 About Authors:
About Authors:
SHAMBHAWI*, M SHIVASHANKAR
School of Advanced Sciences, Pharmaceutical chemistry division,
VIT University, VELLORE
India
ABSTRACT:
Chitosan has been the subject of interest for its use as a polymeric drug carrier material in dosage form design due to its appealing properties such as biocompatibility, biodegradability, low toxicity and relatively low production cost from abundant natural sources. Hydrogels are a unique class of macromolecular networks that can hold a large fraction of an aqueous solvent within their structure. The objective of this paper is to give a brief review on the fundamentals and recent advances in chitosan based hydrogel for e.g., thermosensitive hydrogel variants which precludes the need of surgical implantation as well as the description of the release mechanism of bioactive molecules from these hydrogels which traps a drug and then releases the active compound by "Swelling" or expanding inside of specific tissues, thus allowing a higher concentration of the drug in a biodegradable format. This article presents an overview of the newest developments and applications of Chitosan based hydrogel for controlled drug delivery system.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1417
Introduction
Controlled delivery systems provide an alternative approach to regulate the bioavailability of therapeutic agents. Controlled drug delivery system is designed in such a way that when an active medicament is encapsulated in the polymer structure then the release of drug will be in a predetermined way(31,32). Hydrogels have gained considerable interest among various other polymeric system which is used for encapsulation and controlled release of drug(33-41). Hydrogels are cross-linked, three-dimensional hydrophilic networks that swell but not dissolve when brought into contact with water. Hydrogels can be formulated in a variety of physical forms, including slabs, microparticles, nanoparticles, coatings, and films(2). Hydrogels are generally considered biocompatible due to their structural similarity to the macromolecular-based components in the body and minimally invasive administration to the body. Hydrogels are usually formed by a hydrophilic polymer matrix crosslinked chemically through covalent bonds or physically through hydrogen bonds, crystallized domains or hydrophobic interactions. By changing the composition and concentration of the macromers, the degradation rate and permeability of the hydrogel can be altered(3). Chitosan is a polyelectrolyte and is obtained from renewable resources(4). It is a linear, semirigid polysaccharide and is biodegradable, biocompatible and of relatively low toxicity. It is a copolymer of N-acetyl D-glucosamine and D-glucosamine (42).Chitosan forms hydrogels by physical crosslinking or chemical cross-linking with glutaraldehyde . Azide derivatized chitosan was reported to form gels by UV irradiation. A chitosan-based thermosensitive gel was prepared by grafting PEG to the chitosan backbone(3). Crosslinking by high temperature and crosslinking by high ph is also used to prepare chitosan based hydrogel(5).Chitosan based hydrogel are commonly used in clinical practice and experimental medicine for a wide range of applications, including biosensors, tissue engineering and regenerative medicine, separation of biomolecules or cells and barrier materials to regulate biological adhesions (35,43,44).
Drug release by hydrogel
Hydrogels have a thermodynamic compatibility with water, which makes them to swell in aqueous media which is used to regulate drug release in reservoir-based, controlled release systems or as carriers in swellable and swelling-controlled release devices. Drug is also released from hydrogel in response to pH, temperature, ionic strength, electric field, or specific analyte concentration differences. In these systems, release can be designed to occur within specific areas of the body (e.g., within a certain pH of the digestive tract) or also via specific sites (adhesive or cell-receptor specific gels via tethered chains from the hydrogel surface) (6)
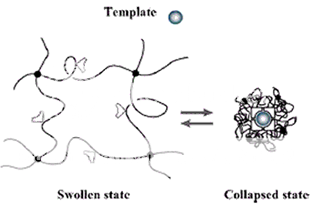
Figure 1: The volume phase transition of the hydrogel -induced by external stimuli (e.g., a change in pH, temperature or electrical field) modifies the relative distance of the functional groups inside the imprinted cavities. This alters their affinity for the template.
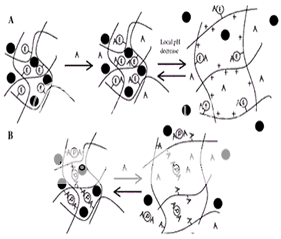
Figure 2: (A) Induced Swelling - As analyte (A) binds, the enzymatic reaction (E denotes covalently attached enzyme) produces a local pH decrease. For the cationic hydrogel, which is weakly basic, the result is ionization, swelling, and release of drug, peptide, or protein (filled circle). When A decreases in the bulk concentration, the gel shrinks. (B) Loss of Effective Cross-links - Analyte competes for binding positions with the protein (P). As free analyte binds to the protein, effective cross-links are reversibly lost and release occurs(6) .
