is the usual line of treatment?
Total Respondents = 40 (multiple responses)
|
Line of Treatment |
Responses |
% of Responses |
|
Lifestyle modification |
0 |
0 |
|
Pharmacological |
13 |
25.49 |
|
Manoeuvres only |
2 |
3.92 |
|
Pharmacological and Manoeuvres |
36 |
70.58 |
Table 7: Responses for usual line of treatment in Vertigo management
Interpretation:
· Pharmacological and Manoeuvres is the most preferred line of treatment amongst 70.58% Neurologists for effective Vertigo management.
· 25.49% Neurologists also resort to Pharmacological therapy for treating Vertigo.
8) In pharmacological treatment what is your drug of choice?
Total Respondents = 40 (multiple responses)
|
Molecule |
Responses |
% of Responses |
|
Betahistine |
40 |
86.96 |
|
Cinnarizine |
0 |
0 |
|
Dimenhydrinate |
3 |
6.52 |
|
Combination |
2 |
4.35 |
|
Any other |
1 |
2.17 |
Table 8: Responses for molecule management in Vertigo management pharmacologically
Interpretation:
· Betahistine is the most preferred drug of choice for treating vertigo amongst 86.96% Neurologists.
· 6.52% Neurologists prescribed Dimenhydrinate and 4.35% Neurologists prefer to use a combination of drugs (Betahistine with Dimenhydrinate / Cinnarizine) for treating Vertigo.
9) May I know your usual treatment approach?
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table 9: Chart showing the type of Betahistine dose prescribed for treating Vertigo
Total Respondents = 40
|
|
8 mg |
16mg |
24mg |
24mg C.R |
48 mg C.R |
|
OD |
-- |
-- |
-- |
4 |
-- |
|
BID |
-- |
4 |
8 |
-- |
-- |
|
TID |
17 |
3 |
4 |
-- |
-- |
Interpretation:
· 42.5% of the Neurologists prefer to give 8mg TID dose of Betahistine to patients to treat vertigo.
· 20% of the Neurologists prescribe 24mg BID dosage, while 10% of them prefer 16mg BID or 24 mg TID dose, for pharmacological therapy with Betahistine for Vertigo.
· Only 10% of Neurologists prescribes 24mg C.R once a day formulation (Betahistine O.D) for treatment in of Vertigo.
· Also, 7.5% Neurologists prescribe 16mg TID of Betahistine to their patients.
Duration:
Total Respondents = 40
|
Duration |
Responses |
% of responses |
|
1-2 weeks |
30 |
75 |
|
3-4 weeks |
7 |
17.5 |
|
4-8 weeks |
3 |
7.5 |
Table 10: Responses for duration of pharmacological therapy for Vertigo
Interpretation:
· 1-2 weeks is the most preferred duration of therapy with Betahistine amongst 75% Neurologists.
· 17.5% Neurologists opted for 3-4 weeks of therapy with Betahistine, while 7.5% preferred 4-8 weeks.
10) Do you titrate dosage?
Total Respondents = 40
|
Response to question |
Responses |
% of Responses |
|
Yes |
36 |
90 |
|
No |
4 |
10 |
Table 12: Responses for dosage titration in treating Vertigo
Interpretation:
· 90% of the Neurologists were found to titrate the dosage in Vertigo treatment.
· With the usual approach being tapering down the dosage in patients where symptoms are relieved and do not recur, and then putting it off. But in some recurrent cases of vertigo the dosage was increased.
· Where as 10% of the Neurologists did not titrate the dosage.
11) Doctor, are you aware of once a day Betahistine preparation?
Total Respondents = 40
|
Response to question |
Responses |
% of Responses |
|
Yes |
38 |
95 |
|
No |
2 |
5 |
Table 13: Responses for Betahistine O.D awareness
Interpretation:
· Majority (95%) of the Neurologists were found to be aware of Betahistine once a day preparation. Only 5% Neurologists were still unaware of Betahistine O.D formulation.
12) If Yes, have you used?
Total Respondents = 40
|
Response to question |
Responses |
% of Responses |
|
Yes |
22 |
55 |
|
No |
18 |
45 |
Table 14: Responses for Betahistine O.D usage
Interpretation:
· 55% of the sampled Neurologists, were found to use Betahistine O.D formulation.
· 45% of the Neurologists did not use Betahistine O.D formulation, they reportedly used the conventional doses.
13) If answer to Q11 is Yes, what is preferred strength & duration?
Fig 14: Pie chart showing choice of Betahistine once a day preparation usage
Total Respondents = 22 (out of 40 Neurologists)
|
Strength |
Duration |
Responses |
% of Responses |
Overall % |
|
24 mg |
1 week |
11 |
50 |
77.27 |
|
2 weeks |
5 |
22.73 |
||
|
4 weeks |
1 |
4.54 |
||
|
48 mg |
1 week |
0 |
0 |
22.73 |
|
2 weeks |
3 |
13.64 |
||
|
4 weeks |
2 |
9.09 |
||
Table 15: Responses for preferred strength of Betahistine O.D usage
Interpretation:
· Amongst all Neurologists those who use Betahistine once a day preparation, 77.27% mostly prescribe 24mg C.R, while 22.73% use 48mg C.R formulation.
· The duration for which 24mg C.R is prescribed the most is 1 week by 50% Neurologists, followed by 2 weeks amongst 22.73% Neurologists.
· 13.64% Neurologists use 48mg C.R for 2 weeks.
· 9.09% Neurologists use 48mg C.R for 4 weeks.
14) If used, how do you use the OD formulation?
Total Respondents = 40
|
Response to question |
Responses |
% of Responses |
|
Start with OD |
27 |
67.5 |
|
Only Maintenance with OD |
13 |
32.5 |
Table 16: Responses for preferred use of once a day formulation
Interpretation:
67.5% of the Neurologists prefer to use O.D formulation as a start up dose, while the rest (32.5%) preferred to use it as a maintenance dose only.
15) Is the usage of OD preparation indication specific?
Total Respondents = 40
|
Response to question |
Responses |
% of Responses |
|
Yes |
7 |
17.5 |
|
No |
33 |
82.5 |
Table 17: Responses for use of once a day formulation indication specifically
Interpretation:
· Majority (82.5%) of the Neurologists do not use O.D formulation particularly specific to any indication.
· Only 17.5% of Neurologists use it specific to any particular condition.
16) If yes, which is the indication and any specific reasons?
17.5% of the Neurologists use O.D formulation in Acute cases of vertigo. They also prefer to use it in patients with severe symptoms.
17) Do you also use two anti-vertigo molecules like morning/evening dose?
Total Respondents = 40
|
Response to question |
Responses |
% of Responses |
|
Yes |
4 |
10 |
|
No |
36 |
90 |
Table 18: Responses for usage of two Anti-vertigo molecules
Interpretation:
· 90% of the Neurologists do not use two Anti-Vertigo molecules together.
· Only 10% of the Neurologists use two Anti-vertigo molecules together as a morning-evening dose.
If Yes, your usual approach
10% of the Neurologists use Betahistine with Dimenhydrinate or Cinnarizine.
18) Do you think, combination of Betahistine with Dimenhydrinate is required? If yes, for which indication and condition is it useful?
Total Respondents = 40
|
Response |
Number of Responses |
% of response |
|
Yes |
15 |
37.5 |
|
No |
4 |
10 |
|
Maybe |
5 |
12.5 |
|
Not tried |
16 |
40 |
Table 19: Responses for usage of combination of Betahistine with Dimenhydrinate
Interpretation:
· 40% of the Neurologists have not tried this combination, but 37.5% said it to be a potent and viable combination.
· They were further aided by 12.5% of doctors who felt it maybe a fruitful combination.
· 10% of Neurologists feel that this would not serve to be a good combination.
CONSULTING PHYSICIANS
CONSULTING PHYSICIANS (SAMPLE SIZE = 60)
· The incidence of 10-15% patients was found to be a majority amongst 43.33% Consulting Physicians, who have administered anti-vertigo medications.
· The % of female patients who seek anti-vertigo medications were found to be on the higher side than Male patients amongst Consulting Physicians.
· 66.67% of the Consulting Physicians felt that a 40:60 (Male:Female) ratio is most observed in their practice.
· 66.67% Consulting Physicians reported that 40-50 years was the prevalent category of the patients.
· It was revealed through the study that 88.33% of the Consulting Physicians, observed patients with Secondary to pre-existing ailment.
· Diabetes Mellitus (53.76%) is the most common secondary to pre-existing ailment, followed by Hypertension & Cardiac problems (34.41%) amongst the Consulting Physicians.
· BPPV is the most common cause of Vertigo, followed by Labyrinthitis and Vestibular Neuronitis amongst the sampled Consulting Physicians.
· Pharmacological therapy is the most preferred line of treatment amongst 68.92% Consulting Physicians for effective Vertigo management.
· Betahistine is the most preferred drug of choice for treating vertigo amongst 83.58% Consulting Physicians.
· 33.33% of the Consulting Physicians prefer to treat with 8mg TID dose of Betahistine for vertigo patients.
· 1-2 weeks is the most preferred duration of therapy amongst 73.33% Consulting Physicians.
· 95% of the Consulting Physicians were found to titrate the dosage in Vertigo treatment with.
· With the usual approach being tapering down the dosage in patients where symptoms are relieved and do not recur, and then putting it off. But in some recurrent cases of vertigo the dosage is increased.
· 98.33% of the Consulting Physicians were found to be aware of Betahistine once a day preparation.
· 53.33% of the Consulting Physicians did not use Betahistine O.D formulation, they reportedly used the conventional doses.
· Consulting Physicians those who use Betahistine once a day preparation, mostly (60.71%) prescribe 24mg C.R, while 39.29% use 48mg C.R formulation.
· 32.14% of Consulting Physicians prescribe 24mg C.R for 1 week, followed by 32.14% of Consulting Physicians for 2 weeks.
· 53.33% of the Consulting Physicians prefer to use O.D formulation as a start up dose, while the rest (46.67%) preferred to use it as a maintenance dose only.
· 96.67% of the Consulting Physicians do not use O.D formulation particularly specific to any indication.
· 78.33% of the Consulting Physicians do not use two Anti-Vertigo molecules together.
· 45% of the Consulting Physicians have not tried this combination, but 45% also said it to be a potent and viable combination.
· They were further aided by 6.67% of doctors who felt it maybe a fruitful combination.
1. In your practice what is the incidence of patients who need anti-vertigo medication?
Total Respondents = 60
|
Category |
Responses |
Percentage of responses |
|
5-10% |
11 |
18.33 |
|
10-15% |
26 |
43.33 |
|
15-25% |
20 |
33.33 |
|
More than 25% |
3 |
5 |
Table 20: Responses for incidence of patients requiring Anti-Vertigo Medication per week
Interpretation:
· The incidence of 10-15% patients was found to be a majority amongst 43.33% Consulting Physicians, who have administered anti-vertigo medications.
· The incidence of 15-25% & 5-10% patients were also reported amongst 33.33% & 18.33% Consulting Physicians respectively.
· Only 5% of the Consulting Physicians reported of more than 25% patients requiring anti-vertigo medication.
2. May I know the approximate bifurcation of patients gender wise (in %)?
Total Respondents = 60
|
% of Patients |
Approx Ratio |
Responses |
% of Responses |
|
Male |
40 |
40 |
66.67 |
|
Female |
60 |
||
|
Male |
50 |
14 |
23.33 |
|
Female |
50 |
||
|
Male |
30 |
5 |
8.33 |
|
Female |
70 |
||
|
Male |
20 |
1 |
1.67 |
|
Female |
80 |
||
Table 21: Responses for gender bifurcation of patients requiring Anti-Vertigo Medication per week
Interpretation:
· The % of female patients who seek anti-vertigo medications were found to be on the higher side than Male patients amongst Consulting Physicians.
· 66.67% of the Consulting Physicians felt that a 40:60 (Male:Female) ratio is most observed in their practice.
· 30:70 (Male:Female) ratio was observed amongst 8.33% Consulting Physicians.
· 1.67% Consulting Physicians observed an equal ratio of 50:50 (Male:Female) in their practice.%.
