About Author:
Anitha Nidadavolu
Department of Industrial Pharmacy
Chalapathi Institute of Pharmceutical Sciences
Chalapathi Nagar, Lam, Guntur-522034,
Andhra Pradesh, India.
anitha058@gmail.com
{ DOWNLOAD AS PDF }
Abstract:
In the present study, an attempt was made to develop and characterize once daily sustained release pellets of highly water soluble drug Venlafaxine Hydrochloride, which is an antidepressant of serotonin-nor epinephrine reuptake inhibitor (SNRI). Compatibility studies by FTIR spectroscopy observed Venlafaxine HCl was compatible with all the excipients used. These pellets were prepared in three stages. In drug loading stage (powder layering technique with pan coater), drug was loaded on non-pareil sugar spheres by using Mannitol, Microcrystalline powder (MCCP) as diluents and PVP K30 as binder. The concentration of Venlafaxine HCl was kept constant. Four preliminary batches of drug loaded pellets prepared by varying concentrations of disintegrant Crospovidone INF-10 (D1- D4) i.e. 1.5%, 3%, 4.5%, 6%. Optimized formulation was selected based on percentage yield, drug content (assay) and found D3- 4.5% as best. In barrier coating stage(wurster process with fluidized bed coater) drug loaded pellets of D3 were coated by different concentrations of film former HPMC E3 (B1- B3) i.e.4%, 6%, 8%. Among them, B2- 6% found as best. In SR coating stage (wurster process with fluidized bed coater) barrier pellets of B2 were coated by varying concentrations of release rate retarding polymer Ethyl cellulose EC 7 cps (S1- S4) i.e. 2%, 5%, 6%, 8%. These EC (S1- S4) formulations were characterized fordrug content (assay), particle size distribution, friability,flow properties, surface morphology (SEM) and dissolution profile.In vitro dissolution studies were carried out by USP dissolution apparatus Type-II and compared with innovator Effexor XR®. Among all formulations S4(8%) was best, followed first order kinetics and found to release the drug over a sustained period of time up to 24 hrs. The release exponent (n values) for all found in the range of n > 1, indicated that the drug transport mechanism by super case-II transport. The optimized S4 formulation was found as pharmaceutically equivalent to innovator due to similarity (f2 =77.77) in drug release profile. As per ICH guidelines, accelerated stability studies conducted and there was no significant difference in physicochemical parameters (p < 0.05), indicated that the optimized S4 formulation was stable.
REFERENCE ID: PHARMATUTOR-ART-2090
Introduction:
Multi -particulate drug delivery systems are the most accepted and extensively used dosage form as they offer numerous advantages over single unit dosage forms like improved bioavailability because of increased surface area, reduced inter subject variation, more even and predictable distribution and transportation and reduced chances of dose dumping.[1, 2, 3] The primary benefit of a sustained release dosage form compared to a conventional dosage form is the uniform drug plasma concentration and therefore uniform therapeutic effect. [4]Over the past two decades, sustained release dosage forms have made significant progress in terms of clinical efficacy and patient compliance.
Pelletization is one of the most promising techniques for the multi-particulate drug delivery systems. Pelletization involves the process of renovation of fine powder or granules of bulk drugs and the excipients into small, free flowing, spherical units in size between 0.5-1.5 mm, referred to as pellets. Pellets can be divided into desired dosage strength without process or formulation changes and also allows the combined delivery of two or more bioactive agents that may or may not be chemically compatible, at the same site or at different sites within the gastrointestinal tract. [5, 6] They offer higher degree of flexibility in the design and development of oral dosage form like suspension, tablet and capsule. [7]Extended release formulations are designed to allow at least two fold reduction in dosing frequency or significant increase in patient compliance or therapeutic performance when compared to a conventional immediate release dosage form. Sustained release pharmaceutical pellet is one of the most popular approaches among the various types of extended release dosage forms as it offers several manufacturing and biopharmaceutical advantages. [5]The spherical shape and low surface area to volume ratio of pellets are advantageous for uniform film coating.
Depression is the most common of the affective disorders (defined as disorders of mood rather than disturbances of thought or cognition); it may range from a very mild condition, bordering on normality, to severe (psychotic) depression accompanied by hallucinations and delusions. Antidepressants work by balancing brain neurotransmitters level to ease depression. They can be used alone or in combination with other medications.
Venlafaxine HCl is a structurally novel hydroxycycloalkanephenethyl bicyclic antidepressant structurally differs from other currently available anti-depressants and is usually categorized as a serotonin-norepinephrine reuptake inhibitor (SNRI) but it has been referred to as a serotonin-norepinephrine-dopamine reuptake inhibitor. Venlaflaxine HCl is the first drug to be marketed that inhibits both noradrenaline and 5-HT reuptake without actions in other receptors.[8] Venlafaxine HCl has short elimination half-life of 5±2 hrs and its recommended daily dose is 75-225 mg/day. [9] Hence it requires twice or thrice dosing per day leads to chances of missing a dose. In such case the formulation releasing the drug in sustained manner will aid the patient to adhere to strict medication routine by avoiding the need to take the dosage form 2 or 3 times daily. Therefore, Venlafaxine hydrochloride is suitable candidate for development of a once daily sustained release dosage form to reduce the frequency of administration and to improve the patient compliance.
It is a white crystalline solid. Venlafaxine HCl is completely absorbed from the GIT, undergoes extensive first pass metabolism and having low bioavailability (10 - 45%). It belongs to BCS class I, having high solubility and high permeability, freely soluble in water (572 mg/ml). These biopharmaceutical and physicochemical properties reveal that Venlafaxine HCl is an ideal candidate to develop into sustained release pellets.