Drug delivery systems
The global market for advanced drug delivery systems was more than €37.9 billion in 2000 and is estimated to grow and reach €75B by 2005 (i.e., controlled release €19.8B, needle-less injection €0.8B, injectable/implantable polymer systems €5.4B, transdermal €9.6B, transnasal €12.0B, pulmonary €17.0B, transmucosal €4.9B, rectal €0.9B, liposomal drug delivery €2.5B, cell/gene therapy €3.8B, miscellaneous €1.9B). Developments within this market are continuing very fast, especially in the area of alternatives to injected macromolecules, as drug formulations seek to cash in on the €6.2B worldwide market for genetically engineered protein and peptide drugs and other biological therapeutics(6). The global market for advanced drug delivery systems amounted to $134.3 billion in 2008, and was projected to increase to $139 billion in 2009. The estimate for 2014 is $196.4 billion, for a compound annual growth rate (CAGR) of 7.2% in the 5-year period. The largest segment of the market is targeted drug delivery, which reached $50.9 billion in 2009 and is expected to increase to $80.2 billion in 2014, for a CAGR of 9.5%. Sustained-release products have the second-largest market share, with estimated sales of $36.1 billion in 2009 and $45.8 billion in 2014, for a CAGR of 4.9%(29)
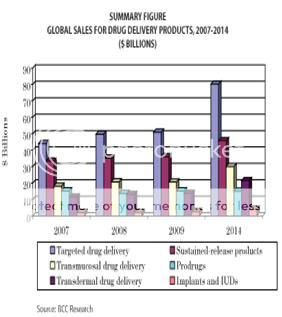
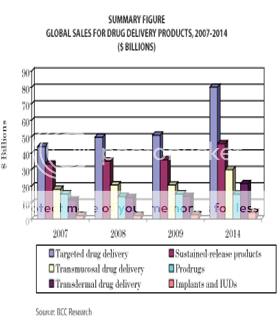
Figure 3: Global sales for drug delivery products
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Drug Delivery Carriers
The main goal while designing a system is to obtain systems with optimized drug loading and release properties, long shelf life and low toxicity, enhanced drug targeting specificity, improved treatment absorption rate and providing protection for pharmaceuticals against biochemical degradation(45,46).During the past decade, novel polymeric microspheres, polymer micelles, and hydrogel type materials have been shown to be effective. Polymer drug delivery nano scale systems are based on ‘nano carriers’ which are composed of mixing polymeric chemical compounds with drugs to form complex, large molecules which carry the drug across physiological barriers(8).Micelles are formed by self assembly of amphiphilic block copolymers(5-50nm) in aqueous solutions. The drugs can be physically entrapped in the core of block copolymer micelles and transported at concentrations that can exceed their intrinsic water solubility. Liposomes are a form of vesicles that consist either of many, few, or just one phospholipid bilayer(6). Niosomes are non ionic surfactant liposome. Dendrimers are nanometer sized, highly branched and monodisperse macromolecules with symmetrical architecture. Nanoparticles are either vesicular or matrix system in which drug is either confined in a cavity or dispersed in the matrix. In recent years biodegradable nanoparticles have attracted considerable attention as potential drug delivery devices in view of their application in the controlled release of drugs in targeting particular organs and tissues, as carriers of DNA in gene therapy, and in their ability to deliver proteins, and genes through the peroral route. Hydrogels as drug delivery carrier can be very promising materials if combined with the technique of molecular imprinting. Liquid crystals can be made to form different geometries, with alternative polar and non polar layers (i.e., a lamellar phase) where aqueous drug solutions can be included(6)
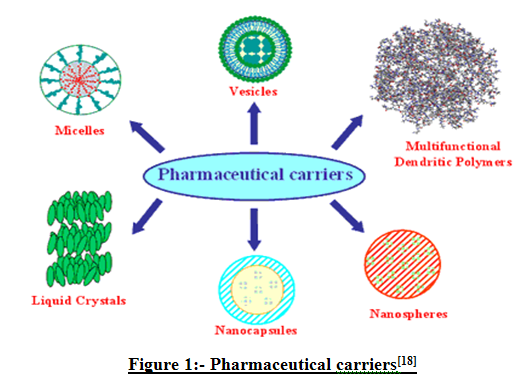
Figure 4: Pharmaceutical carriers
Recent advances in chitosan based hydrogel drug delivery system
Chitosan and chitosan-based hydrogels have two advantageous characteristics that enhance DDSs: pH sensitivity and mucoadhesive properties which has find various applications in drug administration through various routes like transdermal, oral, subcutaneous(7), nasal, buccal and vaginal(9).Here we are going to discuss various recent work done in chitosan based hydrogel drug delivery system.
Protein delivery
A recent work was done for the development of a new injectable drug delivery system for proteins sustained release. A series of in situ forming hydrogels derived from oxidized carboxy methyl cellulose (OCMC) and N-succinyl-chitosan (NSC) were prepared by Schiff base reaction. The in vitro cytotoxicity studies showed that the OCMC/NSC hydrogels were non-cytotoxic and preserved the viability of the entrapped cells. This newly described OCMC/NSC hydrogels demonstrated attractive properties and would be a suitable injectable and biodegradable system for the delivery of protein drugs(10).
Wound delivery
Chitosan based hydrogel as a potential wound dressing has been reported recently. There is a demand for a wound dressing which can be removed from the application site easily without causing any pain or discomfort. A new formulation consisting of thiolated chitosan with poly(N-isopropyl acrylamide) loaded with ciprofloxacin was reported. The thermoresponsive material was cytocompatible and was found to modulate the release of the incorporated ciprofloxacin in a sustained fashion reflecting its suitability to protect a wound for a prolonged period. The combination of thiolated chitosan with poly(N-isopropyl acrylamide) and ciprofloxacin showed antibacterial properties to the virulent bacteria E coli supporting its potential as a wound dressing(11).A composite hydrogel sheetcomposed of alginate, chitin/chitosan and fucoidan (ACF-HS) wasproduced from blended powder. ACF-HS possesses many advantagesas a wound dressing for the repair of healing-impairedwounds, such as exudate absorption, immobilization and activationof growth factors in exudates, non-cytotoxicity, and simple applicationand removal from the wound(18) .
Tissue engineering
Thermosensitive polymer hydrogels are of greatinterest in therapeutic delivery and tissue engineering as injectable depot systems. It was prepared by grafting an appropriate amount of PEG onto the chitosan backbone and studied for drug release in vitro usingbovine serum albumin (BSA) as a model protein which allowed the safeincorporation of bioactive molecules for a broad range of medical applications, particularly for sustained in vivo drug releaseand tissue engineering(12).Injectable scaffolds are promising substrates for tissue engineering because of the in vivo culture environment, minimal invasion and low cost, and so on (47, 48). Various materials including microspheres and hydrogels have been employed as injectable scaffolds.The chitosan hydrogel formed at a lower initiator concentration,e.g., 5 mM APS/TMEDA, degrades rapidlyeither in lysozyme solution or in the case of chondrocyteencapsulation, but does not degrade in PBS solution duringan 18 d-incubation. In vitro chondrocyte encapsulation demonstratesthat the cells can survive in the chitosan hydrogeland possess normal morphology through a 12 d-cultureperiod, although the overall cell number is decreased asdetected by DNA assay(21) . A novel porous PVA-chitosan based hydrogel has been developed which has the desired structure and pore size and enhances chondrogenesis of implanted cells. PVA-chitosan based hydrogel scaffold shows great potential as a cell carrier for cartilage tissue engineering(27).Chitosan/carboxymethyl chitosan hydrogel exhibits better overall mechanical properties and electrical sensitivity, suggesting its great potential for microsensor and actuator applications, especially in the biomedical field(28) .