3. Is it more predominant in any particular age group?
Total Respondents = 60
|
Age of Patients |
Responses |
% of Responses |
|
Below 20 years |
0 |
0 |
|
20-40 years |
3 |
5 |
|
40-50 years |
40 |
66.67 |
|
Above 50 years |
17 |
28.33 |
Table 22: Responses for prevalent age group of patients requiring Anti-Vertigo Medication per week
Interpretation:
· 66.67% Consulting Physicians reported that 40-50 years was the prevalent category of the patients.
· Patients above 50 years age was reported with 28.33% Consulting Physicians
· 5% Consulting Physicians found 20-40 years category to be more prevalent in their practice, while there were no patients reported below 20 years.
4. Of these patients reported, do you get most patients as primary vertigo/dizziness patients or it is secondary to some pre-existing ailment?
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Total Respondents = 60
|
Type |
Responses |
% of Responses |
|
Primary |
7 |
11.67 |
|
Secondary |
53 |
88.33 |
Table 23: Responses for category of patient having secondary to pre-existing ailment
Interpretation:
· It was revealed through the study that 88.33% of the Consulting Physicians, observed patients with Secondary to pre-existing ailment.
· Only 11.67% of Consulting Physicians observed Vertigo patients with Primary Vertigo.
5. If it is secondary to pre-existing ailment, what is the most common pre-existing disease/disorder you see?
Total Respondents = 53 multiple responses (out of 60 Consulting Physicians)
|
Disorder |
Responses |
% of Responses |
|
Diabetes Mellitus |
50 |
53.76 |
|
Hypertension & Cardiac problems |
32 |
34.41 |
|
ENT problems |
3 |
3.23 |
|
Spondylosis |
4 |
4.30 |
|
Otitis Media |
1 |
1.08 |
|
Vit B12 deficiency |
3 |
3.23 |
Table 24: Responses for patents having most common secondary to pre-existing ailment
Interpretation:
· Diabetes Mellitus (53.76%) is the most common secondary to pre-existing ailment, followed by Hypertension & Cardiac problems (34.41%) amongst the Consulting Physicians.
· Spondylosis (4.3%) along with ENT problems(3.23%) were also reported amongst Consulting Physicians respectively.
· 1.08% Consulting Physicians felt Otitis Media could be the pre-exiting case along with 3.23% Consulting Physicians who indicated Vit B12 Deficiency.
6. What are the most common causes of Vertigo that you see in your practice?
Total Respondents = 60
|
Cause |
Frequency of Ranks |
|||||
|
Rank 1 |
Rank 2 |
Rank 3 |
Rank 4 |
Rank 5 |
Rank 6 |
|
|
BPPV |
53 |
5 |
|
1 |
|
|
|
Labyrinthitis |
4 |
38 |
4 |
2 |
|
|
|
Vestibular Neuronitis |
2 |
9 |
14 |
2 |
|
|
|
Meniere’s Disease |
|
5 |
13 |
2 |
|
|
|
Spondylosis |
1 |
1 |
7 |
|
1 |
|
|
Migraine |
|
|
|
|
2 |
|
Table 25: Table depicting the most common cause of Vertigo as ranked
Interpretation:
· BPPV is the most common cause of Vertigo, followed by Labyrinthitis and Vestibular Neuronitis amongst the sampled Consulting Physicians.
· Meniere’s Disease and Spondylosis were the other causes reported by the Consulting Physicians (in decreasing order of occurrence).
· Migraine is the least significant cause of Vertigo.
7. What is the usual line of treatment?
Total Respondents = 60 (multiple responses)
|
Line of Treatment |
Responses |
% of Responses |
|
Lifestyle modification |
1 |
1.35 |
|
Pharmacological |
51 |
68.92 |
|
Manoeuvres only |
1 |
1.35 |
|
Pharmacological and Manoeuvres |
21 |
28.38 |
Table 26: Responses for usual line of treatment in Vertigo management
Interpretation:
· Pharmacological therapy is the most preferred line of treatment amongst 68.92% Consulting Physicians for effective Vertigo management.
· 28.38% Consulting Physicians also resort to Pharmacological & Manoeuvres therapy for treating Vertigo.
8. In pharmacological treatment what is your drug of choice?
Total Respondents = 60 (multiple responses)
|
Molecule |
Responses |
% of Responses |
|
Betahistine |
56 |
83.58 |
|
Cinnarizine |
5 |
7.46 |
|
Dimenhydrinate |
1 |
1.49 |
|
Combination |
5 |
7.46 |
|
Any other |
0 |
0 |
Table 27: Responses for molecule management in Vertigo management pharmacologically
Interpretation:
· Betahistine is the most preferred drug of choice for treating vertigo amongst 83.58% Consulting Physicians.
· 1.49% Consulting Physicians prescribed Dimenhydrinate and 7.46% Consulting Physicians prefer to use a combination of drugs (Betahistine with Dimenhydrinate/Cinnarizine) or Cinnarizine solely for treating Vertigo.
9. May I know your usual treatment approach?
|
|
8 mg |
16mg |
24mg |
24mg C.R |
48 mg C.R |
|
OD |
-- |
-- |
-- |
4 |
1 |
|
BID |
4 |
14 |
3 |
-- |
-- |
|
TID |
20 |
4 |
8 |
-- |
-- |
Table 28: Chart showing the type of Betahistine dose prescribed for treating Vertigo
Total Respondents = 60
Interpretation:
· 33.33% of the Consulting Physicians prefer to give 8mg TID dose of Betahistine to patients to treat vertigo.
· 23.33% of the Consulting Physicians prescribe 16mg BID dosage, while 13.33% of them prefer 24mg TID, for pharmacological therapy with Betahistine for Vertigo.
· Only 6.67% of Consulting Physicians prescribes 24mg C.R once a day formulation (Betahistine O.D) for treatment in of Vertigo.
· 6.67% of Consulting Physicians prescribe 8mg BID & 5% of Consulting Physicians use 24mg as a BID dose for treating Vertigo.
· Also, 1.67% Consulting Physicians prescribes 48mg OD of Betahistine to their patients.
Duration:
Total Respondents = 60
|
Duration |
Responses |
% of responses |
|
1-2 weeks |
44 |
73.33 |
|
3-4 weeks |
16 |
26.67 |
|
4-8 weeks |
0 |
0 |
Table 29: Responses for duration of pharmacological therapy for Vertigo
Interpretation:
· 1-2 weeks is the most preferred duration of therapy amongst 73.33% Consulting Physicians.
· 26.66% Consulting Physicians opted for 3-4 weeks therapy with Betahistine, while no one opted for more than 4 weeks
10. Do you titrate dosage?
Total Respondents = 60
|
Response to question |
Responses |
% of Responses |
|
Yes |
57 |
95 |
|
No |
3 |
5 |
Table 30: Responses for dosage titration in treating Vertigo
Interpretation:
· 95% of the Consulting Physicians were found to titrate the dosage in Vertigo treatment with.
· With the usual approach being tapering down the dosage in patients where symptoms are relieved and do not recur, and then putting it off. But in some recurrent cases of vertigo the dosage was increased.
· Where as 5% of the Consulting Physicians did not titrate the dosage.
11. Doctor, are you aware of once a day Betahistine preparation?
Total Respondents = 60
|
Response to question |
Responses |
% of Responses |
|
Yes |
59 |
98.33 |
|
No |
1 |
1.67 |
Table 31: Responses for Betahistine O.D awareness
Interpretation
· Majority (98.33%) of the Consulting Physicians were found to be aware of Betahistine once a day preparation.
· Only 1.67% Consulting Physicians were still unaware of Betahistine O.D formulation.
12. If Yes, have you used?
Fig 29: Bar chart showing Betahistine once a day usage
Total Respondents = 60
|
Response to question |
Responses |
% of Responses |
|
Yes |
28 |
46.67 |
|
No |
32 |
53.3 |
Table 32: Responses for Betahistine O.D usage
Interpretation:
· 53.33% of the Consulting Physicians did not use Betahistine O.D formulation, they reportedly used the conventional doses.
· 46.67% of the sampled Consulting Physicians, were found to use Betahistine O.D formulation.
13. If answer to Q11 is Yes, what is preferred strength & duration?
Fig 30: Pie chart showing choice of Betahistine once a day preparation usage
Total Respondents = 28 (out of 60 Consulting Physicians)
|
Strength |
Duration |
Responses |
% of Responses |
Overall % |
|
24 mg |
1 week |
8 |
28.57 |
60.71 |
|
2 weeks |
9 |
32.14 |
||
|
4 weeks |
0 |
0 |
||
|
48 mg |
1 week |
5 |
17.86 |
39.29 |
|
2 weeks |
5 |
17.86 |
||
|
4 weeks |
1 |
3.57 |
||
Interpretation:
· Consulting Physicians those who use Betahistine once a day preparation, mostly (60.71%) prescribe 24mg C.R, while 39.29% use 48mg C.R formulation.
· 32.14% of Consulting Physicians prescribe 24mg C.R for 1 week, followed by 32.14% of Consulting Physicians for 2 weeks.
· No Consulting Physician use 24mg OD formulation for 4 weeks
· 17.86% of Consulting Physicians use 48mg OD formulation for 1 week and 2 weeks respectively to treat Vertigo.
· No Consulting Physician use 48mg OD formulation for 4 weeks
14. If used, how do you use the OD formulation?
Total Respondents = 60
|
Response to question |
Responses |
% of Responses |
|
Start with OD |
32 |
53.33 |
|
Only Maintenance with OD |
28 |
46.67 |
Table 34: Responses for preferred use of once a day formulation
Interpretation:
· 53.33% of the Consulting Physicians prefer to use O.D formulation as a start up dose, while the rest (46.67%) preferred to use it as a maintenance dose only.
15. Is the usage of OD preparation indication specific?
Fig 32: Pie chart showing use of once a day preparation usage indication specifically
Total Respondents = 60
|
Response to question |
Responses |
% of Responses |
|
Yes |
2 |
3.33 |
|
No |
58 |
96.67 |
Table 35: Responses for use of once a day formulation indication specifically
Interpretation:
· Majority (96.67%) of the Consulting Physicians do not use O.D formulation particularly specific to any indication.
· Only 3.33% of Consulting Physicians use it specific to any particular condition.
16. If yes, which is the indication and any specific reasons?
3.33% of the Consulting Physicians use O.D formulation in Acute cases of vertigo. They also prefer to use it in patients with severe symptoms.
17. Do you also use two anti-vertigo molecules like morning/evening dose?
Fig 33: Pie chart showing use of two Anti-Vertigo molecules
Total Respondents = 60
|
Response to question |
Responses |
% of Responses |
|
Yes |
13 |
21.67 |
|
No |
47 |
78.33 |
Table 36: Responses for usage of two Anti-vertigo molecules
Interpretation:
· 78.33% of the Consulting Physicians do not use two Anti-Vertigo molecules together.
· Only 21.67% of the Consulting Physicians use two Anti-vertigo molecules together as a morning-evening dose.
If Yes, your usual approach
Sometimes Betahistine is prescribed with Dimenhydrinate or Cinnarizine.
18. Do you think, combination of Betahistine with Dimenhydrinate is required? If yes, for which indication and condition is it useful?
Total Respondents = 60
|
Response |
Number of Responses |
% of response |
|
Yes |
27 |
45 |
|
No |
2 |
3.33 |
|
Maybe |
4 |
6.67 |
|
Not tried |
27 |
45 |
Table 37: Responses for usage of combination of Betahistine with Dimenhydrinate
Interpretation:
· 45% of the Consulting Physicians have not tried this combination, but 45% also said it to be a potent and viable combination.
· They were further aided by 6.67% of doctors who felt it maybe a fruitful combination.
· 3.33% of Consulting Physicians feel that this would not serve to be a good combination.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CARDIOLOGISTS
CARDIOLOGISTS(SAMPLE SIZE = 20)
· The incidence of, 15-25% patients was found to be a majority amongst 40% Cardiologists, who have administered anti-vertigo medications.
· The % of female patients who seek anti-vertigo medications were found to be on the higher side amongst Cardiologists.
· 55% of the Cardiologists felt that a 40:60 (Male:Female) ratio is most observed in their practice.
