{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
Narinder Singh*, Raminder kaur
Department of Pharmaceutics
CT institute of Pharmaceutical Sciences,
Jalandhar, Punjab, India
*Pharmacist.narinder@gmail.com
ABSTRACT
Current experimental investigation is dedicated to formulated alginate floating microbeads of Isoniazid as a model drug by ion cross-linking method or ion gelation technique using calcium chloride as cross-linking agent. Drug effectiveness might be influenced by blood plasma protein binding. The less bound drug is more viable it can cross cell membrane and diffuse. Protein binding can impact the drug’s biological half-life in the body. Isoniazid was first generation antibiotic which are used for the resistance is an obstacle to the treatment of tuberculosis disease and latent tuberculosis infection. The permeability of Isoniazid study using different membrane (Egg membrane and cellophane membrane). The IN VITRO drug release studies showed the optimized formulation of drug release 91 % in cellophane membrane and 88 % in egg membrane at 60 mins. The percentage buoyancy and swelling index of microbeads F1-F4 respectively 53-72% and 56-76%. The drug entrapment efficiency of the microbeads prepared from sodium alginate alone were found to be in the range of 52-71%.Experimental report revealed that Isoniazid loaded microbeads may act as ideal nano formulation with elaborated studies.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2576
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 4 Received On: 07/02/2018; Accepted On: 10/02/2018; Published On: 01/04/2018 How to cite this article: Singh N, Kaur R; Preparation and Evaluation of Isoniazid Microbeads by using Different Membrane; PharmaTutor; 2018; 6(4); 39-44; http://dx.doi.org/10.29161/PT.v6.i4.2018.39 |
INTRODUCTION
The site‐specific drug delivery to the target sites can possibly reduce the side effect and most favored technique for administrating therapeutically agents for foundational impacts since it is a characteristic and cost effective to manufacturing process. Oral route is the most usually utilized for the drug administration (Patil et al., 2009). Microspheres are little spherical particles, with diameters across in the micrometer extend (regularly 1μm to 1000 μm). Microspheres are some of the time alluded to as microparticles. Microspheres can be fabricated from different normal and synthetic materials (Agnihotri et al., 2014). Most drugs bind reversibly to plasma proteins to some degree. The division of drug bound can change with plasma drug concentration or dose of drug administered. Likewise the plasma protein concentration of the patient ought to be considered. If a patient has a low plasma protein concentration then for any given dose of drug the concentration in free drug may be higher than expected. Tuberculosis is an extremely infectious determined infection caused by organisms of the Mycobacterium tuberculosis complex. Although mainly a pulmonary pathogen M. tuberculosis can cause disease in almost any part of the body and M. tuberculosis has maximum insubstantiality rate than any other communicable disease. There are many complications in treatment. Acute tuberculosis is normally preserved with a multidrug therapy methodology, including pyrazinamide or rifampicin but Isoniazid mono therapy is typically used in the administration of inactive tuberculosis (Kumar et al., 2014).
Materials and Methods
Materials
Isoniazid was obtained from Balaji drugs, India. Sodium alginate was obtained from S.D. Fine Chemicals Ltd., India. Calcium chloride was obtained from S.D. Fine Chemicals Ltd., India. All other chemicals or reagents used were of analytical grade.
Method for preparation of Beads Containing Isoniazid
The beads containing isoniazid was prepared by using ionotropic -gelation method. Required amount of sodium alginate was dissolved in 50 ml distilled water with constant stirring. Add accurately weighed quantity of isoniazid were added to sodium alginate solution. The final mixture containing sodium alginate was stirred at different rpm continuously for 15 min until the homogeneous and stable suspension remained formed. Then, the suspension was dropped by dropped 18G needle into 10 % (w/v) calcium chloride solution (100 ml).The added droplets were retained for 10 min in the calcium chloride solution to complete the curing reaction. The prepared beads were filtered. The dried beads containing isoniazid were stored in desiccators until used (Rajesh et al., 2003; Das and Murraya., 2006; Radha et al.,2012).
Table 1: Composition of various isoniazid beads
|
Formulation |
(Isoniazid)mg |
Sodium alginate (mg) |
Calcium chloride (10%w/v)(ml) |
RPM |
|
F1 |
100 |
100 |
q.s |
1500 |
|
F2 |
100 |
200 |
q.s |
2000 |
|
F3 |
100 |
300 |
q.s |
3000 |
|
F4 |
100 |
400 |
q.s |
2500 |
Percentage Yield
The percentage of production yield was calculated from the weight of dried microspheres recovered from each batch and the sum of the initial weight of starting materials (Hemalatha et al.,2014). The percentage yield was calculated using the following formula
Percentage Yield= Practical yield/Theoretical yield*100
Drug entrapment efficiency
Accurately weighed 50 mg of prepared beads from each batch were taken separately and were crushed using pestle and mortar. The crushed powders were placed in 50 ml of Phosphate Buffer 7.4 and kept for 24h with occasionally shaking. After the stipulated time, the mixture was stirred at 600 rpm for 30 min on a magnetic stirrer. The polymer debris formed after disintegration of bead was removed by filtering through Whatman filter paper. After that drug content in the filtrate samples were determined using a UV spectrophotometer by measuring absorbance at λ max of 262nm.
