{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
UPENDRA KUMAR SINGH*, Mr. Sammer Rastogi, Dr. Manish Kumar Yadav
*MASTER OF PHARMACY in QUALITY ASSURANCE
School of Pharmacy, Lloyd Institute of Management and Technology
Uttar Pradesh, India
* upendra.singh81@gmail.com
ABSTRACT Quality Risk Management (QRM) is a key component for access the product quality. For any pharmaceutical product, Quality Risk Management shall be applied to aim that raising the level of protection for the patient by the reduction of the risk to which that patient is exposed at the time he /she receives a drug product. This general objective can only be achieved by implemented policy of Quality Risk Management (QRM) on the product and process design and its lifecycle. The concept of risk management was first applied in the financial and insurance sectors. This concept was systematically transferred and applied in the pharmaceutical industries in 2005 with the International Conference on Harmonization (ICH) and its publication of the ICH guideline Q9 on “Quality Risk Management”. The European Commission added this guideline as Annex 20 to the EU GMP guide in March 2008. This research was explored the risk identification, risk assessment and development scientific risk control measures during transportation of API from API manufacturing site to user site (formulation plant).
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2530
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 5, Issue 10 Received On: 16/06/2017; Accepted On: 23/06/2017; Published On: 01/10/2017 How to cite this article: Singh UK, Rastogi S, Yadav MK; Quality Risk Management (QRM) of active pharmaceutical ingredients during transportation by using FMEA tools and methodology; PharmaTutor; 2017; 5(10); 23-29 |
INTRODUCTION
Risk is defined as a combination of the probability that damage will occurs and the extent of that damage. These researches are describing that to lay down a procedure to identify failure modes and analyze, assess & control the risk associated with transportation of API from API manufacturing site to user site (formulation plant). Risk Management is done through scientific approach in order to achieve quality of product and to prevent of the entry of falsified drug in drug product & control measures and monitoring during transportation of Active Pharmaceutical Ingredients.
RISK ASSESSMENT APPROACH
The risk based approach is a basic element of the revised EU GMP guidelines on “Principle of Good Distribution Practice of active substances for medicinal products for human use”.
Quality risk management should ensure that the evaluation of risk to quality is based on scientific knowledge and experience with the process. Failure Modes and Effects Analysis (FMEA) is proactive method for evaluating a drug product to identify where and how it might impact and to assess the relative impact of different failures, in order to identify the parts of the process that are most in need of change. FMEA includes review of the following:
• Failure modes (What could go wrong?)
• Failure causes (Why would the failure happen?)
• Failure effects (What would be the consequences of each failure?)
Risk analysis is performed into the following process steps:
• Risk Assessment: Risk analysis
• Risk Control: Risk acceptance
• Risk Review: Processes and procedure
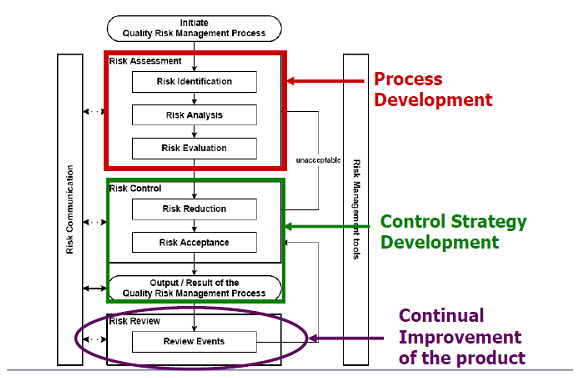
Figure 1: Overview of a typical quality risk management process
Methodology for risk Analysis and evaluation of risk in the supply chain transport:
By applying the FMEA tool and methodology the following aspects are evaluated to assess the risk and evaluation of risk during transportation of API from API manufacturing site to user site (formulation plant site).
• Determine which functions represent potential “Failure Modes” or points of potential failure.
• Determine the worst potential “Effect” or consequences of each of the failure modes.
• Determine the “Contributory Factors” for each failure mode
• Identify any “Controls” in the transportation method / process. Controls are components of the process which (a) reduce the likelihood of a contributory factor or a failure mode, (b) reduce the severity of an effect, or (c) detect the occurrence of a Failure Mode or Contributory Factor before it leads to the adverse outcome (Effect). Example of control measure is: Good Distribution Practices (GDP), validated transport, Assign transportation controls and alarm systems for temperature excursion.
• Rate of severity (consequences of failure) (S), Probability of occurrence (likelihood) (O) and rate the effectiveness of each “Detection Control” (D) details is given in term of degree in the below table 1 “ Degree of Risk Ranking”.
• Calculate the Risk Priority Number (RPN) as per below;
RPN = S x O x D
• The product of three rating is the Risk Priority Number (RPN) for that Contributory Factor e.g. If severity rating is 3, occurrence rating is 2 and detection level is 1, then RPN=3×2×1=6
• Calculate the overall Risk Priority Number (RPN) by addition of each failure mode Risk Priority Number.