· 56.52% Cardiologists reported that 40-50 years was the prevalent category of the patients.
· Majorly 85% of Cardiologists observed Vertigo patients with Secondary to pre-existing ailment.
· Diabetes Mellitus is the most common secondary to pre-existing ailment reported by Cardiologists, followed by Hypertension & Cardiac problems (28.13%) amongst the Cardiologists.
· BPPV is the most common cause of Vertigo, followed by Labyrinthitis and Vestibular Neuronitis amongst the sampled Cardiologists.
· Pharmacological therapy is the most preferred line of treatment amongst 68.18% Cardiologists for effective Vertigo management.
· Betahistine is the most preferred drug of choice for treating vertigo amongst 100% Cardiologists.
· 55 % of the Cardiologists prefer to give 8mg TID dose of Betahistine to patients to treat vertigo.
· 1-2 weeks is the most preferred duration of therapy with Betahistine amongst 65% Cardiologists.
· 80% of the Cardiologists were found to titrate the dosage in Vertigo treatment.
· Majority (80%) of the Cardiologists were found to be aware of Betahistine once a day preparation.
· 70% of the Cardiologists did not use Betahistine O.D formulation, they reportedly used the conventional doses.
· Amongst all Cardiologists those who use Betahistine once a day preparation, 66.67% mostly prescribe 24mg C.R, while 33.33% use 48mg C.R formulation.
· The duration for which 24mg C.R is prescribed the most, is equally by 33.33% Cardiologists for 1 week and 2 weeks respectively.
· 60% of the Cardiologists prefer to use O.D formulation as a start up dose, while the rest (40%) preferred to use it as a maintenance dose only.
· Majority (95%) of the Cardiologists do not use O.D formulation particularly specific to any indication.
· 90% of the Cardiologists do not use two Anti-Vertigo molecules together.
· 45% of the Cardiologists have not tried this combination, but 45% said it to be a potent and viable combination.
· They were further aided by 5% of doctors who felt it maybe a fruitful combination.
1. In your practice what is the incidence of patients who need anti-vertigo medication?
Total Respondents = 20
|
Category |
Responses |
Percentage of responses |
|
5-10% |
5 |
25 |
|
10-15% |
5 |
25 |
|
15-25% |
8 |
40 |
|
More than 25% |
2 |
10 |
Table 38: Responses for incidence of patients requiring Anti-Vertigo Medication per week
Interpretation:
· The incidence of, 15-25% patients was found to be a majority amongst 40% Cardiologists, who have administered anti-vertigo medications.
· The incidence of 5-10% & 10-15% patients were also reported amongst 25% Cardiologists respectively.
· Only 10% of the Cardiologists reported of more than 25% patients requiring anti-vertigo medication.
2. May I know the approximate bifurcation of patients gender wise (in %)?
Total Respondents = 20
|
% of Patients |
Approx Ratio |
Responses |
% of Responses |
|
Male |
40 |
11 |
55 |
|
Female |
60 |
||
|
Male |
50 |
5 |
25 |
|
Female |
50 |
||
|
Male |
30 |
4 |
20 |
|
Female |
70 |
||
Table 39: Responses for gender bifurcation of patients requiring Anti-Vertigo Medication per week
Interpretation:
· The % of female patients who seek anti-vertigo medications were found to be on the higher side amongst Cardiologists.
· 55% of the Cardiologists felt that a 40:60 (Male:Female) ratio is most observed in their practice.
· 30:70 (Male:Female) ratio was observed amongst 25% Cardiologists.
· 20% Cardiologists observed an equal ratio of 50:50 (Male:Female) in their practice.
3. Is it more predominant in any particular age group?
Total Respondents = 20 (Multiple responses)
|
Age of Patients |
Responses |
% of Responses |
|
Below 20 years |
0 |
0 |
|
20-40 years |
0 |
0 |
|
40-50 years |
13 |
56.52 |
|
Above 50 years |
10 |
43.48 |
Table 40: Responses for prevalent age group of patients requiring Anti-Vertigo Medication per week
Interpretation:
· 56.52% Cardiologists reported that 40-50 years was the prevalent category of the patients.
· Patients above 50 years age was reported with 43.48% Cardiologists, while there were no patients reported below 40 years.
4. Of these patients reported, do you get most patients as primary vertigo/dizziness patients or it is secondary to some pre-existing ailment?
Total Respondents = 20
|
Type |
Responses |
% of Responses |
|
Primary |
3 |
15 |
|
Secondary |
17 |
85 |
Table 41: Responses for category of patient having secondary to pre-existing ailment
Interpretation:
· It was revealed through the study that only, 15% of the Cardiologists, observed patients with Primary Vertigo.
· Majorly 85% of Cardiologists observed Vertigo patients with Secondary to pre-existing ailment.
5. If it is secondary to pre-existing ailment, what is the most common pre-existing disease/disorder you see?
Total Respondents = 17 multiple responses (out of 20 Cardiologists)
|
Disorder |
Responses |
% of Responses |
|
Diabetes Mellitus |
14 |
43.75 |
|
Hypertension & Cardiac problems |
9 |
28.13 |
|
ENT problems |
3 |
9.38 |
|
Spondylosis |
4 |
12.5 |
|
Thyroid |
2 |
6.25 |
Table 42: Responses for patents having most common secondary to pre-existing ailment
Interpretation:
· Diabetes Mellitus (43.75%) is the most common secondary to pre-existing ailment reported by Cardiologists, followed by Hypertension & Cardiac problems (28.13%) amongst the Cardiologists.
· Spondylosis along with ENT problems were also reported in12.5% & 9.38% Cardiologists respectively.
· 6.25% Cardiologists felt Thyroid could be the secondary to pre-existing cause of Vertigo.
6. What are the most common causes of Vertigo that you see in your practice?
|
Cause |
Frequency of Ranks |
|||||
|
Rank 1 |
Rank 2 |
Rank 3 |
Rank 4 |
Rank 5 |
Rank 6 |
|
|
BPPV |
20 |
|
|
|
|
|
|
Labyrinthitis |
|
12 |
2 |
|
|
|
|
Vestibular Neuronitis |
|
2 |
5 |
1 |
|
|
|
Spondylosis |
|
2 |
4 |
2 |
|
|
|
Meniere’s Disease |
|
|
2 |
1 |
|
|
Table 43: Table depicting the most common cause of Vertigo as ranked
Total Respondents = 20
Interpretation:
· BPPV is the most common cause of Vertigo, followed by Labyrinthitis and Vestibular Neuronitis amongst the sampled Cardiologists.
· Spondylosis & Meniere’s Disease were the other causes reported by the Cardiologists (in decreasing order of occurrence).
7. What is the usual line of treatment?
Total Respondents = 20 (multiple responses)
|
Line of Treatment |
Responses |
% of Responses |
|
Lifestyle modification |
0 |
0 |
|
Pharmacological |
15 |
68.18 |
|
Manoeuvres only |
0 |
0 |
|
Pharmacological and Manoeuvres |
7 |
31.82 |
Table 44: Responses for usual line of treatment in Vertigo management
Interpretation:
· Pharmacological therapy is the most preferred line of treatment amongst 68.18% Cardiologists for effective Vertigo management.
· 31.82% Cardiologists also resort to Pharmacological & Manoeuvres therapy for treating Vertigo.
· Lifestyle Modification and only Manoeuvres were not opted by any Cardiologists.
8. In pharmacological treatment what is your drug of choice?
Total Respondents = 20 (multiple responses)
|
Molecule |
Responses |
% of Responses |
|
Betahistine |
20 |
100 |
|
Cinnarizine |
0 |
0 |
|
Dimenhydrinate |
0 |
0 |
|
Combination |
0 |
0 |
|
Any other |
0 |
0 |
Table 45: Responses for molecule management in Vertigo management pharmacologically
Interpretation:
· Betahistine is the most preferred drug of choice for treating vertigo amongst 100% Cardiologists.
9. May I know your usual treatment approach?
|
|
8 mg |
16mg |
24mg |
24mg C.R |
48 mg C.R |
|
OD |
-- |
-- |
-- |
-- |
-- |
|
BID |
1 |
3 |
6 |
-- |
-- |
|
TID |
10 |
-- |
-- |
-- |
-- |
Table 46: Chart showing the type of Betahistine dose prescribed for treating Vertigo
Total Respondents = 20
Interpretation:
· 55 % of the Cardiologists prefer to give 8mg TID dose of Betahistine to patients to treat vertigo.
· 30% of the Cardiologists prescribe 24mg BID dosage, while 15% of them prefer 16mg BID, for pharmacological therapy with Betahistine for Vertigo.
· Only 5% of Cardiologists prescribes 8mg BID for treatment in of Vertigo.
· Out of all the Cardiologists sampled none were seen to prescribe OD formulation of Betahistine to their patients.
Duration:
Total Respondents = 20
|
Duration |
Responses |
% of responses |
|
|
1-2 weeks |
13 |
65 |
|
|
3-4 weeks |
7 |
35 |
Interpretation:
· 1-2 weeks is the most preferred duration of therapy with Betahistine amongst 65% Cardiologists.
· 35% Cardiologists opted for 3-4 weeks of therapy with Betahistine.
10. Do you titrate dosage?
Total Respondents = 20
|
Response to question |
Responses |
% of Responses |
|
Yes |
16 |
80 |
|
No |
4 |
20 |
Table 48: Responses for dosage titration in treating Vertigo
Interpretation:
· 80% of the Cardiologists were found to titrate the dosage in Vertigo treatment.
· With the usual approach being tapering down the dosage in patients where symptoms are relieved and do not recur, and then putting it off. But in some recurrent cases of vertigo the dosage was increased.
· Where as 20% of the Cardiologists did not titrate the dosage.
11. Doctor, are you aware of once a day Betahistine preparation?
Total Respondents = 20
|
Response to question |
Responses |
% of Responses |
|
Yes |
16 |
80 |
|
No |
4 |
20 |
Table 49: Responses for Betahistine O.D awareness
Interpretation:
· Majority (80%) of the Cardiologists were found to be aware of Betahistine once a day preparation.
· Only 20% Cardiologists were still unaware of Betahistine O.D formulation.
12. If Yes, have you used?
Total Respondents = 20
|
Response to question |
Responses |
% of Responses |
|
Yes |
6 |
30 |
|
No |
14 |
70 |
Table 50: Responses for Betahistine O.D usage
Interpretation:
· 70% of the Cardiologists did not use Betahistine O.D formulation, they reportedly used the conventional doses.
· 30% of the sampled Cardiologists, were found to use Betahistine O.D formulation.
13. If answer to Q11 is Yes, what is preferred strength & duration?
Total Respondents = 6 (out of 20 Cardiologists)
|
Strength |
Duration |
Responses |
% of Responses |
Overall % |
|
24 mg |
1 week |
2 |
33.33 |
66.67 |
|
2 weeks |
2 |
33.33 |
||
|
4 weeks |
0 |
0 |
||
|
48 mg |
1 week |
1 |
16.67 |
33.33 |
|
2 weeks |
1 |
16.67 |
||
|
4 weeks |
0 |
0 |
||
Table 51: Responses for preferred strength of Betahistine O.D usage
Interpretation:
· Amongst all Cardiologists those who use Betahistine once a day preparation, 66.67% mostly prescribe 24mg C.R, while 33.33% use 48mg C.R formulation.
· The duration for which 24mg C.R is prescribed the most, is equally by 33.33% Cardiologists for 1 week and 2 weeks respectivey.
· 16.67% Cardiologists use 48mg C.R for 1 week and 2 weeks respectively.
14. If used, how do you use the OD formulation?
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Total Respondents = 20
|
Response to question |
Responses |
% of Responses |
|
Start with OD |
12 |
60 |
|
Only Maintenance with OD |
8 |
40 |
Table 52: Responses for preferred use of once a day formulation
Interpretation:
· 60% of the Cardiologists prefer to use O.D formulation as a start up dose, while the rest (40%) preferred to use it as a maintenance dose only.
15. Is the usage of OD preparation indication specific?
Total Respondents = 20
|
Response to question |
Responses |
% of Responses |
|
Yes |
1 |
5 |
|
No |
19 |
95 |
Table 53: Responses for use of once a day formulation indication specifically
Interpretation:
· Majority (95%) of the Cardiologists do not use O.D formulation particularly specific to any indication.