The EE of beads was calculated using this following formula.
EE = (Actual drug content in beads/ Theoretical drug content in beads) × 100
Determination of buoyancy
50 mg of microbeads were placed in 100 ml simulated gastric fluid (SGF, pH 7.4). The mixture was stirred at 100 rpm on a magnetic stirrer. After 2 h, the floating and settled microbeads were collected separately, dried at 400°C and weighed.
Buoyancy= (Weight of floating Microbeads/ Weight of floating Microbeads+ Weight of settled Microbeads) x 100
Swelling study
Swelling ratio of different dried microbeads were determined gravimetrically in simulated gastric fluid pH 7.4 .The microbeads were removed periodically from the solution, blotted to remove excess surface liquid and weighed on balance. Swelling ratio (% w/v) was determined.
In-vitro drug release study using different membrane
Preparation of dialysis membrane
The dialysis membrane (Mol wt 12000 KD) was soaked overnight in a buffer in order to open the pores of the membrane. After period of 24 hours dialysis membrane was taken out and was used for the in-vitro drug release.
Preparation of egg membrane
An egg shell was kept in concentrated HCL solution for about five minutes. The outer layer dissolved leaving a membrane was removed and washed with distilled water and kept in buffer solution pH 7.4
In- Vitro drug release
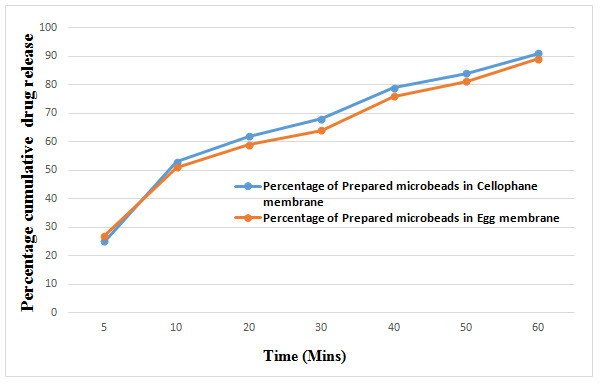
A dialysis membrane and egg membrane was used to monitor Isoniazid release from the Microbeads. Dialysis membrane with molecular weight cut off 12,000 KD was used to determine the release of isoniazid from Microbeads and egg membrane with protein. For determining the In-vitro drug release the 7.4 phosphate buffer solution was prepared. The dialysis membrane was soaked overnight in a buffer in order to open the pores of the membrane. The dialysis membrane was taken out from the buffer and was tied at one end. The membrane was introduced in the buffer medium. At frequent time intervals 1 ml aliquot was withdrawn and after sufficient dilution was analyzed spectrophotometrically at 262 nm. The sample Withdrawn stayed replaced via an equal quantity of fresh 7.4 phosphate Buffer saline. The results relieved that isoniazid release rate in Cellophane membrane and egg membrane were demonstrates slight distinction of the release pattern. Drug release from Cellophane membrane demonstrated comparatively greater drug permeability than using egg membrane barrier. This might be because of the distinction of synthetic and biological barrier use. The drug release profile is presented graphically.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Results and Discussion
UV Spectroscopy (λ max determination)
The Standard Solution of Isoniazid pH 7.4 Phosphate buffer (10µg/ml) was Scanned (200-400) nm by using Double Beam UV Spectrophotometer manufacturer by Shimadzu Corporation .The λ max was found to be 262 nm.
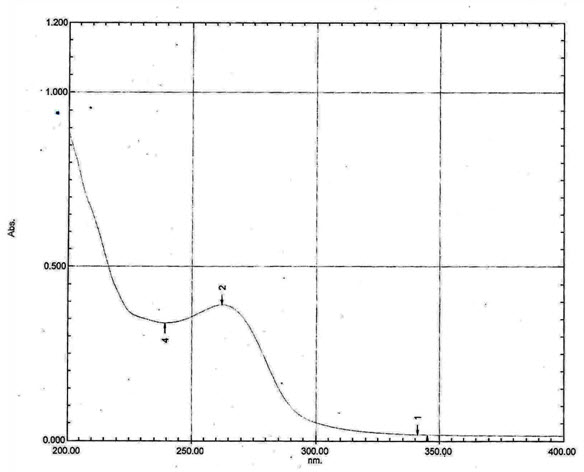
Figure 1: UV spectrum of Isoniazid in pH 7.4 Phosphate buffer Saline (10 µg/ml)
Melting Point
The melting point of the pure drug was found to be in the range of 282-283º C which is in accordance with reference value.
FTIR
The FTIR (Fourier Transformed Infrared spectroscopy) absorption spectrum of the pure drug was taken in the range of 4000-400 cm using KBr pellet method.