Skin delivery
Novel chitosan based polyelectrolyte complexes (PEC) were developed to be applied on skin and this has shown very good properties for application in the skin andrepresents a very promising formulation for further incorporation of drugs for topical and transdermaladministration. The development was based on the combination of chitosan andtwo polyacrylic acid (PAA) polymers with different crosslinkers and crosslinking densities. The interactionbetween the polymers was maximized controlling the pH, and by forming the films at a pH value closeto the pKa of the respective components as identified by potentiometric and turbidimetric titration(13) .
Vaccine delivery
Silica nanoparticles (SNP) containing the model antigen ovalbumin (OVA) were incorporated into a thermosensitive chitosan hydrogel, and the resulting formulation investigated for its potential to act as a particulate sustained release vaccine delivery system. Approximately 16% of total protein was released in a particulate form over a 14-day period, while approximately 35% was released as soluble antigen. Chitosan gels containing OVA-loaded SNP and the adjuvant QA showed a significantly greater ability to induce CD4+ T cell proliferation than chitosan gel containing soluble OVA and QA, indicating the future promise for such a system(14) .
Colon delivery
Targeting of drugs specifically to the colon is advantageous in the treatment of diseases such as amoebiasis, Crohn’s disease, ulcerative colitis, and colorectal cancer. In addition, it has shown great potential in the oral delivery of therapeutic peptides and proteins, which are unstable in the upper part of the gastrointestinal (GI) tract. chitosan (CS)–dextran sulfate (DS) nanoparticles coated iron oxide as drug carriers was detected using magnetic resonance imaging (MRI) technique. The 5- aminosalicylic acid (5-ASA) was chosen as model drug molecule. CS–DS hydrogels were formulated by a complex coacervation process under mild conditions. The CS–DS hydrogel developed based on the modulation of ratio show promise as a system for controlled delivery of drug detectable using magnetic resonance imaging technique (MRI)(15) .
Interpenetrating network
A Capecitabine-loaded semi-interpenetrating network hydrogel microsphere of chitosan poly(ethylene oxideg-acrylamide) was synthesized by emulsion crosslinking using glutaraldehyde. Poly(ethylene oxide)was grafted with polyacrylamide by free radical polymerization using ceric ammonium nitrate as a redox initiator. A development of novel pH-sensitive interpenetrating polymeric network (IPN) microgels (MGs) based on chitosan, acrylamide-grafted-poly(vinyl alcohol) and hydrolyzed acrylamide-grafted-poly(vinyl alcohol) that are crosslinked with glutaraldehyde and used in the controlled release (CR) of cefadroxil an antibiotic drug was reported IPN matrices of this study were able to extend the release rates from conventional dosage release times to more than 10 h(22) .
Gastroretentive delivery
A new extended release gastroretentive multiparticulate deliverysystem was designed by incorporation of the hydrogel beads made of chitosan. As the first part of a continued research on conversion of N-sulfonato-N,O carboxy methyl chitosan(NOCCS) to useful biopolymer-based materials, large numbers of carboxylic functional groups were introduced onto NOCCS by grafting with polymethacrylic acid (PMAA). The equilibrium swelling studies were carried out in enzyme-free simulated gastric and intestinal fluids (SGF and SIF, respectively). Also, the satranidazole as a model drug was entrapped in nano-gels and in vitro release profiles were established separately in both enzyme-free SGF and SIF. The drug release was found to be faster in SIF. The drug release profiles indicate that the drug release depends on their degree of swelling and cross-linking(17) .
Nasal delivery
The role ofchitosan microspheres as a nasal drug delivery system has beenwidely studied in a variety of drugs, such as proteins, peptides, andvaccines (Alpar et al., 2005; Gavini et al., 2006; Illum et al., 2002; Jainet al., 2004; Kang et al., 2006; Sankar et al., 2001; van der Lubben et al., 2003; Varshosaz et al., 2004). Chitosan microspheres asnasal delivery vehicles of vaccines in vivo. Diverse chitosan microspheres wereprepared through different methods and were evaluatedas vaccine delivery vehicles for controlled drug release and theenhanced protection and permeation of the antigens in the nasalmucosa. Thechemically modified chitosan microspheres or chitosan microspherescombined with other adjuvants were shown to have an increased immunostimulatory or adjuvant effect in nasal vaccine delivery(19) .
Colon-specific drug delivery
Chitosan has been widely utilized as drug delivery systems for low molecular drugs, peptides and genes (49,50,51). Chitosan-based delivery systems have been widely studied for colonic drug targeting since this system can protect therapeutic agents from the hostile conditions of the upper gastrointestinal tract and release the entrapped agents specifically at the colon through degradation of the glycosidic linkages of chitosan by colonic microflora (52,53).Oral delivery of the absorption enhancer along with poorly absorbable drugs using chitosan capsules could improve the absorption characteristics of the drugs (54).Varshosaz et al. reported chitosan microspheres coated with cellulose acetate butyrate, prepared by the emulsion-solvent evaporation technique, for delivery of 5-ASA into the colon (55).The authors found that decreasing the coat content and increasing the molecular weight of chitosan increased its bioadhesion significantly. Chitosan-Ca-alginate microparticles have also been used for colonspecific delivery of 5-ASA (56) .Recently, Jain et al. prepared hydrogel microspheres of chitosan grafted with vinyl polymers for the controlled and targeted delivery of 5-ASA to the colon, which exhibited better therapeutic effects(57).Chitosan can be insoluble at acidic fluids through chemical cross-linking of the microsphere with aldehydes, it is not effective in preventing the release of the encapsulated drugs. To alleviate this problem, Alonso et al. developed microencapsulated chitosan microspheres coated with enteric coating materials (58).The potential of this microsphere was evaluated using sodium diclofenac (SD), an anti-inflammatory drug. SD was entrapped into the chitosan cores by the spray drying method, Onishi et al. prepared Eudragit®-coated microspheres composed of chitosan succinylprednisolone conjugates (Ch–SP) using the sonication method (59).Most of the loaded drugs were released in the colon, an environment rich in bacterial enzymes that degrade the chitosan. nanoparticular systems for colon-specific delivery of metronidazole were reported by Elzatahry and Eldin (60).Hyaluronic acid-coupled chitosan nanoparticles bearing 5-fluorouracil (5-FU) were also prepared by an ionotropic gelation method for the effective delivery of the drug to the colon tumors (61).These nanoparticles showed enhanced cellular uptake by HT-29 colon cancer cells compared to the uncoupled nanoparticles(20) .