· Only 5% of Cardiologists use it specific to any particular condition.
16. If yes, which is the indication and any specific reasons?
5% of the Cardiologists use O.D formulation in Acute cases of vertigo. They also prefer to use it in patients with severe symptoms.
17. Do you also use two anti-vertigo molecules like morning/evening dose?
Total Respondents = 20
|
Response to question |
Responses |
% of Responses |
|
Yes |
2 |
10 |
|
No |
18 |
90 |
Table 54: Responses for usage of two Anti-vertigo molecules
Interpretation:
· 90% of the Cardiologists do not use two Anti-Vertigo molecules together.
· Only 10% of the Cardiologists use two Anti-vertigo molecules together as a morning-evening dose
If Yes, your usual approach
Sometimes Betahistine is prescribed with Dimenhydrinate or Cinnarizine.
18. Do you think, combination of Betahistine with Dimenhydrinate is required? If yes, for which indication and condition is it useful?
Total Respondents = 20
|
Response |
Number of Responses |
% of response |
|
Yes |
9 |
45 |
|
No |
1 |
5 |
|
Maybe |
1 |
5 |
|
Not tried |
9 |
45 |
Table 55: Responses for usage of combination of Betahistine with Dimenhydrinate
Interpretation:
· 45% of the Cardiologists have not tried this combination, but 45% said it to be a potent and viable combination.
· They were further aided by 5% of doctors who felt it maybe a fruitful combination.
· 5% of Cardiologists feel that this would not serve to be a good combination.
E.N.T PHYSICIANS & SURGEONS
E.N.T( SAMPLE SIZE = 35)
· The incidence of, 15-25% patients was found to be a majority amongst 40% E.N.T doctors, who have administered anti-vertigo medications.
· The % of female patients who seek anti-vertigo medications were found to be on the higher side amongst E.N.T Doctors.
· 51.43% of the E.N.T Doctors felt that a 40:60 (Male:Female) ratio is most observed in their practice.
· 51.43% E.N.T Doctors reported that 40-50 years was the prevalent category of the patients.
· 51.53% of E.N.T Doctors observed Vertigo patients with Secondary to pre-existing ailment
· Diabetes Mellitus is the most common secondary to pre-existing ailment amongst 47.36% E.N.T doctors, followed by Hypertension & Cardiac problems in 28.95% and Spondylosis (10.52%) amongst the E.N.T Doctors.
· BPPV is the most common cause of Vertigo, followed by Vestibular Neuronitis and Labyrinthitis amongst the sampled E.N.T Doctors.
· Pharmacological and Manoeuvres is the most preferred line of treatment amongst 49.28% E.N.T Doctors for effective Vertigo management.
· Betahistine) is the most preferred drug of choice for treating vertigo amongst 61.11% E.N.T Doctors followed by 14.81% who use Cinnarizine.
· 48.57% of the E.N.T Doctors prefer to give 8mg TID dose of Betahistine to patients to treat vertigo.
· 1-2 weeks is the most preferred duration of therapy with Betahistine amongst 62.86% E.N.T Doctors
· 88.57% of the E.N.T Doctors were found to titrate the dosage in Vertigo treatment.
· With the usual approach being tapering down the dosage in patients where symptoms are relieved and do not recur, and then putting it off. But in some recurrent cases of vertigo the dosage was increased.
· All of the E.N.T doctors (100%) were found to be aware of Betahistine once a day preparation.
· 57.14% of the E.N.T Doctors did not use Betahistine O.D formulation, they reportedly used the conventional doses.
· Amongst all E.N.T Doctors those who use Betahistine once a day preparation, 73.33% mostly prescribe 24mg C.R, while 26.67% use 48mg C.R formulation.
· The duration for which 24mg C.R is prescribed the most is 1 week by 53.33% E.N.T Doctors, followed by 2 weeks amongst 20% E.N.T Doctors.
· Majority of the E.N.T doctors (62.86%) prefer to use O.D formulation as a start up dose, while the rest (37.14%) preferred to use it as a maintenance dose only.
· Majority (85.71%) of the E.N.T doctors do not use O.D formulation particularly specific to any indication.
· 14.29% of the E.N.T doctors use O.D formulation in Acute cases of vertigo. They also prefer to use it in patients with severe symptoms.
· 85.71% of the E.N.T Doctors do not use two Anti-Vertigo molecules together.
· 40% of the E.N.T Doctors have not tried this combination, but 54.29% said it to be a potent and viable combination.
They were further aided by 2.86% of doctors who felt it maybe a fruitful combination.
1. In your practice what is the incidence of patients who need anti-vertigo medication?
Total Respondents = 35
|
Category |
Responses |
Percentage of responses |
|
5-10% |
1 |
2.86 |
|
10-15% |
8 |
22.86 |
|
15-25% |
14 |
40 |
|
More than 25% |
12 |
34.28 |
Table 56: Responses for incidence of patients requiring Anti-Vertigo Medication per week
Interpretation:
· The incidence of, 15-25% patients was found to be a majority amongst 40% E.N.T doctors, who have administered anti-vertigo medications.
· 34.28% of the E.N.T doctors reported of more than 25% patients requiring anti-vertigo medication
· The incidence of 10-15% & 5-10% patients were also reported amongst 22.86% & 2.86% E.N.T doctors respectively.
2. May I know the approximate bifurcation of patients gender wise (in %)?
Table 57: Responses for gender bifurcation of patients requiring Anti-Vertigo Medication per week
Total Respondents = 35
|
% of Patients |
Approx Ratio |
Responses |
% of Responses |
|
Male |
40 |
18 |
51.43 |
|
Female |
60 |
||
|
Male |
50 |
4 |
11.43 |
|
Female |
50 |
||
|
Male |
30 |
13 |
37.14 |
|
Female |
70 |
||
Interpretation:
· The % of female patients who seek anti-vertigo medications were found to be on the higher side amongst E.N.T Doctors.
· 51.43% of the E.N.T Doctors felt that a 40:60 (Male:Female) ratio is most observed in their practice.
· 30:70 (Male:Female) ratio was observed amongst 37.14% E.N.T Doctors.
· 11.43% E.N.T Doctors observed an equal ratio of 50:50 (Male:Female) in their practice.
3. Is it more predominant in any particular age group?
Total Respondents = 35
|
Age of Patients |
Responses |
% of Responses |
|
Below 20 years |
0 |
0 |
|
20-40 years |
1 |
2.86 |
|
40-50 years |
18 |
51.43 |
|
Above 50 years |
16 |
45.71 |
Table 58: Responses for prevalent age group of patients requiring Anti-Vertigo Medication per week
Interpretation:
· 51.43% E.N.T Doctors reported that 40-50 years was the prevalent category of the patients.
· 2.86% E.N.T Doctors found 20-40 years category to be more prevalent in their practice.
· Patients above 50 years age was reported with 45.71% E.N.T Doctors, while there were no patients reported below 20 years.
4. Of these patients reported, do you get most patients as primary vertigo/dizziness patients or it is secondary to some pre-existing ailment?
Total Respondents = 35
|
Type |
Responses |
% of Responses |
|
Primary |
17 |
48.57 |
|
Secondary |
18 |
51.43 |
Table 59: Responses for category of patient having secondary to pre-existing ailment
Interpretation:
· 51.53% of E.N.T Doctors observed Vertigo patients with Secondary to pre-existing ailment
· It was revealed through the study that 48.57% of the E.N.T Doctors, observed patients with Primary Vertigo.
5. If it is secondary to pre-existing ailment, what is the most common pre-existing disease/disorder you see?
Total Respondents = 18 multiple responses (out of 35 E.N.T doctors)
|
Disorder |
Responses |
% of Responses |
|
Diabetes Mellitus |
18 |
47.36 |
|
Hypertension & Cardiac problems |
11 |
28.95 |
|
Thyroid |
2 |
5.26 |
|
Spondylosis |
4 |
10.52 |
|
Otitis Media |
3 |
7.89 |
Table 60: Responses for patents having most common secondary to pre-existing ailment
Interpretation:
· Diabetes Mellitus is the most common secondary to pre-existing ailment amongst 47.36% E.N.T doctors, followed by Hypertension & Cardiac problems in 28.95% and Spondylosis (10.52%) amongst the E.N.T Doctors.
· 7.89% reported Otits Media & 5.26% reported Thyroid as the most common secondary to pre-existing ailment respectively.
6. What are the most common causes of Vertigo that you see in your practice?
Total Respondents =35
|
Cause |
Frequency of Ranks |
|||||
|
Rank 1 |
Rank 2 |
Rank 3 |
Rank 4 |
Rank 5 |
Rank 6 |
|
|
BPPV |
33 |
1 |
|
|
|
|
|
Vestibular Neuronitis |
2 |
9 |
12 |
5 |
1 |
|
|
Labyrinthitis |
1 |
12 |
7 |
5 |
|
|
|
Meniere’s Disease |
|
12 |
9 |
2 |
2 |
|
|
Spondylosis |
|
1 |
2 |
2 |
2 |
|
|
Migraine |
|
|
|
4 |
1 |
|
Table 61: Table depicting the most common cause of Vertigo as ranked
Interpretation:
· BPPV is the most common cause of Vertigo, followed by Vestibular Neuronitis and Labyrinthitis amongst the sampled E.N.T Doctors.
· Meniere’s Disease and Spondylosis were the other causes reported by the E.N.T Doctors (in decreasing order of occurrence).
· Migraine is the least significant cause of Vertigo.
7. What is the usual line of treatment?
Fig 57: Bar chart showing the preference in line of treatment for Vertigo management
Total Respondents = 35 (multiple responses)
|
Line of Treatment |
Responses |
% of Responses |
|
Lifestyle modification |
1 |
1.45 |
|
Pharmacological |
33 |
47.82 |
|
Manoeuvres only |
1 |
1.45 |
|
Pharmacological and Manoeuvres |
34 |
49.28 |
Table 62: Responses for usual line of treatment in Vertigo management
Interpretation:
· Pharmacological and Manoeuvres is the most preferred line of treatment amongst 49.28% E.N.T Doctors for effective Vertigo management.
· 47.82% E.N.T Doctors also resort to Pharmacological therapy for treating Vertigo.
· 1.45% E.N.T doctors reported Lifestyle modification and Manoeuvres only respectively for vertigo management.
8. In pharmacological treatment what is your drug of choice?
Fig 58: Bar chart showing the preference in line of treatment for Vertigo management
Total Respondents = 35 (multiple responses)
|
Molecule |
Responses |
% of Responses |
|
Betahistine |
33 |
61.11 |
|
Cinnarizine |
8 |
14.81 |
|
Dimenhydrinate |
4 |
7.41 |
|
Combination |
7 |
12.96 |
|
Any other |
2 |
3.71 |
Table 63: Responses for molecule management in Vertigo management pharmacologically
Interpretation:
· Betahistine) is the most preferred drug of choice for treating vertigo amongst 61.11% E.N.T Doctors followed by 14.81% who use Cinnarizine.
· 7.41% E.N.T Doctors prescribed Dimenhydrinate and 12.96% E.N.T Doctors prefer to use a combination of drugs (Betahistine with Dimenhydrinate / Cinnarizine) to treating Vertigo.
· 3.71% use other drugs (Dimenhydrinate only)
9. May I know your usual treatment approach?
Total Respondents = 35
|
|
8 mg |
16mg |
24mg |
24mg C.R |
48 mg C.R |
|
OD |
-- |
-- |
-- |
1 |
-- |
|
BID |
-- |
4 |
5 |
-- |
-- |
|
TID |
17 |
5 |
1 |
-- |
-- |
Table 64: Chart showing the type of Betahistine dose prescribed for treating Vertigo
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Interpretation:
· 48.57% of the E.N.T Doctors prefer to give 8mg TID dose of Betahistine to patients to treat vertigo.
· 14.29% of the E.N.T Doctors prescribe 24mg BID dosage, while 11.43% of them prefer 16mg BID, for pharmacological therapy with Betahistine for Vertigo.