• Calculate the Risk Assessment rating by assigning Risk Level (RL) as follows:
Sum of RPN for each failure mode
RL = ------------------------------------------------------x 100
Sum of highest RPN for each failure mode (920)#
#calculated as per highest RPN for each failure mode in table 1 degree of risk ranking(5x5x5+5x5x5+5x5x5+5x5x5+5x5x5+5x5x5+5x5x5+3x5x3) = 920
• API shall be chosen as per Risk Level Rating for consequences-
a) RL ≥ 70 %: Severe – API shall not be used for drug product manufacturing and supply is hold & intimate to vendor for CAPA implemented
b) 50 % <RL <70 % : Major – Risk reduction shall be done additional control implementation during transportation
c) 25 % <RL < 50 %: Moderate – Manage by routine monitoring process
d) Less than 25 %: Minor – No action required, risk is adequate and acceptable.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table 1: Degree of Risk Ranking
| RISK FACTORS | ||
| Failure Mode: Product stability profiles | Description | Risk Score |
| Failure Mode: Product stability profiles | ||
|
Severity; S (Consequences of failure) |
Material has a shelf life longer than 18 months and no environmental controls are required for transport. |
1 |
|
Material has a shelf life less than 18 months and no environmental controls are required for transport. |
3 |
|
|
Material has a shelf life less than 18 months and environmental controls are required for transport. |
5 |
|
|
Probability of occurrence ; O (Potential failure Cause) |
Failure unlikely |
1 |
|
Occasional failure |
3 |
|
|
High failure |
5 |
|
|
Probability of Detectability ; D (ability to control measures) |
Accelerated and stress (mimicking transportation condition) stability is performed and product storage is in temperature controlled. |
1 |
|
Only accelerated stability is performed and product storage is in temperature controlled. |
3 |
|
|
Accelerated and stress (mimicking transportation condition) stability is not performed and product storage is not in temperature controlled. |
5 |
|
|
Failure Mode: Product (API) Physicochemical properties |
||
|
Severity; S (Consequences of failure) |
Product is not sensitive to light, temperature and humidity. |
1 |
|
Product is sensitive to light but not temperature and humidity. |
2 |
|
|
Product is sensitive to light and moisture but not temperature. |
3 |
|
|
Product is sensitive to temperature, humidity and light exposure |
5 |
|
|
Probability of occurrence ; O (Potential failure Cause) |
Failure unlikely |
1 |
|
Occasional failure |
3 |
|
|
High failure |
5 |
|
|
Probability of Detectability ; D (ability to control measures) |
Control measures are not required. |
1 |
|
Material is packed in air tight closed container and temperature controlled vehicle is used for material supply. |
2 |
|
|
Material is packed in air tight closed container and temperature controlled vehicle is not used for material supply. |
3 |
|
|
Material is not packed in air tight closed container and temperature controlled vehicle is not used for material supply |
5 |
|
|
Failure Mode: Material Warehousing site |
||
|
Severity ; S (Consequences of failure) |
Environment controlled storage facility is not required for storage of product (API). |
1 |
|
Environment controlled storage facility is required for storage of product (API). |
5 |
|
|
Probability of occurrence ; O (Potential failure Cause) |
Failure unlikely |
1 |
|
Occasional failure |
3 |
|
|
High failure |
5 |
|
|
Probability of Detectability ; D (ability to control measures) |
Environment controlled qualified facility with free from accumulation of dust, dirt, waste and site is audited by International regulatory authority i.e. USFDA, EUGMP or alternative equivalent standard approved facility. |
1 |
|
Environment controlled qualified facility with free from accumulation of dust, dirt, waste and site is not audited by International regulatory authority i.e. USFDA, EUGMP or alternative equivalent standard approved facility. |
3 |
|
|
Warehousing facility is not qualified for environment condition and site is not audited by International regulatory authority i.e. USFDA, EUGMP or alternative equivalent standard approved facility. |
5 |
|
|
Failure Mode: Repacking activity performed by supplier |
||
|
Severity ; S (Consequences of failure) |
Material is directly supplied from manufacturing site warehouse. |
1 |
|
Material is supplied by the supplier / wholesaler without repacking activities. |
3 |
|
|
Material is supplied by the supplier / wholesaler with repacking activities. |
5 |
|
|
Probability of occurrence ; O (Potential failure Cause) |
Failure unlikely |
1 |
|
Occasional failure |
3 |
|
|
High failure |
5 |
|
|
Probability of Detectability ; D (ability to control measures) |
Supplier / agent have not repacks the material and the environment monitoring is conducted for storage of material. |
1 |
|
Supplier / agent have repacks the material and the environment monitoring are conducted for storage of material. |
3 |
|
|
Supplier / agent have repacks the material and the environment monitoring are not conducted for storage of material. |
5 |
|
|
Failure Mode: Transportation route profile |
||
|
Severity ; S (Consequences of failure) |
Road freight |
1 |
|
Air Freight |
3 |
|
|
Sea Travel |
5 |
|
|
Probability of occurrence ; O (Potential failure Cause) |
Failure unlikely |
1 |
|
Occasional failure |
3 |
|
|
High failure |
5 |
|
|
Probability of Detectability ; D (ability to control measures) |
Transportation route / methods are suitable for protective against ambient temperature & humidity condition. |
1 |
|