Liver targeted drug delivery
Liver-targeting systems employ passive trapping of microparticles by reticuloendothelium or active targeting based on recognition between hepatic receptor and ligand-bearing particulates (62).Recently, Liu et al. prepared polyion complex micelles (PIC micelles) based on methoxy poly (ethylene glycol) (PEG)-graftchitosan and lactose-conjugated PEG-graft-chitosan for liver-targeted delivery of diammonium glycyrrhizinate (DG) (63).DG has been used in the treatment of chronic hepatitis and immunodeficiency virus infection. Pharmacokinetic experiments carried out using rats showed that the area under the curve (AUC) values of DG for PIC micelles were higher than that for DG injection. The lactose-conjugated PIC (Lac- PIC) micelles delivered more DG to the liver than conventional PIC micelles, indicating that Lac-PIC micelles were promising liver targeted nanocarriers for DG. Ping et al. conjugated glycyrrhizin (GL) to the surface of chitosan nanoparticles (CS-NP)s, prepared by an ionic gelation process (64).These nanoparticles were developed for a drug delivery system targeting the liver through a specific interaction between GL and hepatocytes(20) .
Kidney and lung targeted delivery
For some drugs like non steroidal anti inflammatory drugs(NSAIDS) Kidney-targeted drug delivery is critical To reduce extra-renal toxicity of the drug and to improve its therapeutic efficiency for diseases occurring at the kidney(65).Several strategies have been proposed for drug targeting to the kidney in the form of drug-carrier conjugates(66-68).Recently, Zhang et al. reported that.LMWC with a proper molecular weight could be applied as a promising carrier for renal targeting. Lung cancer is one of the most prevalent cancers and is the leading cause of cancer mortality in the developed world (69).Shim et al. prepared chitosan-modified poly(lactic coglycolic acid) nanoparticles containing paclitaxel (C-NPs-paclitaxel) with a mean diameter of 200–300 nm by a solvent evaporation method (70).The study demonstrated that the in vitro uptake of the nanoparticles by a lung cancer cell line (A549) was significantly increased by chitosan modification(20) .
Cancer targeted drug delivery
Designing a distinct carrier system that encapsulates a large quantity of drugs and specifically targets tumor cells is indispensable for successful cancer therapy. Passive targeting by using chitosan drug conjugates, cross linked chitosan nanoparticles, chitosan based polyelectolye complex, self assembled chitosan nanoparticles and PEGylated chitosan nanoparticles have shown to have enhanced permeability and retention (EPR) effect(20) . Active targeting can be achieved by chemical modification of nanosized drug carriers with targeting components that precisely recognize and specifically interact with receptors on the targeted tissue (71,72).Researchers developed an efficient drug delivery system comprised of (a) active chemotherapeutic drug, (b) targeting moiety, and (c) a nano-sized carrier made up of polymers or lipids. In this system, the therapeutic agents are physically entrapped in the carrier. Physical targeting using Chitosan-based stimuli-sensitive formulations and chitosan based magnetic nanoparticles are also developed(20). Capecitabine, an anticancer drug, was successfully loaded into microspheres by changing experimental variables such as grafting ratio of the graft copolymer, ratio of the graft copolymer to chitosan, amount of crosslinking agent and percentage of drug loading in order to optimize process variables on drug encapsulation efficiency, release rates, size and morphology of the microspheres. Capecitabine was successfully encapsulated into semi-IPN microspheres and percentage of encapsulation efficiency varied from 79 to 87. In vitro release studies were performed in simulated gastric fluid (pH 1.2) for the initial 2 h, followed by simulated intestinal fluid (pH 7.4) until complete dissolution. The release of capecitabine was continued up to 10 h(16) . A recent work has performed in which o-carboxymethyl chitosan nanocarrier was found effective for the delivery of curcumin to cancer cells. Cytotoxicity studies by MTT assay indicated that curcumin-O-CMC Nps were toxic to cancer and non-toxic to normal cells(23) .
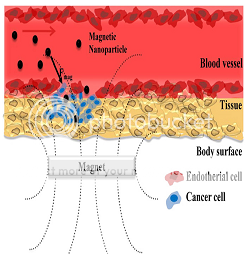
Figure 5: Schematic representation of magnetic nanoparticle-based drug delivery system.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Oral delivery
Preparation of CTS hydrogels and sponges carrying diclofenac (DCF), an anti-inflammatorydrug is reported where chitosan was used as an excipient in oral formulations and as a drug delivery vehicle for burnt painful injuries(24).A novel lyophilized chitosan hydrogel complex for the controlled release of a highly water soluble drug, niacinamide was developed where CS-M, demonstrated enhanced characteristics indicating its potential to be used as a controlled release excipient in oral drug formulations(25).Hydrogel Based Oral Controlled Drug DeliverySystem for Antihypertensive Drug was developed where it has been shown that the action was sustained for 8 hrs which leadsto achieve an effective therapy with low dosage of the drug, to reduce the frequency of medication(26) .