· Only 2.86% of E.N.T Doctors prescribes 24mg C.R once a day formulation (Betahistine O.D) for treatment in of Vertigo.
· Also, 14.29% E.N.T Doctors prescribe 16mg TID of Betahistine to their patients.
Duration:
Total Respondents = 35
|
Duration |
Responses |
% of responses |
|
1-2 weeks |
22 |
62.86 |
|
3-4 weeks |
12 |
34.29 |
|
4-8 weeks |
1 |
2.86 |
Table 65: Responses for duration of pharmacological therapy for Vertigo
Interpretation:
· 1-2 weeks is the most preferred duration of therapy with Betahistine amongst 62.86% E.N.T Doctors.
· 34.29% E.N.T Doctors opted for 3-4 weeks of therapy with Betahistine, while 2.86% preferred 4-8 weeks.
10. Do you titrate dosage?
Total Respondents = 35
|
Response to question |
Responses |
% of Responses |
|
Yes |
31 |
88.57 |
|
No |
4 |
11.43 |
Table 66: Responses for dosage titration in treating Vertigo
Interpretation:
· 88.57% of the E.N.T Doctors were found to titrate the dosage in Vertigo treatment.
· With the usual approach being tapering down the dosage in patients where symptoms are relieved and do not recur, and then putting it off. But in some recurrent cases of vertigo the dosage was increased.
· Where as 11.43% of the E.N.T Doctors did not titrate the dosage.
11. Doctor, are you aware of once a day Betahistine preparation?
Fig 61: Pie chart showing Betahistine once a day preparation awareness
Total Respondents = 35
|
Response to question |
Responses |
% of Responses |
|
Yes |
35 |
100 |
|
No |
0 |
0 |
Table 67: Responses for Betahistine O.D awareness
Interpretation:
· All of the E.N.T doctors (100%) were found to be aware of Betahistine once a day preparation.
12. If Yes, have you used?
Total Respondents = 35
|
Response to question |
Responses |
% of Responses |
|
Yes |
15 |
42.86 |
|
No |
20 |
57.14 |
Table 68: Responses for Betahistine O.D usage
Interpretation:
· 57.14% of the E.N.T Doctors did not use Betahistine O.D formulation, they reportedly used the conventional doses.
· 42.86% of the sampled E.N.T Doctors, were found to use Betahistine O.D formulation.
13. If answer to Q11 is Yes, what is preferred strength & duration?
Total Respondents = 15 (out of 35 E.N.T doctors)
|
Strength |
Duration |
Responses |
% of Responses |
Overall % |
|
24 mg |
1 week |
8 |
53.33 |
73.33 |
|
2 weeks |
3 |
20 |
||
|
4 weeks |
0 |
0 |
||
|
48 mg |
1 week |
2 |
13.33 |
26.67 |
|
2 weeks |
1 |
6.67 |
||
|
4 weeks |
1 |
6.67 |
||
Table 69: Responses for preferred strength of Betahistine O.D usage
Interpretation:
· Amongst all E.N.T Doctors those who use Betahistine once a day preparation, 73.33% mostly prescribe 24mg C.R, while 26.67% use 48mg C.R formulation.
· The duration for which 24mg C.R is prescribed the most is 1 week by 53.33% E.N.T Doctors, followed by 2 weeks amongst 20% E.N.T Doctors.
· 13.33% E.N.T Doctors use 48mg C.R for 1 week.
· 6.67% E.N.T Doctors use 48mg C.R for 2 weeks and 4 weeks repectively.
14. If used, how do you use the OD formulation?
Total Respondents = 35
|
Response to question |
Responses |
% of Responses |
|
Start with OD |
22 |
62.86 |
|
Only Maintenance with OD |
13 |
37.14 |
Table 70: Responses for preferred use of once a day formulation
Interpretation:
Majority of the E.N.T doctors (62.86%) prefer to use O.D formulation as a start up dose, while the rest (37.14%) preferred to use it as a maintenance dose only.
15. Is the usage of OD preparation indication specific?
Total Respondents = 35
|
Response to question |
Responses |
% of Responses |
|
Yes |
5 |
14.29 |
|
No |
30 |
85.71 |
Table 71: Responses for use of once a day formulation indication specifically
Interpretation:
· Majority (85.71%) of the E.N.T doctors do not use O.D formulation particularly specific to any indication.
· Only 14.29% of E.N.T doctors use it specifically against particular indication
16. If yes, which is the indication and any specific reasons?
14.29% of the E.N.T doctors use O.D formulation in Acute cases of vertigo. They also prefer to use it in patients with severe symptoms.
17. Do you also use two anti-vertigo molecules like morning/evening dose?
Total Respondents = 35
|
Response to question |
Responses |
% of Responses |
|
Yes |
5 |
14.29 |
|
No |
30 |
85.71 |
Table 72: Responses for usage of two Anti-vertigo molecules
Interpretation:
· 85.71% of the E.N.T Doctors do not use two Anti-Vertigo molecules together.
· Only 14.29% of the E.N.T Doctors use two Anti-vertigo molecules together as a morning-evening dose
If Yes, your usual approach
Sometimes Betahistine is prescribed with Dimenhydrinate or Cinnarizine.
18. Do you think, combination of Betahistine with Dimenhydrinate is required? If yes, for which indication and condition is it useful?
Total Respondents = 60
|
Response |
Number of Responses |
% of response |
|
Yes |
19 |
54.29 |
|
No |
1 |
2.86 |
|
Maybe |
1 |
2.86 |
|
Not tried |
14 |
40 |
Table 73: Responses for usage of combination of Betahistine with Dimenhydrinate
Interpretation:
· 40% of the E.N.T Doctors have not tried this combination, but 54.29% said it to be a potent and viable combination.
· They were further aided by 2.86% of doctors who felt it maybe a fruitful combination.
· 2.86% of E.N.T Doctors feel that this would not serve to be a good combination.
DIABETOLOGISTS
DIABETOLOGISTS(SAMPLE SIZE = 10)
· The incidence of 10-15% patients who seek anti-vertigo medications was found to be a majority amongst 40 % Diabetologists.
· The % of female patients who seek anti-vertigo medications were found to be on the higher side amongst Diabetologists.
· 50% of the Diabetologists felt that a 40:60 (Male:Female) ratio is most observed in their practice.
· 70% Diabetologists reported that 40-50 years was the prevalent category of the patients.
· It was revealed through the study that 90% of the Diabetologists, observed patients with secondary to pre-existing ailment.
· Diabetes Mellitus (47.39%) is the most common secondary to pre-existing ailment, followed by Spondylosis and Hypertension & Cardiac problems amongst 21.05% Diabetologists.
· BPPV is the most common cause of Vertigo, followed by Labyrinthitis and Meniere’s Disease amongst the sampled Diabetologists.
· Pharmacological therapy is the most preferred line of treatment amongst 70% Diabetologists for effective Vertigo management.
· Betahistine) is the most preferred drug of choice for treating vertigo amongst 90.9% Diabetologists.
· 30% of the Diabetologists prefer to give 8mg TID dose of Betahistine to patients to treat vertigo.
· 1-2 weeks is the most preferred duration of therapy with Betahistine amongst 90% Diabetologists.
· 90% of the Diabetologists were found to titrate the dosage in Vertigo treatment.
· With the usual approach being tapering down the dosage in patients where symptoms are relieved and do not recur, and then putting it off. But in some recurrent cases of vertigo the dosage was increased.
· Majority (90%) of the Diabetologists were found to be aware of Betahistine once a day preparation. Only 10% Diabetologists were still unaware of Betahistine O.D formulation.
· 60% of the Diabetologists did not use Betahistine O.D formulation, they reportedly used the conventional doses.
· Amongst all Diabetologists those who use Betahistine once a day preparation, 100% prescribe 24mg C.R, while none use 48mg C.R formulation.
· The duration for which 24mg C.R is prescribed the most is 2 weeks by 100% Diabetologists.
· 70% of the Diabetologists prefer to use O.D formulation as a start up dose, while the rest (30%) preferred to use it as a maintenance dose only.
· Majority (90%) of the Diabetologists do not use O.D formulation particularly specific to any indication.
· 80% of the Diabetologists do not use two Anti-Vertigo molecules together.
· 40% of the Diabetologists have not tried this combination, but 40% said it to be a potent and viable combination.
· They were further aided by 10% of doctors who felt it maybe a fruitful combination.
1. In your practice what is the incidence of patients who need anti-vertigo medication?
Total Respondents = 10
|
Category |
Responses |
Percentage of responses |
|
5-10% |
3 |
30 |
|
10-15% |
4 |
40 |
|
15-25% |
2 |
20 |
|
More than 25% |
1 |
10 |
Table 74: Responses for incidence of patients requiring Anti-Vertigo Medication per week
Interpretation:
· The incidence of 10-15% patients who seek anti-vertigo medications was found to be a majority amongst 40 % Diabetologists.
· 30% of the Diabetologists observed incidence of 5-10% of patients.
· 20% of Diabetologists had observed 15-25% patients in their practice. While only 10% Diabetologists had more than 25% patients requiring anti-vertigo medication
2. May I know the approximate bifurcation of patients gender wise (in %)?
Total Respondents = 10
|
% of Patients |
Approx Ratio |
Responses |
% of Responses |
|
Male |
40 |
5 |
50 |
|
Female |
60 |
||
|
Male |
50 |
2 |
20 |
|
Female |
50 |
||
|
Male |
30 |
3 |
30 |
|
Female |
70 |
||
Table 75: Responses for gender bifurcation of patients requiring Anti-Vertigo Medication per week
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Interpretation:
· The % of female patients who seek anti-vertigo medications were found to be on the higher side amongst Diabetologists.
· 50% of the Diabetologists felt that a 40:60 (Male:Female) ratio is most observed in their practice.
· 30:70 (Male:Female) ratio was observed amongst 30% Diabetologists.
· 20% Diabetologists observed an equal ratio of 50:50 (Male:Female) in their practice.
3. Is it more predominant in any particular age group?
Total Respondents = 10
|
Age of Patients |
Responses |
% of Responses |
|
Below 20 years |
0 |
0 |
|
20-40 years |
0 |
|
|
40-50 years |
5 |
50 |
|
Above 50 years |
5 |
50 |
Table 76: Responses for prevalent age group of patients requiring Anti-Vertigo Medication per week
Interpretation:
· 70% Diabetologists reported that 40-50 years was the prevalent category of the patients.
· 17.5% Diabetologists found 20-40 years category to be more prevalent in their practice.
· Patients above 50 years age was reported with 12.5% Diabetologists, while there were no patients reported below 20 years.
4. Of these patients reported, do you get most patients as primary vertigo/dizziness patients or it is secondary to some pre-existing ailment?
Total Respondents = 40
|
Type |
Responses |
% of Responses |
|
Primary |
1 |
10 |
|
Secondary |
9 |
90 |
Table 77: Responses for category of patient having secondary to pre-existing ailment
Interpretation:
· It was revealed through the study that only 10% of the Diabetologists, observed patients with Primary Vertigo.
· 90% of Diabetologists observed Vertigo patients with Secondary to pre-existing ailment.
5. If it is secondary to pre-existing ailment, what is the most common pre-existing disease/disorder you see?
Total Respondents = 9 Multiple Responses (out of 10 Diabetologists)
|
Disorder |
Responses |
% of Responses |
|
Diabetes Mellitus |
9 |
47.39 |
|
Hypertension & Cardiac problems |
4 |
21.05 |
|
Otitis Media |
2 |
10.53 |
|
Spondylosis |
4 |
21.05 |
Table 78: Responses for patents having most common secondary to pre-existing ailment
Interpretation:
· Diabetes Mellitus (47.39%) is the most common secondary to pre-existing ailment, followed by Spondylosis and Hypertension & Cardiac problems amongst 21.05% Diabetologists.
· Otitis Media was also reported in 10.53% Diabetologists.