Transportation route / methods are not suitable for protective against ambient temperature & humidity condition. |
5 |
|
|
Failure Mode: Transportation mode & Transportation time |
||
|
Severity ; S (Consequences of failure) |
Temperature controlled container / vehicle with less than one week transportation time. |
1 |
|
Temperature uncontrolled container / vehicle with less than one week transportation time. |
3 |
|
|
Temperature uncontrolled container / vehicle with more than one week transportation time. |
5 |
|
|
Probability of occurrence ; O (Potential failure Cause) |
Failure unlikely |
1 |
|
Occasional failure |
3 |
|
|
High failure |
5 |
|
|
Probability of Detectability ; D (ability to control measures) |
No additional control measures required |
1 |
|
Temperature controlled vehicle with temperature control monitoring. |
3 |
|
|
Temperature controlled vehicle without temperature control monitoring. |
5 |
|
|
Failure Mode: Handling of product at port |
||
|
Severity ; S (Consequences of failure) |
Product / API are not imported. |
1 |
|
Imported material is not time and temperature sensitive product /API. |
3 |
|
|
Imported material is time and temperature sensitive product /API. |
5 |
|
|
Probability of occurrence ; O (Potential failure Cause) |
Failure unlikely |
1 |
|
Occasional failure |
3 |
|
|
High failure |
5 |
|
|
Probability of Detectability ; D (ability to control measures) |
Suitable temperature controlled storage facility available and avoid & monitoring to exposure of product in adverse condition during stuffing. |
1 |
|
Suitable temperature controlled storage facility available but monitoring is not done to exposure of product in adverse condition during stuffing. |
3 |
|
|
Suitable temperature controlled storage facility is not available and monitoring is not done to exposure of product in adverse condition during stuffing. |
5 |
|
|
Failure Mode: Temperature monitoring during transportation |
||
|
Severity ; S (Consequences of failure) |
Temperature monitoring is done during transportation |
1 |
|
Temperature monitoring is not done during transportation |
3 |
|
|
Probability of occurrence ; O (Potential failure Cause) |
Failure unlikely |
1 |
|
Occasional failure |
3 |
|
|
High failure |
5 |
|
|
Probability of Detectability ; D (ability to control measures) |
Temperature monitoring device i.e. data logger is calibrated. |
1 |
|
Temperature monitoring device i.e. data logger is not calibrated. |
3 |
|
Risk assessment was carried out for API during transportation as per below table 2.
A case study, Aceclofenac BP is manufactured by the ABC Ltd. Gujrat and API supplied to directly XYZ Ltd. Himachal Pradesh. The material has been transported through road permit with temperature controlled container. As per BP monograph the storage condition of Aceclofenac is “Store in air tight container, protect from light”. The accelerated and long term stability study was performed by the API manufacturer and shelf life assigned for 4 yrs. Stress stability (mimicking transportation condition) study was not performed by the API manufacturer.
TABLE 2: Supply Chain Risk Assessment for API at Transport

ADVATAGE OF RISK MANAGEMENT IN TRANSPORT:
A risk based approach of the individual segment of supply chain i.e. transport can be performed to assure the drug (API) is supplied to user i.e. manufacturing plant site with quality and efficacy. On the basis of risk level the different action plan will be developed to control the risk and their effectiveness within the scope of risk monitoring must be reviewed.
Under the aspect of drug quality, a risk based approach can be used to determine the scope and depth of the necessary qualification of API supplier, transport packing and transport mode. On this basis, additional action for validation and monitoring of transport process can be determined.
SUMMARY
From the above evaluation of risk assessment based on FMEA it was summarized that the various critical steps were expected to occur at each stage of supply chain, were adequate to reduce the associated risk. Methods of risk management can be beneficially employed to identify, assess and control risk. It becomes clear that potential risks change from one transport to the other. The result of a risk analysis can serve as the basis of qualification of transport vehicles, transport packaging and supplier; they can supply important starting points for the qualification of transport service providers or they can from the foundation of a monitoring concept.
REFERENCES
• World Health Organization WHO Technical Report series, no. 957, 2010 annex 5 WHO Good Distribution Practices For Pharmaceutical Products
• Information from European Union Institutions, Bodies, Offices And Agencies European Commission Guidelines , 5 november 2013, On Good Distribution Practice of Medicinal Products for Human Use (text with EEA relevance) (2013/C 343/01)
• International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH Harmonised Tripartite Guideline Quality Risk Management Q9 Current Step 4 version dated 9 November 2005.
• International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH Harmonised Tripartite Guideline Pharmaceutical Development Q8(R2) Current Step 4 version dated August 2009.
• EU GMP Guidelines on 19 march 2015 Principle of Good Distribution Practice of active substances for medicinal products for human use.
• A guide to supply chain risk management for the pharmaceutical and medical device industries and their suppliers v.1.0 2010 the chartered quality institute pharmaceutical quality group website at pqg.org.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