Drug candidate for drug delivery
Biodegradable nanoparticles are frequently used to improve the therapeutic value of various water soluble/insoluble medicinal drugs and bioactive molecules by improving bioavailability, solubility and retention time (71). These nanoparticle–drug formulation reduces the patient expenses, and risks of toxicity (72). Nanoencapsulation of medicinal drugs (nanomedicines) increases drug efficacy, specificity, tolerability and therapeutic index of corresponding drugs (73-78). Several disease related drugs/bioactive molecules are successfully encapsulated to improve bioavailability, bioactivity and control delivery (79-81). Nanomedicines of the dreadful diseases like cancer (82), AIDS (83), diabetes (84), malaria (85) , prion disease (86)and tuberculosis (87)are in different trial phase for the testing and some of them are commercialized(88-89). Nanomedicine formulation depends on the choice of suitable polymeric system having maximum encapsulation (higher encapsulation efficiency), improvement of bioavailability and retention time. The desired nanomedicines are generally achieved by hit and trial method (no specific rule) however, the encapsulation process with polymeric nanoparticles are in more advance condition in comparison to other nanoparticle systems. Azab et al. developed a chitosan-based hydrogel cross-linked with glutaraldehyde and loaded with 131Inorcholesterol (131I-NC), and tested the hydrogel in a breast cancer xenograft mouse model. This hydrogel showed a reduction in the progression rate of the tumor, and prevented 69% of tumor recurrence and metastatic spread(1). Patel et al.demonstrated the selective release mechanism in vivo using a chitosan-PEO semi-IPN hydrogel. This work showed the localized delivery of the amoxicillin and metronidazole antibiotics to the stomach. chitosan-based bioadhesive PEC hydrogels loaded with 5 fluorouracil (effective against colon carcinomas) and insulin (effective against diabetes mellitus) showed selective release in the intestine. Chitosan-based hydrogel DDSs loaded with acetaminophen, mesalazine (5-ASA), sodium diclofenac, and insulin showed satisfactory uptake within the colon. The chitosan polymer itself was found to be degraded by the microflora of the colon, offering a degradation mechanism that leads to controlled drug release. Cao et al. developed a chitosan-based thermosensitive in situ hydrogel for ocular drug delivery and tested it in rabbits. Using the microdialysis method of analysis, the C(max) of timolol maleate released from the hydrogel was 11.2μg/ml, two-fold higher than that of the conventional eye drop. Furthermore, the hydrogel had a greater capacity to reduce the intra-ocular pressure (IOP). Glimepride, a third generation oral antidiabetic sulfonylurea drug has shown potential for effective delivery by chitosan hydrogel release. In vivo application in mice showed consistent therapeutic efficacy over 48h, suggesting possible effectiveness in the clinic. Recently, Park et al. developed a chitosan hydrogel scaffold impregnated with bFGF-loaded microspheres that can accelerate wound closure in the treatment of chronic ulcers(1).Chitosan-based superporous hydrogels of rosiglitazone can be used as a gastroretentive drug delivery system in view of their swelling characteristics in acidic pH(4).Forregional delivery, paclitaxel an anticancer drug has been formulated in biodegradablepolymeric microspheres, hydrogels, surgicalpastes, and implants. Camptothecin is an inhibitor of the DNA replicating enzymetopoisomerase I, leading to the production of a double-strandDNA break during replication and resulting in cell death if thebreak is not repaired. Camptothecin has been formulated in biodegradablepolymeric implant devices, microspheres and hydrogels. Camptothecin was loaded into a controlled-release polymer (ethylene-vinyl acetate co-polymer; EVAc) for brain tumor treatment. It was shown that local controlled delivery by this polymer system significantly extended survival(5) .
Future opportunities and challenges
Nanoparticles and nanoformulations have already been applied as drug delivery systems with great success; and nanoparticulate drug delivery systems have still greater potential for many applications, including anti-tumor therapy, gene therapy, and AIDS therapy, radiotherapy, in the delivery of proteins, antibiotics, virostatics, vaccines and as vesicles to pass the blood - brain barrier. Nanoparticles provide massive advantages regarding drug targeting, delivery and release and, with their additional potential to combine diagnosis and therapy, emerge as one of the major tools in nanomedicine. The main goals are to improve their stability in the biological environment, to mediate the bio distribution of active compounds, improve drug loading, targeting, transport, release, and interaction with biological barriers. The cytotoxicity of nanoparticles or their degradation products remains a major problem, and improvements in biocompatibility obviously are a major concern of future research(6) .
Conclusion
Targeted delivery of drugs is critical in improving therapeutic efficacy and minimizing side effects. Many approaches are currently available to deliver the drugs to the specific site of action. For the development of targeted delivery systems, chitosan and its derivatives possess various advantages such as biocompatibility, biodegradability, mucoadhesivity, and other unique biological properties(20).Recently, environmental stimulation has become an intense area of research forthe development of unique materials that can(1) form safely within thebody negating the need for surgery,(2) trigger drug release at local sitespreventing systemic toxicity, and (3) degrade in a controlled manner foreffective, long-term drug release. Indeed, chitosan hasreceived significant attention in the development of injectable, in situgelling systems for tumor treatment and tissue regeneration purposesand as a delivery vehicle in oral and ophthalmic delivery systems.We hope that these current advancements will yield next generation delivery systems as we gain a further understanding of thedynamics of mixed chitosan chain networks. With an understanding ofthe fundamental loading and release criteria of varying therapeutics ,wewill be able to adapt delivery systems for different drug formulations, release conditions, and treatment intervals. Once these design parameters have been established, cheap, non-toxic, and efficient chitosanhydrogel drug delivery systems can move closer to clinical availability(7). Overall, it is evident that chitosan and its derivatives are useful carriers for low molecular drugs requiring targeted delivery.
References
1. Chitosan-based hydrogels for controlled, localized drug delivery.Narayan Bhattarai, Jonathan Gunn, Miqin Zhang. Advanced Drug Delivery Reviews 62 (2010) 83–99
2. Hydrogels in Controlled Drug Delivery Systems.Fariba Ganji and Ebrahim Vasheghani-Farahani.Iranian Polymer Journal.18 (1), 2009, 63-88
3. Protein Delivery Using Chitosan Derivatives.K. Kumari, P. P. Kundu, and C.S. Cho1, J. Chitin Chitosan 13(4), 181-188 (2008)181
4. Development of a Gastroretentive Drug Delivery. System based on Superporous Hydrogel. N Vishal Gupta and HG Shivakumar. Tropical Journal of Pharmaceutical Research June 2010; 9 (3): 257-264
5. Injectable chitosan hydrogels for localised cancer therapy. Hang Thu Ta a, Crispin R. Dass b, Dave E. Dunstan a. Journal of Controlled Release 126 (2008) 205–216
6. Recent advances in novel drug delivery systems. costas kaparissides, Sofia alexandridou, Katerina Kotti and Chaitidou. Journal of nanotechnology online. DOI: 10.2240/azojono0111.