6. What are the most common causes of Vertigo that you see in your practice?
Total Respondents = 10
|
Cause |
Frequency of Ranks |
|||||
|
Rank 1 |
Rank 2 |
Rank 3 |
Rank 4 |
Rank 5 |
Rank 6 |
|
|
BPPV |
7 |
2 |
|
|
|
|
|
Labyrinthitis |
1 |
3 |
2 |
|
|
|
|
Meniere’s Disease |
1 |
2 |
|
3 |
|
|
|
Spondylosis |
|
3 |
|
2 |
|
|
|
Vestibular Neuronitis |
|
|
5 |
|
|
|
|
Migraine |
|
|
|
|
1 |
|
Table 79: Table depicting the most common cause of Vertigo as ranked
Interpretation:
· BPPV is the most common cause of Vertigo, followed by Labyrinthitis and Meniere’s Disease amongst the sampled Diabetologists.
· Spondylosis, and Vestibular Neuronitis were the other causes reported by the Diabetologists (in decreasing order of occurrence).
· Migraine is the least significant cause of Vertigo.
7. What is the usual line of treatment?
Total Respondents = 10 (multiple responses)
|
Line of Treatment |
Responses |
% of Responses |
|
Lifestyle modification |
0 |
0 |
|
Pharmacological |
7 |
70 |
|
Manoeuvres only |
0 |
0 |
|
Pharmacological and Manoeuvres |
3 |
30 |
Table 80: Responses for usual line of treatment in Vertigo management
Interpretation:
· Pharmacological therapy is the most preferred line of treatment amongst 70% Diabetologists. for effective Vertigo management.
· 30% Diabetologists. also resort to Pharmacological and Manoeuvres therapy for treating Vertigo.
· Lifestyle modification and only manoeuvres were not opted by the Diabetologists.
8. In pharmacological treatment what is your drug of choice?
Total Respondents = 10 (multiple responses)
|
Molecule |
Responses |
% of Responses |
|
Betahistine |
10 |
90.9 |
|
Cinnarizine |
0 |
0 |
|
Dimenhydrinate |
1 |
9.09 |
|
Combination |
0 |
0 |
|
Any other |
0 |
0 |
Table 81: Responses for molecule management in Vertigo management pharmacologically
Interpretation:
· Betahistine) is the most preferred drug of choice for treating vertigo amongst 90.9% Diabetologists.
· 9.09% Diabetologists prescribed Dimenhydrinate.
· None of the sampled Diabetologists prefer to use a combination of drugs or any other drugs for treating Vertigo.
9. May I know your usual treatment approach?
Total Respondents = 10
|
|
8 mg |
16mg |
24mg |
24mg C.R |
48 mg C.R |
|
OD |
-- |
-- |
-- |
2 |
-- |
|
BID |
-- |
1 |
1 |
-- |
-- |
|
TID |
3 |
3 |
-- |
-- |
-- |
Table 82: Chart showing the type of Betahistine dose prescribed for treating Vertigo
Interpretation:
· 30% of the Diabetologists prefer to give 8mg TID dose of Betahistine to patients to treat vertigo.
· 10% of the Diabetologists prescribe 24mg BID dosage, while 10% of them prefer 16mg BID, for pharmacological therapy with Betahistine for Vertigo.
· Only 20% of Diabetologists prescribes 24mg C.R once a day formulation (Betahistine O.D) for treatment in of Vertigo.
· Also, 30% Diabetologists prescribe 16mg TID of Betahistine to their patients.
Duration:
Total Respondents = 10
|
Duration |
Responses |
% of responses |
|
1-2 weeks |
9 |
90 |
|
3-4 weeks |
1 |
10 |
|
4-8 weeks |
0 |
0 |
Table 83: Responses for duration of pharmacological therapy for Vertigo
Interpretation:
· 1-2 weeks is the most preferred duration of therapy with Betahistine amongst 90% Diabetologists.
· 10% Diabetologists opted for 3-4 weeks of therapy with Betahistine, while none of the sampled Diabetologists preferred 4-8 weeks therapy with Betahistine.
10. Do you titrate dosage?
Fig 77 : Bar chart showing dosage titration in Vertigo treatment
Total Respondents = 10
|
Response to question |
Responses |
% of Responses |
|
Yes |
9 |
90 |
|
No |
1 |
10 |
Table 84: Responses for dosage titration in treating Vertigo
Interpretation:
· 90% of the Diabetologists were found to titrate the dosage in Vertigo treatment.
· With the usual approach being tapering down the dosage in patients where symptoms are relieved and do not recur, and then putting it off. But in some recurrent cases of vertigo the dosage was increased.
· Where as 10% of the Diabetogists did not titrate the dosage.
11. Doctor, are you aware of once a day Betahistine preparation?
Total Respondents = 10
|
Response to question |
Responses |
% of Responses |
|
Yes |
9 |
90 |
|
No |
1 |
10 |
Table 85: Responses for Betahistine O.D awareness
Interpretation:
· Majority (90%) of the Diabetologists were found to be aware of Betahistine once a day preparation. Only 10% Diabetologists were still unaware of Betahistine O.D formulation.
12. If Yes, have you used?
Fig 79: Bar chart showing Betahistine once a day usage
Total Respondents = 40
|
Response to question |
Responses |
% of Responses |
|
Yes |
4 |
40 |
|
No |
6 |
60 |
Table 86: Responses for Betahistine O.D usage
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Interpretation:
· 40% of the sampled Diabetologists, were found to use Betahistine O.D formulation.
· 60% of the Diabetologists did not use Betahistine O.D formulation, they reportedly used the conventional doses.
13. If answer to Q11 is Yes, what is preferred strength & duration?
Fig 80: Pie chart showing choice of Betahistine once a day preparation usage
Total Respondents = 4 (out of 10 Diabetologists)
|
Strength |
Duration |
Responses |
% of Responses |
Overall % |
|
24 mg |
1 week |
0 |
0 |
100 |
|
2 weeks |
4 |
100 |
||
|
4 weeks |
0 |
0 |
||
|
48 mg |
1 week |
0 |
0 |
0 |
|
2 weeks |
0 |
0 |
||
|
4 weeks |
0 |
0 |
||
Table 87: Responses for preferred strength of Betahistine O.D usage
Interpretation:
· Amongst all Diabetologists those who use Betahistine once a day preparation, 100% prescribe 24mg C.R, while none use 48mg C.R formulation.
· The duration for which 24mg C.R is prescribed the most is 2 weeks by 100% Diabetologists.
· None of the sampled Diabetologists were found to prescribe Betahistine 48mg O.D formulation.
14. If used, how do you use the OD formulation?
Total Respondents = 10
|
Response to question |
Responses |
% of Responses |
|
Start with OD |
7 |
70 |
|
Only Maintenance with OD |
3 |
30 |
Table 88: Responses for preferred use of once a day formulation
Interpretation:
· 70% of the Diabetologists prefer to use O.D formulation as a start up dose, while the rest (30%) preferred to use it as a maintenance dose only.
15. Is the usage of OD preparation indication specific?
Total Respondents = 10
|
Response to question |
Responses |
% of Responses |
|
Yes |
1 |
10 |
|
No |
9 |
90 |
Table 89: Responses for use of once a day formulation indication specifically
Interpretation:
· Majority (90%) of the Diabetologists do not use O.D formulation particularly specific to any indication.
· Only 10% of Diabetologists use it specific to any particular condition.
16. If yes, which is the indication and any specific reasons?
10% of the Diabetologists use O.D formulation in Acute cases of vertigo. They also prefer to use it in patients with severe symptoms.
17. Do you also use two anti-vertigo molecules like morning/evening dose?
Total Respondents = 10
|
Response to question |
Responses |
% of Responses |
|
Yes |
2 |
20 |
|
No |
8 |
80 |
Table 90: Responses for usage of two Anti-vertigo molecules
Interpretation:
· 80% of the Diabetologists do not use two Anti-Vertigo molecules together.
· Only 20% of the Diabetologists use two Anti-vertigo molecules together as a morning-evening dose
If Yes, your usual approach
Sometimes Betahistine is prescribed with Dimenhydrinate or Cinnarizine.
18. Do you think, combination of Betahistine with Dimenhydrinate is required? If yes, for which indication and condition is it useful?
Total Respondents = 10
|
Response |
Number of Responses |
% of response |
|
Yes |
4 |
40 |
|
No |
1 |
10 |
|
Maybe |
1 |
10 |
|
Not tried |
4 |
40 |
Table 91: Responses for usage of combination of Betahistine with Dimenhydrinate
Interpretation:
· 40% of the Diabetologists have not tried this combination, but 40% said it to be a potent and viable combination.
· They were further aided by 10% of doctors who felt it maybe a fruitful combination.
· 10% of Diabetologists feel that this would not serve to be a good combination
1. In your practice what is the incidence of patients who need anti-vertigo medication?
Fig 85: Bar chart showing incidence of patients requiring Anti-Vertigo Medication per week
Total Respondents = 165
|
Category |
Responses |
Percentage of responses |
|
5-10% |
28 |
16.97 |
|
10-15% |
64 |
38.79 |
|
15-25% |
52 |
31.52 |
|
More than 25% |
21 |
12.72 |
Table 92: Responses for incidence of patients requiring Anti-Vertigo Medication per week
Interpretation:
· 38.79 % (majority) of doctors observed 10-15% patients requiring anti-vertigo medications.
· 15-25% incidence of vertigo patients were reported to 31.52% of doctors.
· 16.97% doctors observed 5-10% patients for vertigo treatment in their practice.
· Only 12.72% of doctors observed more than 25% patients.
· The incidence of 10-15% patients was found to be a majority amongst 52.5% Neurologists, 43.33% Consulting Physicians and amongst 40 % Diabetologists who have administered anti-vertigo medications.
· The incidence of, 15-25% patients was found to be a majority amongst 40% Cardiologists and 40% E.N.T doctors, who have administered anti-vertigo medications.
2. May I know the approximate bifurcation of patients gender wise (in %)?
Fig 86: Bar chart showing gender bifurcation of patients requiring Anti-Vertigo Medication per week
Total Respondents = 165
|
% of Patients |
Approx Ratio |
Responses |
% of Responses |
|
Male |
40 |
99 |
60 |
|
Female |
60 |
||
|
Male |
50 |
31 |
18.79 |
|
Female |
50 |
||
|
|
|
|
|
|
Male |
30 |
34 |
20.61 |
|
Female |
70 |
||
|
Male |
20 |
1 |
0.61 |
|
Female |
80 |
||
Table 93: Responses for gender bifurcation of patients requiring Anti-Vertigo Medication per week
Interpretation:
· The % of female patients who seek anti-vertigo medications were found to be on the higher side amongst sampled doctors.
· 60% of the sampled doctors felt that a 40:60 (Male:Female) ratio is most observed in their practice.
· 30:70 (Male:Female) ratio was observed amongst 20.61% Sampled doctors.
· 18.79% sampled doctors observed an equal ratio of 50:50 (Male:Female) in their practice.
· 62.5% of the Neurologists, 66.67% of the Consulting Physicians, 55% of the, 51.43% and 50% of the Diabetologists observed that a 40:60 (Male:Female) ratio is most common in their practice.
3. Is it more predominant in any particular age group?
Total Respondents = 165 Multiple responses
|
Age of Patients |
Responses |
% of Responses |
|
Below 20 years |
0 |
0 |
|
20-40 years |
11 |
6.51 |
|
40-50 years |
104 |
61.54 |
|
Above 50 years |
54 |
31.95 |
Table 94: Responses for prevalent age group of patients requiring Anti-Vertigo Medication per week
Interpretation:
· 61.54% Sampled doctors reported that 40-50 years was the prevalent category of the patients.
· 6.51% Sampled doctors found 20-40 years category to be more prevalent in their practice.
· Patients above 50 years age was reported with 31.95% Sampled doctors, while there were no patients reported below 20 years.
· 70% Neurologists, 66.67% Consulting Physicians, 56.52% Cardiologists, 51.43% E.N.T Doctors & 70% Diabetologists reported that 40-50 years was the prevalent category of the patients.
4. Of these patients reported, do you get most patients as primary vertigo/dizziness patients or it is secondary to some pre-existing ailment?
Total Respondents = 165
|
Type |
Responses |
% of Responses |
|
Primary |
57 |
34.55 |
|
Secondary |
108 |
65.45 |
Table 95: Responses for category of patient having secondary to pre-existing ailment
Interpretation:
· It was revealed through the study that 34.55% of the sampled doctors, observed patients with Primary Vertigo.