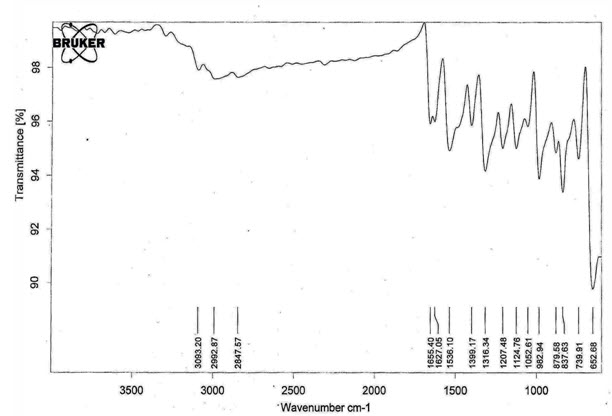
Figure 2: FTIR Spectrum of Isoniazid
|
IR Absorption Band (cm-1) (Experimental) |
IR Absorption Band (cm -1) (Literature) |
Functional Groups
|
|
3093.2 |
3100-3000 |
N-H Asymmetric Stretching |
|
2992.8 |
2990-2900 |
C-H Symmetric Stretching |
|
1655.4 |
1689-1471 |
C=O stretching |
|
1627.0 |
1635 |
C=N Asymmetric Stretching |
|
1536.1 |
1540 |
Pyridine Nitrogen |
|
1399.1 |
1342 |
C-N Stretching |
|
1207.4 |
1210 |
Ring C-C-H Asymmetric Bending |
|
1124.7 |
1140 |
N-X Stretching ( X = NH2) |
|
1052.6 |
1020 |
Ring C-C-N Asymmetric Bending |
|
982.9 |
924 |
Ring C-C-N Symmetric Bending |
|
879.5 |
888 |
Ring C-N-C Bending |
|
739.9 |
745 |
Ring C-C-N Asymmetric Bending |
Entrapment Efficiency
The Entrapment Efficiency of Isoniazid loaded Microbeads were performed in triplicate different Formulation from F1 to F4 exposed in Figure: 3

Figure 3: Entrapment efficiency of isoniazid from Microbeads
Percentage Buoyancy and Swelling Index
The Percentage Buoyancy and Swelling Index of Isoniazid loaded Microbeads were performed in triplicate different Formulation from F1 to F4 exposed in Figure: 4
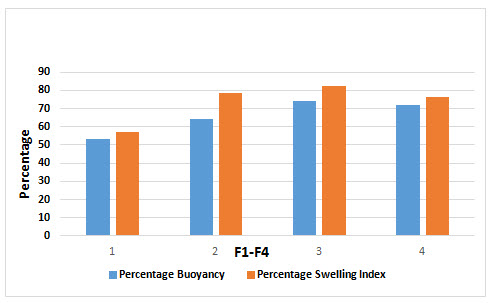
Figure 4: Percentage Buoyancy and Swelling Index of Isoniazid from Microbeads
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
IN-VITRO drug release
IN-VITRO release study of drug from Microbeads solution in 7.4 Phosphate buffer using different membrane obtained between Percentage cumulative drug release and Time (mins). Figure: 5
CONCLUSION
Current experimental work was an attempt to prepare a reconstituted microbeads of ant tubercular drug. Prepared Isoniazid microbeads were having glossy, good Buoyancy and Swelling Index, good entrapment efficiency and controlled drug release. The drug release of INH study with two different membrane which evidently indicate INH has extensively bind with cellophane membrane and the drug release was prolonged manner. The release design of drug from the two membranes shows slight difference due to biological and artificial variance
REFERENCES
• Agnihotri S.A., Mallikarjuna N.N.and Aminabhavi TM; Recent advances on chitosan-based micro- and nanoparticles in drug delivery; J. Control. Release; 2004; vol.100: 5-28.
• Das M.K and Murraya D; Evaluation Of Dilitiazem Hydrochloride Loaded Mucoadhesive Microspheres Prepared By Emulsification -Internal Gelation Technique; Acta Polonia Pharmaceutica Drug Research; 2008; vol. 65 No.2: 249-259.
• Hemalatha C.H., Vasavi G.,Ananda kumar CH. and Sriram N; Formulation And Development Of Gliclazide Microspheres For Pharmaceutical Evaluations; Int. j. of advanced pharmaceutics;2014; vol.4 No.2: 83-92.
• Kumar H.S.N., Parumasivam T., Jumaat F., Ibrahim P., Asmawi MZ. And Sadikun A; Synthesis and evaluation of isonicotinoyl hydrazine derivatives as antimycobacteria and anticancer agents; Med. Chem. Res; 2014; vol.23:269–279.
• Patil G.B., Deshmukh P.K., Belgamwar V.S. and Fursule RA; Chitosan coated mucoadhesive multiparticulate drug delivery system for Gliclazide; Asian Journal of Pharmaceutical and Clinical Research; 2009; vol.2 No.2: 62-68.
• Radha G.V.N.,Samantha L., Swetha Y.and Praveen K; Formulation and in vitro evaluation of mucoadhesive microspheres of nifedipine; J.of pharmaceutical and scientific innovation; 2012; 1(5): 39-43.
• Rajesh K.S., Khanrah A. and Biswanath S.A; Release of Ketoprofen from Alginate Microparticles Containing Film forming Polymer; J. Sci. Ind. Res., 2003; vol. 62 No.10: 987.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