7. Chitosan-based hydrogels for controlled, localized drug delivery.Narayan Bhattarai, Jonathan Gunn, Miqin Zhang. Advanced Drug Delivery Reviews 62 (2010) 83–99
8. Hydrogels as Potential Nano-Scale. Drug Delivery Systems. Mohammad Reza Saboktakin, Ph. D of Nano Chemistry
9. Chitosan based hydrogels for the drug delivery system. Murugesh shivashankar, Badal kumar mandal, Roshini yerappagari,Venkatesan Praveen kumar. Murugesh shivashankar et al IRJP 2(2) 2011 1-6.
10. An injectable oxidized carboxymethylcellulose/N-succinyl-chitosan hydrogel system for protein delivery.Shaoyu Lü, Mingzhu Liu∗, Boli Ni. Chemical Engineering Journal 160 (2010) 779–787
11. Drug loaded thermoresponsive and cytocompatible chitosan based hydrogel as a potential wound dressing.C. Radhakumarya, Molly Antontyb, K. Sreenivasana,. Carbohydrate Polymers 83 (2011) 705 713
12. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release Narayan Bhattaraia, Hassna R. Ramaya, Jonathan Gunna, Frederick A. Matsenb, Miqin Zhanga,b,T. Journal of Controlled Release 103 (2005) 609–624
13.Films based on chitosan polyelectrolyte cpmplexes for skin drug delivery: Development and characterization. Journal of membrane science 320(2008)268-279.
14. In vitro and in vivo investigation of thermosensitive chitosan hydrogels containing silica nanoparticles for vaccine delivery. Sarah Gordona, Elena Teichmanna, Katherine Younga, Kim Finnieb, Thomas Radesa, Sarah Hooka. European Journal of Pharmaceutical Sciences 41 (2010) 360–368
15. Synthesis and characterization of superparamagnetic chitosan–dextran sulfate hydrogels as nano carriers for colon-specific drug delivery. Mohammad Reza Saboktakina,b, Roya Tabatabaiea, Abel Maharramovb, Mohammad Ali Ramazanovb. Carbohydrate Polymers 81 (2010) 372–376.
16. Novel interpenetrating network chitosan-poly(ethylene oxide-g-acrylamide) hydrogel microspheres for the controlled release of capecitabine. Sunil A. Agnihotri, Tejraj M. Aminabhavi. International Journal of Pharmaceutics 324 (2006) 103–115
17. Synthesis and characterization of biodegradable chitosan beads as nano-carriers for local delivery of satranidazole. Mohammad Reza Saboktakin, Roya M. Tabatabaiea, Abel Maharramovb, Mohammad Ali Ramazanovb. Carbohydrate Polymers 81 (2010) 726–731
18. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings Kaoru Murakami a, Hiroshi Aoki b, Shingo Nakamura c, Shin-ichiro Nakamura d, Megumi Takikawa d, Motoaki Hanzawa a, Satoko Kishimoto e, Hidemi Hattori e, Yoshihiro Tanaka e, Tomoharu Kiyosawa d, Yasunori Sato a, Masayuki Ishihara e. Biomaterials 31 (2010) 83–90
19. Application of chitosan microspheres for nasal delivery of vaccines. Mi Lan Kang a, Chong Su Cho b, Han Sang Yoo a,. Biotechnology Advances 27 (2009) 857–865
20. Targeted delivery of low molecular drugs using chitosan and its derivatives. Jae Hyung Park a,c, Gurusamy Saravanakumar a, Kwangmeyung Kim b,Ick Chan Kwon. Advanced Drug Delivery Reviews 62 (2010) 28–41
21. Covalently crosslinked chitosan hydrogel: Properties of in vitro degradation and chondrocyte encapsulation. Yi Hong a, Haiqing Song b, Yihong Gong a, Zhengwei Mao a, Changyou Gao a, Jiacong Shen. Acta Biomaterialia 3 (2007) 23–31
22. Novel chitosan-based pH-sensitive interpenetrating network microgels for the controlled release of cefadroxil .K.S.V. Krishna Rao a, B. Vijaya Kumar Naidu b, M.C.S. Subha a, M. Sairam b,T.M. Aminabhavi b. Carbohydrate Polymers 66 (2006) 333–344
23. Efficient water soluble O-carboxymethyl chitosan nanocarrier for the delivery of curcumin to cancer cells. A. Anithaa, S. Mayaa, N. Deepaa, K.P. Chennazhia, S.V. Naira, H. Tamurab, R. Jayakumara. Carbohydrate Polymers 83 (2011) 452–461
24. Chitosan-based delivery systems for diclofenac delivery: preparation and characterization. Simina Dreve, Irina Kacso, Ioan Bratu and Emil Indrea. Journal of Physics: Conference Series 182 (2009) 012065
25. Characterization of a novel lyophilized chitosan hydrogel complex for the controlled release of a highly water soluble drug, niacinamide .Chan Ma1 and Sunil Prabhu2. International Journal of Drug Delivery 3 (2011) 55-63
26. Formulation and Evaluation of Hydrogel Based Oral Controlled Drug Delivery System for Antihypertensive Drug. Pavithra .T.K1, Harshitha .R1, Panneer .K1,Renuka .S2, Prakash Rao .B2, Narendra .C2. Pavithra et al / Journal of Pharmaceutical Science and Technology Vol. 2 (8), 2010,276-283
27. Porous PVA-Chitosan Based Hydrogel as an Extracellular Matrix Scaffold for Cartilage Regeneration. AA. Abbas1, SY Lee1, L. Selvaratnam2, N. Yusof3, T. Kamarul1. European Cells and Materials Vol. 16. Suppl. 2, 2008 (page 50)
28. Chitosan-based electroactive hydrogel Jing Shang, Zhengzhong Shao, Xin Chen. Polymer 49 (2008) 5520–5525
29. Biodegradable polymeric nanoparticles based drug delivery systems. Avnesh Kumari, Sudesh Kumar Yadav, Subhash C. Yadav∗. Colloids and Surfaces B: Biointerfaces 75 (2010) 1–18
30. Advanced Drug Delivery Systems: New Developments, New Technologies .Report Code: PHM006G, Published: August 2009
31. L.B.-P.Donald LeeWise, Handbook of pharmaceutical controlled release technology,2000.
32. V. Jogani, K. Jinturkar, T. Vyas, A. Misra, Recent patents review on intranasal administration for CNS drug delivery, Recent Pat. Drug. Deliv. Formul. 2 (2008)25–40.