· Majority (65.45%) of sampled doctors observed Vertigo patients with Secondary to pre-existing ailment.
· It was revealed through the study that 72.5% of the Neurologists, observed patients with Primary Vertigo.
· It was observed through the study that 88.33% of the Consulting Physicians, 85% of Cardiologists, 51.53% of E.N.T Doctors and 90% of the Diabetologists, observed patients with secondary to pre-existing ailment.
5. If it is secondary to pre-existing ailment, what is the most common pre-existing disease/disorder you see?
Fig 89: Bar chart showing incident patients having most common secondary to pre-existing ailment
Total Respondents = 108 multiple responses (out of 165 Sampled doctors)
|
Disorder |
Responses |
% of Responses |
|
Diabetes Mellitus |
103 |
51.24 |
|
Hypertension & Cardiac problems |
58 |
28.86 |
|
ENT problems |
2 |
0.99 |
|
Spondylosis |
26 |
12.94 |
|
Thyroid |
4 |
1.99 |
|
Otitis Media |
5 |
2.49 |
|
Vit B12 deficiency |
3 |
1.49 |
Table 96: Responses for patents having most common secondary to pre-existing ailment
Interpretation:
· Diabetes Mellitus (51.24%) is the most common secondary to pre-existing ailment, followed by Hypertension & Cardiac problems (28.86%) amongst the Sampled doctors.
· 12.94% doctors opted for spondylosis followed by 2.49% for Otitis Media
· 1.99% doctors reported Thyroid and 1.49% for Vit B12 defi. As secondary ailment
6. What are the most common causes of Vertigo that you see in your practice?
Total Respondents = 165
|
Cause |
Frequency of Ranks |
|||||
|
Rank 1 |
Rank 2 |
Rank 3 |
Rank 4 |
Rank 5 |
Rank 6 |
|
|
BPPV |
152 |
9 |
|
1 |
|
|
|
Labyrinthitis |
6 |
66 |
21 |
10 |
|
|
|
Vestibular Neuronitis |
9 |
35 |
38 |
9 |
1 |
|
|
Meniere’s Disease |
1 |
19 |
25 |
9 |
5 |
|
|
Spondylosis |
1 |
22 |
19 |
6 |
4 |
1 |
|
Vertebral Ischemia |
|
|
2 |
|
|
|
|
Migraine |
|
|
|
7 |
4 |
|
Table 97: Table depicting the most common cause of Vertigo as ranked
Interpretation:
· BPPV is the most common cause of Vertigo, followed by Vestibular Neuronitis and Labyrinthitis amongst the sampled Sampled doctors.
· Spondylosis, Meniere’s Diseases and Migraine were the other causes reported by the Sampled doctors (in decreasing order of occurrence).
· Vertebral Ischemia is the least significant cause of Vertigo.
· Diabetes Mellitus is the most common secondary to pre-existing ailment amongst 44% Neurologists, followed by Spondylosis (40%).
· Diabetes Mellitus (53.76%) is the most common secondary to pre-existing ailment, followed by Hypertension & Cardiac problems (34.41%) amongst the Consulting Physicians.
· Diabetes Mellitus (43.75%) is the most common secondary to pre-existing ailment reported by Cardiologists, followed by Hypertension & Cardiac problems (28.13%) amongst the Cardiologists.
· Diabetes Mellitus is the most common secondary to pre-existing ailment amongst 47.36% E.N.T doctors, followed by Hypertension & Cardiac problems in 28.95% and Spondylosis (10.52%) amongst the E.N.T Doctors.
· Diabetes Mellitus (47.39%) is the most common secondary to pre-existing ailment, followed by Spondylosis and Hypertension & Cardiac problems amongst 21.05% Diabetologists.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
7. What is the usual line of treatment?
Total Respondents = 165 (multiple responses)
|
Line of Treatment |
Responses |
% of Responses |
|
Lifestyle modification |
2 |
0.88 |
|
Pharmacological |
120 |
53.12 |
|
Manoeuvres only |
3 |
1.33 |
|
Pharmacological and Manoeuvres |
101 |
44.69 |
Table 98: Responses for usual line of treatment in Vertigo management
Interpretation:
· 53.12% Sampled doctors majorly resort to Pharmacological therapy for treating Vertigo.
· Pharmacological and Manoeuvres is preferred line of treatment amongst 44.69% Sampled doctors for effective Vertigo management.
· 0.88% doctors suggest a Lifestyle modification & 1.33% doctors suggest only manoeuvres in Vertigo management.
· Pharmacological % Manoeuvres is the most preferred line of treatment amongst 70.58% Neurologists & 49.28% E.N.T Doctors for effective Vertigo management.
· Pharmacological therapy is the most preferred line of treatment amongst 68.92% Consulting Physicians, 68.18% Cardiologists & 70% Diabetologists for treating Vertigo.
8. In pharmacological treatment what is your drug of choice?
Total Respondents = 165 (multiple responses)
|
Molecule |
Responses |
% of Responses |
|
Betahistine |
161 |
80.5 |
|
Cinnarizine |
13 |
6.5 |
|
Dimenhydrinate |
9 |
4.5 |
|
Combination |
14 |
7 |
|
Any other |
3 |
1.5 |
Table 99: Responses for molecule management in Vertigo management pharmacologically
Interpretation:
· Betahistine) is the most preferred drug of choice for treating vertigo amongst 80.5% sampled doctors.
· 4.5 % Sampled doctors prescribed Dimenhydrinate and 7% Sampled doctors prefer to use a combination of drugs (Betahistine with Dimenhydrinate / Cinnarizine) to treating Vertigo.
· 6.5% doctors prescribe Cinnarizine while the rest 1.5% prescribes other medication (diuretics etc).
9. May I know your usual treatment approach?
|
|
8 mg |
16mg |
24mg |
24mg C.R |
48 mg C.R |
|
OD |
-- |
-- |
-- |
11 |
1 |
|
BID |
5 |
26 |
23 |
-- |
-- |
|
TID |
67 |
15 |
13 |
-- |
-- |
Table 100: Chart showing the type of Betahistine dose prescribed for treating Vertigo
Total Respondents = 165
Interpretation:
• 40.61% of the Sampled doctors prefer to give 8mg TID dose of Betahistine to patients to treat vertigo.
• 7.88% of the Sampled doctors prescribe 24mg BID dosage, while 15.76% of them prefer 16mg BID o& 13.94% doctors prefer 24 mg TID dose, for pharmacological therapy with Betahistine for Vertigo.
• Only 6.67% of Sampled doctors prescribes 24mg C.R once a day formulation (Betahistine O.D) for treatment in of Vertigo.
• Also, 0.61% Sampled doctors prescribe 16mg TID of Betahistine to their patients.
• Betahistine is the most preferred drug of choice for treating vertigo amongst 86.96% Neurologists, 83.58% Consulting Physicians, 100% Cardiologists, 61.11% E.N.T Doctors & 90.9% Diabetologists.
• 42.5% of the Neurologists, 33.33% of the Consulting Physicians, 55 % of the Cardiologists, 48.57% of the E.N.T Doctors & 30% of the Diabetologists prefer to give 8mg TID dose of Betahistine to patients to treat vertigo.
Duration:
Total Respondents = 165
|
Duration |
Responses |
% of responses |
|
|
1-2 weeks |
118 |
71.52 |
|
|
3-4 weeks |
43 |
26.06 |
|
|
4-8 weeks |
4 |
2.42 |
Interpretation:
· 1-2 weeks is the most preferred duration of therapy with Betahistine amongst 71.52% Sampled doctors.
· 26.06% Sampled doctors opted for 3-4 weeks of therapy with Betahistine, while 2.42% preferred 4-8 weeks.
· 1-2 weeks is the most preferred duration of therapy with Betahistine amongst 75% Neurologists, 73.33% Consulting Physicians, 65% Cardiologists, 62.86% E.N.T Doctors & 90% Diabetologists.
10. Do you titrate dosage?
Total Respondents = 165
|
Response to question |
Responses |
% of Responses |
|
Yes |
149 |
90.3 |
|
No |
16 |
9.7 |
Table 102: Responses for dosage titration in treating Vertigo
Interpretation:
· 90.3% of the Sampled doctors were found to titrate the dosage in Vertigo treatment.
· With the usual approach being tapering down the dosage in patients where symptoms are relieved and do not recur, and then putting it off. But in some recurrent cases of vertigo the dosage was increased.
· Where as 9.7% of the Sampled doctors did not titrate the dosage.
· 90% of the Neurologists, 95% of the Consulting Physicians, 80% of the Cardiologists, 88.57% of the E.N.T Doctors & 90% of the Diabetologists were found to titrate the dosage in Vertigo treatment.
11. Doctor, are you aware of once a day Betahistine preparation?
Total Respondents = 165
|
Response to question |
Responses |
% of Responses |
|
Yes |
157 |
95.15 |
|
No |
8 |
4.85 |
Table 103: Responses for Betahistine O.D awareness
Interpretation:
· Majority (95.15%) of the Sampled doctors were found to be aware of Betahistine once a day preparation. Only 4.85% Sampled doctors were still unaware of Betahistine O.D formulation.
· 95% of the Neurologists, 98.33% of the Consulting Physicians, 80% of the Cardiologists, 100% of E.N.T doctors & 90% of the Diabetologists were found to be aware of Betahistine once a day preparation.
12. If Yes, have you used?
Total Respondents = 165
|
Response to question |
Responses |
% of Responses |
|
Yes |
75 |
45.45 |
|
No |
90 |
54.55 |
Table 104: Responses for Betahistine O.D usage
Interpretation:
· 45.45% of the sampled doctors, were found to use Betahistine O.D formulation.
· 54.55% of the sampled doctors did not use Betahistine O.D formulation, they reportedly used the conventional doses.
· 55% of the sampled Neurologists, were found to use Betahistine O.D formulation
· 53.33% of the Consulting Physicians, 70% of the Cardiologists, 57.14% of the E.N.T Doctors & 60% of the Diabetologists did not use Betahistine O.D formulation, they reportedly used the conventional doses.
13. If answer to Q11 is Yes, what is preferred strength & duration?
Total Respondents = 75 (out of 165 Sampled doctors)
|
Strength |
Duration |
Responses |
% of Responses |
Overall % |
|
24 mg |
1 week |
29 |
54.71 |
70.67 |
|
2 weeks |
23 |
43.33 |
||
|
4 weeks |
1 |
1.89 |
||
|
|
||||
|
48 mg |
1 week |
8 |
36.36 |
29.33 |
|
2 weeks |
10 |
27.78 |
||
|
4 weeks |
4 |
11.11 |
||
Table 105: Responses for preferred strength of Betahistine O.D usage
Interpretation:
· Amongst all Sampled doctors those who use Betahistine once a day preparation, 70.67% mostly prescribe 24mg C.R, while 29.33% use 48mg C.R formulation.
· The duration for which 24mg C.R is prescribed the most is 1 week by 54.71% Sampled doctors, followed by 2 weeks amongst 43.33% Sampled doctors.
· 36.36% Sampled doctors use 48mg C.R for 2 weeks.
· 27.78% Sampled doctors use 48mg C.R for 4 weeks.
· 1.89% doctors prescribed 24mg C.R for 4 weeks and 11.11% prescribed 48mg C.R for 4 weeks
· Amongst the sample set, the O.D usage is as follows:
a. Neurologists those who use Betahistine once a day preparation, 77.27% mostly prescribe 24mg C.R, while 22.73% use 48mg C.R formulation
b. Consulting Physicians those who use Betahistine once a day preparation, mostly (60.71%) prescribe 24mg C.R, while 39.29% use 48mg C.R formulation.
c. Cardiologists those who use Betahistine once a day preparation, 66.67% mostly prescribe 24mg C.R, while 33.33% use 48mg C.R formulation.
d. E.N.T Doctors those who use Betahistine once a day preparation, 73.33% mostly prescribe 24mg C.R, while 26.67% use 48mg C.R formulation.
e. Diabetologists those who use Betahistine once a day preparation, 100% prescribe 24mg C.R, while none use 48mg C.R formulation.