33. Hoffman AS, Hydrogels for biomedical applications, Adv Drug Deliv Rev, 43, 3-12, 2002.
34. Hoare TR, Kohane DS, Hydrogels in drug delivery: progress and challenges, Polymer, 49, 1993-2007, 2008
35. Brannon-Peppas L, Polymers in controlled drug delivery, medical plastic and biomaterials magazine, November 1997, Medical device link, https://www.devicelink.com/mpb/archive/97/11/003.html, 10 August 2008.
36. Peppas NA, Bures P, Leobandung W, Ichikawa H,Hydrogels in pharmaceutical formulations, Eur J Pharm Biopharm, 50, 27-46, 2000.
37. Baroli B, Hydrogels for tissue engineering and delivery of tissue-inducing substances, J Pharm Sci, 96, 2197-2223, 2007.
38. Ganta S, Devalapally H, Shahiwala A, Amiji M, A review of stimuli-responsive nanocarriers for drug and gene delivery, J Control Rel, 126, 187-204,2008.
39. He Ch, Kim SW, Lee DS, In situ gelling stimuli sensitive block copolymer hydrogels for drug delivery, J Control Rel, 127, 189-207, 2008.
40. Schmaljohann D, Thermo- and pH-responsive polymers in drug delivery, Adv Drug Delivery.Rev, 58, 1655-1670, 2006.
41. Peppas NA, Khare AR, Preparation, structure and diffusional behavior of hydrogels in controlled release, Adv Drug Delivery Rev, 11, 1-35, 1993.
42. Wenming X, Peixin X, Wei W, Qing L. Preparation and antibacterial activity of a water soluble chitosan derivative. Carbohydrate Polym 2002;50: 35-40.
43. Entezami AA, Massoumi B, Artificial muscles, biosensors and drug delivery systems based on conducting polymers: a review, Iran Polym J, 15,13-30, 2006.
44. Ghazizadeh Y, Mirzadeh H, Amanpour S, Ahmadi H, Rabbani Sh, Investigation of effectiveness of chitosan hydrogel to stop bleeding and air leakage from lung fistula: an in vivo study, Iran Polym J, 15, 821-828, 2006.
45.Yamaoka T., Makita Y., Sasatani H., Kim S. I., Kimura Y. ;(2000);" Linear type azocontaining polyurethane as drug-coating material for colon-specific delivery : its properties degradation behavior and utilization for drug formulation"; Journal of Control Release;66:187-197.
46. Van den Mooter G., Samyn C., Kinget R. ;(1992);"Azo polymers for colon-spicific drug delivery";International Journal of Pharmceutical;87:37-46.
47. Tememoff JS, Mikos AG. Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials 2000;21:2405–12.
48. Hou QP, De Bank PA, Shakesheff KM. Injectable scaffolds for tissue regeneration. J Mater Chem 2004;14:1915–23.
49. M. Amidi, S.G. Romeijn, G. Borchard, H.E. Junginger, W.E. Hennink, W. Jiskoot,Preparation and characterization of protein-loaded N-trimethyl chitosan nanoparticles as nasal delivery system, J. Control Release 111 (2006) 107–116.
50. J.H.Kim,Y.S.Kim,K. Park, S. Lee,H.Y.Nam,K.H.Min,H.G. Jo, J.H. Park,K.Choi, S.Y. Jeong, R.W. Park, I.S. Kim, K. Kim, I.C. Kwon, Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice, J. Control Release 127 (2008) 41–49.
51. H.S. Yoo, J.E. Lee, H. Chung, I.C. Kwon, S.Y. Jeong, Self-assembled nanoparticles containing hydrophobically modified glycol chitosan for gene delivery, J. Control.Release 103 (2005) 235–243.
52. R. Hejazi, M. Amiji, Chitosan-based gastrointestinal delivery systems, J. Control Release 89 (2003) 151–165.
53. H. Tozaki, J. Komoike, C. Tada, T. Maruyama, A. Terabe, T. Suzuki, A. Yamamoto, S.Muranishi, Chitosan capsules for colon-specific drug delivery: improvement of insulin absorption from the rat colon, J. Pharm. Sci. 86 (1997) 1016–1021
54. G. Fetih, S. Lindberg, K. Itoh, N. Okada, T. Fujita, F. Habib, P. Artersson, M. Attia, A. Yamamoto, Improvement of absorption enhancing effects of n-dodecyl-beta-Dmaltopyranoside by its colon-specific delivery using chitosan capsules, Int. J. Pharm.293 (2005) 127–135.
55. J. Varshosaz, A. Jaffarian Dehkordi, S. Golafshan, Colon-specific delivery of mesalazine chitosan microspheres, J. Microencapsul. 23 (2006) 329–339.
56. K. Mladenovska, R.S. Raicki, E.I. Janevik, T. Ristoski, M.J. Pavlova, Z. Kavrakovski,M.G. Dodov, K. Goracinova, Colon-specific delivery of 5-aminosalicylic acid from chitosan-Ca-alginate microparticles, Int. J. Pharm. 342 (2007) 124–136.
57. S.K. Jain, A. Jain, Y. Gupta, A. Jain, P. Khare, M. Kannandasan, Targeted delivery of 5-ASA to colon using chitosan hydrogel microspheres, J. Drug Deliv. Sci. Tech. 18 (2008) 315–321.
58. M.L. Lorenzo-Lamosa, C. Remunan-Lopez, J.L. Vila-Jato, M.J. Alonso, Design of microencapsulated chitosan microspheres for colonic drug delivery, J. Control Release 52 (1998) 109–118.
59. T. Oosegi, H. Onishi, Y. Machida, Novel preparation of enteric-coated chitosan–prednisolone conjugate microspheres and in vitro evaluation of their potential as a colonic delivery system, Eur. J. Pharm. Biopharm. 68 (2008) 260–266
60. A.A. Elzatahry, M.S.M. Eldin, Preparation and characterization of metronidazole loaded chitosan nanoparticles for drug delivery application, Polym. Adv. Technol.19 (2008) 1787–1791.
61. A. Jain, S.K. Jain, In vitro and cell uptake studies for targeting of ligand anchored nanoparticles for colon tumors, Eur. J. Pharm. Sci. 35 (2008) 404–416.