· Amongst the sample set the duration of O.D usage is as follows:
a. The duration for which 24mg C.R is prescribed the most is 1 week by 50% Neurologists, followed by 2 weeks amongst 22.73% Neurologists.
b. 32.14% of Consulting Physicians prescribe 24mg C.R for 1 week, followed by 32.14% of Consulting Physicians for 2 weeks
c. The duration for which 24mg C.R is prescribed the most, is equally by 33.33% Cardiologists for 1 week and 2 weeks respectively.
d. The duration for which 24mg C.R is prescribed the most is 1 week by 53.33% E.N.T Doctors, followed by 2 weeks amongst 20% E.N.T Doctors
e. The duration for which 24mg C.R is prescribed the most is 2 weeks by 100% Diabetologists
14. If used, how do you use the OD formulation?
Total Respondents = 165
|
Response to question |
Responses |
% of Responses |
|
Start with OD |
100 |
60.61 |
|
Only Maintenance with OD |
65 |
39.39 |
Table 106: Responses for preferred use of once a day formulation
Interpretation:
· 60.61% of the sampled doctors prefer to use O.D formulation as a start up dose, while the rest (39.39%) preferred to use it as a maintenance dose only.
· 67.5% of the Neurologists, 53.33% of the Consulting Physicians, 60% of the Cardiologists, 62.86% E.N.T doctors & 70% of the Diabetologists prefer to use O.D formulation as a start up dose.
15. Is the usage of OD preparation indication specific?
Total Respondents = 165
|
Response to question |
Responses |
% of Responses |
|
Yes |
16 |
9.7 |
|
No |
149 |
90.3 |
Table 107: Responses for preferred use of once a day formulation
Interpretation:
· Majority (90.3%) of the Sampled doctors do not use O.D formulation particularly specific to any indication.
· Only 9.7% of Sampled doctors use it specific to any particular condition.
· 82.5% of the Neurologists, 96.67% of the Consulting Physicians, 95% of the Cardiologists, 85.71% of the E.N.T doctors & 90% of the Diabetologists do not use O.D formulation particularly specific to any indication
16. If yes, which is the indication and any specific reasons?
9.7% of the Sampled doctors use O.D formulation in Acute cases of vertigo. They also prefer to use it in patients with severe symptoms.
17. Do you also use two anti-vertigo molecules like morning/evening dose?
Total Respondents = 165
|
Response to question |
Responses |
% of Responses |
|
Yes |
26 |
15.76 |
|
No |
139 |
84.24 |
Table 108: Responses for usage of two Anti-vertigo molecules
Interpretation:
· 84.24% of the Sampled doctors do not use two Anti-Vertigo molecules together.
· Only 15.76% of the Sampled doctors use two Anti-vertigo molecules together as a morning-evening dose
· 90% of the Neurologists, 78.33% of the Consulting Physicians, 90% of the Cardiologists, 85.71% of the E.N.T Doctors & 80% of the Diabetologists do not use two Anti-Vertigo molecules together.
If Yes, your usual approach
Sometimes Betahistine is prescribed with Dimenhydrinate or Cinnarizine.
18. Do you think, combination of Betahistine with Dimenhydrinate is required? If yes, for which indication and condition is it useful?
Total Respondents = 165
|
Response |
Number of Responses |
% of response |
|
Yes |
74 |
44.85 |
|
No |
9 |
5.45 |
|
Maybe |
12 |
7.27 |
|
Not tried |
70 |
42.42 |
Table 109: Responses for usage of combination of Betahistine with Dimenhydrinate
Interpretation:
· 42.42% of the sampled doctors have not tried this combination, but 44.85% said it to be a potent and viable combination.
· They were further aided by 7.27% of doctors who felt it maybe a fruitful combination.
· 5.45% of sampled doctors feel that this would not serve to be a good combination.
· 37.5% Neurologists, 45% of Consulting Physicians, 45% Cardiologists, 54.29% E.N.T doctors and 40% Diabetologists said it to be a potent and viable combination
KEY FINDINGS
· The incidence of patients who seek anti-vertigo medications was found to be 10-15% of the total number of patients that a doctor sees in a week.
· The percentage of female patients who seek anti-vertigo medications were found to be the majority amongst doctors in the ratio of 40:60 (Male : Female)
· The incidence of the patients were found to be predominant in the 40-50 years category amongst the doctors.
· The study revealed that majority of patients reported to the doctors had secondary to some pre-existing ailment.
· Diabetes Mellitus, Hypertension, Cervical Spondylosis, Cardiac problems, & Thyroid were the prime secondary to some pre-existing ailment. Even cases of dehydration related vertigo during summer months were reported to doctors.
· BPPV was found out to be the most common cause. Following to it were Labyrinthitis and Vestibular Neuritis as most popular causes. Meniere’s disease and Migraine were the least of the causative factors. Vertebro-Basilar Ischemia and Syncope were also reported as the least effective cause along with Stroke and Cervical Spondylosis.
· Pharmacological and Manoeuvres is the most preferred line of treatment amongst ENT and Neurology doctors. The doctors of other speciality preferred Pharmacological treatment.
· Betahistine is the most preferred drug of choice amongst the doctors.
· Combination of Cinnarizine and Dimenhydrinate with Betahistine was also reported in few cases.
· The usual strength of Betahistine varies depends upon the severity condition. It was found to range in between 8mg-16mg. The average duration of drug administration was around 3-4 weeks, though in some cases 1-2 weeks was also reported.
· Majority of the doctors were found to prescribe 8mg Betahistine TID for 1-2 weeks or 16mg Betahistine BID for 1 week.
· Some doctors (majorly Neurologists) even prescribed the OD formulation on grounds of higher patient compliance. Though, a higher number of doctors reported that TID / BID resulted in higher degree of success in therapy.
· Inability to titrate the dosage and patient’s psychology in taking medicine were the distinct disadvantages of OD formulation.
· The majority of the doctors were found to titrate the dosage.
· Majority of the doctors taper the dose down to 8mg (BID) and then put it off. In certain severe cases it was found that the doctors even tapered up the dosage to 16mg BID.
· Majority of the doctors are aware of once a day Betahistine preparation, which is indicative of good promotion on behalf of the company.
· A good number of the doctors (Neurologists, Cardiologists, C.P) used Betahistine OD.
· Those doctors who are aware of Betahistine OD and use it, basically administer 24mg for 1-2 weeks. They even prescribe 48mg for 3-4 weeks. No doctor responded for 32mg OD preparation.
· Some doctors, complained of unavailability and sighted grounds of improper promotion. Rest of the doctors who do not use Betahistine OD said they were not used to it and never tried it.
· Majority of the doctors prefer to use Betahistine OD preparation as start up dose, while the rest use it to maintain the patients on it.
· The use of Betahistine OD preparation was not indication specific amongst the majority of the doctors. Though few used it for acute cases.
· Majority of the doctors do not use two anti-vertigo molecules.
· A fair share of the doctors felt that it is a potential and a viable combination. They also suggested it should be a good approach to treat Central and Peripheral vertigo. Some used Stemityl 5mg to overcome emetic problems
S.W.O.T Analysis for Betahistine O.D formulation
RECOMMENDATIONS
If the company was to modify its existing Betahistine OD and its position:
• Since OD preparation usage is not majorly indication specific, doctors could be made aware to use Betahistine OD as a start-up dose.
• Doctors who prescribe TID/BID doses should be made aware of OD formulation.
• Discussions regarding, that success rate do not vary with OD doses could be carried out.
• A possible combination with Dimenhydrinate could be formulated (as 45% of customers thought of it as a potent customer)
• Since majority of the patients were 40-50 years old, OD dose with low side effects (gastric problems) could be introduced or lower strength OD formulation could also be introduced.
• Diabetes is the most common secondary to pre-existing ailment along with hypertension and cardiac problems. Doctors of these specialities could be illustrated to co-prescribe Betahistine O.D formulation (with higher patient compliance) to prevent attacks of vertigo.
• Orthopaedic doctors could also be thought of as a potent customer as Spondylosis is a popular cause of Vertigo.
• Since incidence of female patients requiring anti-vertigo medications is higher than male patients, Gynaecological doctors & Endocrinologists could be added into the customer base.
• Majority of the doctors are aware of Betahistine OD preparation, but still they are hesitant to use it. It is due to their preference to the time tested conventional doses. Discussions pertaining to:
i) patient compliance,
ii) changing the customer’s perception: that there is no significant difference in OD and conventional doses (so that they can instil the confidence in the end consumers to change their psychology),
iii) freedom from dosage titration and
iv) elimination of problems pertaining to dosage accumulation could be carried out.
• Effective marketing through journals, internet etc could be mediated to focus on advantages (patient compliance) of OD preparation over conventional doses
• Betahistine (82.65 crores) contributes 27.73% of the entire Somatologicals market share (297.59 crores), widened window of growth.
• The market potential for Betahistine O.D formulation is good. The product is in the introductory phase. To achieve a faster growth a competitive pricing strategy could be framed.
REFERENCES
Books referred:
1. Essentials of Medical Pharmacology; K.D. Tripathi, 5th Edition, 2003, Jaypee Brothers Medical Publishers (P) Ltd.
2. Principles of Anatomy & Physiology; Gerard J. Tortora, Bryan Derrickson, 13th Edition, 2011, Volume – 1 & 2, John Wiley & Sons (Asia) Pte Ltd.
3. Goodman & Gilman’s The Pharmacological Basis of Therapeutics; Laurence L. Brunton, Bruce A. Chabner, Bjorn C. Knollmann, 12th Edition, 2011, McGraw Hill Medical Publishers
4. Harrison’s Principles of Internal Medicine; Anthony S. Fauci, Eugene Braunwald, Dennis L. Kasper, Stephen L. Hauser, 17th Edition, 2008 Volume 1, The McGraw Hill Companies Inc.
5. Drug Facts and Comparisons; Wolters Kluwer Health, 2008 Edition
6. Journals from W.H.O repository
Online / e- References:
1. Meniere’ Disease: Marc A Thorp, Department of Otolaryngology, Corner Brook, Newfoundland, Canada
2. Betahistine in the treatment of vertiginous syndromes: a meta-analysis C. DELLA PEPA, G. GUIDETTI1, M. EANDI Department of Anatomy, Pharmacology and Forensic Medicine, University of Turin; 1 Service of Audio-Vestibology and Vestibular Rehabilitation, A.S.L. (Local Health Service) of Modena, Modena, Italy
3. medibolism.com/medication/betahistine-hydrochloride/
4. High-dosage Betahistine dihydrochloride between 288 and 480 mg/day in patients with severe Menie`re’s disease: a case series Franziska Lezius • Christine Adrion • Ulrich Mansmann • Klaus Jahn • Michael Strupp
Received: 20 February 2011 / Accepted: 11 May 2011 / Published online: 29 May 2011_ Springer-Verlag 2011
5. Review Article: Inner Ear Disease and Benign Paroxysmal Positional Vertigo: A Critical Review of Incidence, Clinical Characteristics and Management
M. Riga,1 A. Bibas,2 J. Xenellis,2 and S. Korres2
1ENT Department, University Hospital of Alexandroupolis, Democritus University of Thrace, Dragana, 68100 Alexandroupolis, Greece
2ENT Department, Hippokrateion General Hospital of Athens and National University of Athens, 11527 Athens, Greece
6. ncbi.nlm.nih.govpmcarticlesPMC2640000tool=pubmed
7. ncbi.nlm.nih.govpmcarticlesPMC2655085
8. ncbi.nlm.nih.govpmcarticlesPMC2866460tool=pmcentrez
9. ncbi.nlm.nih.govpmcarticlesPMC3201005tool=pmcentrez
10. Medical Management of Vestibular Disorders and Vestibular Rehabilitation by Kevin Katzenmeyer & Jeffrey Vrabec
11. betterhealth.vic.gov.au\emergencydepartmentfactsheet\vertigo
12. Vertigo Can Be Treated Easily And Quickly, Science Daily (May 26, 2008)
13. Medical treatment for vertigo, C. Desloovere, Department of Ear, Nose and Throat disease, University Hospital, Catholic University of Leuven, Belgium
FIND IMAGES HERE
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