62. K. Ogawara, M. Yoshida, K. Higaki, T. Kimura, K. Shiraishi, M. Nishikawa, Y.Takakura, M. Hashida, Hepatic uptake of polystyrene microspheres in rats: effect of particle size on intrahepatic distribution, J. Control Release 59 (1999) 15–22.
63. K.W. Yang, X.R. Li, Z.L. Yang, P.Z. Li, F. Wang, Y. Liu, Novel polyion complex micelles for liver-targeted delivery of diammonium glycyrrhizinate: in vitro and in vivo characterization, J. Biomed. Mater. Res. A. 88 (2009) 140–148.
64. A. Lin, Y. Liu, Y. Huang, J. Sun, Z. Wu, X. Zhang, Q. Ping, Glycyrrhizin surfacemodified chitosan nanoparticles for hepatocyte-targeted delivery, Int. J. Pharm.359 (2008) 247–253
65. R. Vriesendorp, A.J. Donker, D. de Zeeuw, P.E. de Jong, G.K. van der Hem, J.R.Brentjens, Effects of nonsteroidal anti-inflammatory drugs on proteinuria, Am.J. Med. 81 (1986) 84–94.
66. K. Suzuki, H. Susaki, S. Okuno, Y. Sugiyama, Renal drug targeting using a vector, alkylglycoside, J. Pharmacol. Exp. Ther. 288 (1999) 57–64.
67. S. Wilk, H. Mizoguchi, M. Orlowski, Gamma-glutamyl dopa: a kidney-specific dopamine precursor, J. Pharmacol. Exp. Ther. 206 (1978) 227–232.
68. M. Haas, A.C. Kluppel, E.S. Wartna, F. Moolenaar, D.K. Meijer, P.E. de Jong, D. de Zeeuw, Drug-targeting to the kidney: renal delivery and degradation of a naproxen–lysozyme conjugate in vivo, Kidney Int. 52 (1997) 1693–1699
69. J.D. Byrne, T. Betancourt, L. Brannon-Peppas, Active targeting schemes for nanoparticle systems in cancer therapeutics, Adv. Drug Deliv. Rev. 60 (2008)1615–1626.
70. T.H. Kim, H.L. Jiang, J.W. Nah, M.H. Cho, T. Akaike, C.S. Cho, Receptor-mediated gene delivery using chemically modified chitosan, Biomed. Mater. 2 (2007)S95–100.
71. [12] D.B. Shenoy, M.M. Amiji, Poly(ethylene oxide)-modified poly(epsiloncaprolactone) nanoparticles for targeted delivery of tamoxifen in breast cancer, Int. J. Pharm. 293 (1–2) (2005) 261–270.
72. A. Glen, The impact of nanotechnology in drug delivery: global developments,Market Anal. Future Prospects (2005), Available from:https://www.nanomarkets.com (cited 31.03.09).
73. T. Safra, et al., Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500mg/m2,Ann. Oncol. 11 (8) (2000) 1029–1033.
74. U. Schroeder, et al., Nanoparticle technology for delivery of drugs across the blood–brain barrier, J. Pharm. Sci. 87 (11) (1998) 1305–1307.
75. R.S. Raghuvanshi, et al., Improved immune response from biodegradable polymer particles entrapping tetanus toxoid by use of different immunization protocol and adjuvants, Int. J. Pharm. 245 (1–2) (2002) 109–121.
76. J. Kreutera, et al., Influence of the type of surfactant on the analgesic effects induced by the peptide dalargin after its delivery across the blood–brain barrier using surfactant-coated nanoparticles, J. Control. Release 49 (1997)
77. A. Fassas, et al., Safety of high-dose liposomal daunorubicin (daunoxome) for refractory or relapsed acute myeloblastic leukaemia, Br. J. Haematol. 122 (1)(2003) 161–163.
78. L. Jean-Christophe, et al., Biodegradable nanoparticles—from sustained release formulations to improved site specific drug delivery, J. Control. Release 39 (1996) 339.
79. A. Budhian, S.J. Siegel, K.I. Winey, Production of haloperidol-loaded PLGA nanoparticles for extended controlled drug release of haloperidol, J. Microencapsul.22 (7) (2005) 773–785.
80. C. Gomez-Gaete, et al., Encapsulation of dexamethasone into biodegradable polymeric nanoparticles, Int. J. Pharm. 331 (2) (2007) 153–159.
81. Q. Cheng, et al., Brain transport of neurotoxin-I with PLA nanoparticles through intranasal administration in rats: a microdialysis study, Biopharm.Drug Disposition 29 (2008) 431.
82. L. Mu, S.S. Feng, A novel controlled release formulation for the anticancer drug paclitaxel (Taxol): PLGA nanoparticles containing vitamin E TPGS, J. Control.Release 86 (1) (2003) 33–48.
83. C. Coester, et al., Preparation of avidin-labelled gelatin nanoparticles as carriers for biotinylated peptide nucleic acid (PNA), Int. J. Pharm. 196 (2) (2000) 147–149.
84. C. Damge, P. Maincent, N. Ubrich, Oral delivery of insulin associated to polymeric nanoparticles in diabetic rats, J. Control. Release 117 (2) (2007)163–170.
85. A.A. Date, M.D. Joshi, V.B. Patravale, Parasitic diseases: liposomes and polymeric nanoparticles versus lipid nanoparticles, Adv. Drug Deliv. Rev. 59 (6)(2007) 505–521.
86. P. Calvo, et al., PEGylated polycyanoacrylate nanoparticles as vector for drug delivery in prion diseases, J. Neurosci. Methods 111 (2) (2001) 151–155.
87. Z. Ahmad, et al., Alginate nanoparticles as antituberculosis drug carriers: formulation development, pharmacokinetics and therapeutic potential, Indian J. Chest Dis. Allied Sci. 48 (3) (2006) 171–176.
88. S.Y. Kim, Y.M. Lee, Taxol-loaded block copolymer nanospheres composed of methoxy poly(ethylene glycol) and poly(epsilon-caprolactone) as novel anticancer drug carriers, Biomaterials 22 (13) (2001) 1697–1704.
89. K.S. Lee, et al., Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer, Breast Cancer Res. Treat. 108 (2) (2008) 241–250
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