Venlafaxine HCl water soluble drug, if not formulated properly, may readily release the drug at a faster rate and produce a toxic concentration of drug on oral administration. So, it is necessary to retard dissolution to ensure sustained release of drug by properselectionofreleaseretarding excipientstoachieveaconstantin vivo inputrateofdrug. Hence it is a challenging task to formulate a suitable pellet dosage form for sustained delivery of highly water soluble drugs with very slow constant release rate. Ethyl cellulose 7cps in combination with HPMC E3 was employed in this research to sustain the drug release for 24hours. Once-a-day SR dosing (for 24h) achieves bioavailability equivalent to that of twice-a-day dosing with IR formulation (for 12h).
Objective:
The primary object of this study was to prepare and in vitro characterization of drug loaded Venlafaxine HCl pellets using powder layering technology and to give functional coating using ethyl cellulose in combination with hydroxy propyl methyl cellulose and to extend the drug release for more than 24 hours. Here, ethyl cellulose acts as a release retarding polymer and hydroxy propyl methyl cellulose acts as a film forming agent.
1. Materials used in the study
|
S.NO |
MATERIALS |
CATEGORY |
|
1 |
Venlafaxine HCl |
Anti-depressant (API) |
|
2 |
Sugar spheres #20/25 |
Non-pareil seeds / inert core |
|
3 |
Crospovidone (INF 10) |
Dissolution aid / Disintegrant |
|
4 |
Sodium Lauryl Sulphate (SLS) |
Lubricant |
|
5 |
Mannitol |
Diluent / Sweetening agent |
|
6 |
Micro Crystalline Cellulose Powder (MCCP) |
Filler / Flow aid |
|
7 |
Poly Vinyl Pyrrolidone ( PVP K30) |
Binder |
|
8 |
Hydroxy Propyl Methyl Cellulose HPMC E3 (hypromellose),3cps |
Film former |
|
9 |
Ethyl Cellulose (EC) 7cps |
Sustained release polymer |
|
10 |
Triethyl citrate |
Plasticizer |
|
11 |
Isopropyl alcohol |
Solvent |
|
12 |
Purified Water |
Solvent |
Preformulation studies:
Characterization of Venlafaxine Hydrochloride: (API)
- Organoleptic characters
- Melting point
- Solubility
- Bulk density and Tapped density
- Carr’s compressibility index
- Hausner’s ratio
- Moisture Content
- Watercontent
- Loss on drying (LOD)
- Particle size
Drug - excipient compatibility studies:
The objective of the compatibility study was to determine the compatibility of Venlafaxine hydrochloride with the excipients incorporated in the formulation.
Physical drug - excipient compatibility studies: [10]
Physical observation of sample was done every week for any color change or lumps formation and flow, for three months stored at 40°C/75% RH.
The physical compatibility of Venlafaxine hydrochloride with various excipients was tested to select suitable excipients for a stable and robust formulation. A blend of the Venlafaxine hydrochloride with the excipients in the suitable ratio was filled in glass vials and was exposed to 40°C/75 % RH .They were observed for any physical change against control samples kept at refrigerated condition of 2-8°C.
2. Protocol for Drug-Excipients Compatibility
|
Batch No |
Drug -Excipient combination |
D:E ratio |
|
1 |
Venlafaxine hydrochloride alone |
- |
|
2 |
Venlafaxine hydrochloride + Sugar spheres |
1:5 |
|
3 |
Venlafaxine hydrochloride + Crospovidone INF -10 |
1:5 |
|
4 |
Venlafaxine hydrochloride + Mannitol |
1:5 |
|
5 |
Venlafaxine hydrochloride + TEC |
1:5 |
|
6 |
Venlafaxine hydrochloride + PVPK30 |
1:5 |
|
7 |
Venlafaxine hydrochloride + HPMC E3 |
1:5 |
|
8 |
Venlafaxine hydrochloride + SLS |
1:5 |
|
9 |
Venlafaxine hydrochloride + EC 7 cps |
1:5 |
|
10 |
Venlafaxine hydrochloride + MCCP |
1:5 |
|
11 |
Venlafaxine hydrochloride + IPA |
1:5 |
FT- IR Spectral Analysis:
FT-IR spectrum of Venlafaxine hydrochloride was compared with FT-IR spectra of Venlafaxine hydrochloride with polymers. Disappearance and shifting of peaks were observed. The scanning range was 4000 to 450 cm-1 and the resolution was 2cm-1.The FT-IR spectra were taken by using FT-IR with ATR module.
Analytical methods:
Preparation of Venlafaxine hydrochloride standard stock solution (100μg/ml) in phosphate buffer (PH 6.8) solution:
A standard stock solution of Venlafaxine hydrochloride was prepared by dissolving accurately weighed 10 mg of Venlafaxine hydrochloride in phosphate buffer (PH 6.8) solution in a 100 ml volumetric flask and the volume was made up to 100 ml by using phosphate buffer(PH 6.8) solution to obtain a stock solution of 100 mg/ml.
UV Spectroscopy (Determination of analytical wavelength i.e. λmax):
The resulting solution containing 10 mg/ml was scanned between 200 and 400 nm. The spectrum is given in Figure 8.1.The λmax was found to be 225 nm and was used as analytical wavelength.
Calibration curve of Venlafaxine hydrochloride:
Linearity:
The linearity was evaluated by linear regression analysis by least squares method. The linearity of method was evaluated by analyzing seven different concentrations (50 ppm-350 ppm) of the working standard solution of Venlafaxine hydrochloride. Calibration graph was plotted against peak area and concentration of solution. In linearity graph, the correlation coefficient (r 2) was found to be 0.999
Preparation of mobile phase:
A degassed mixture of PH 7.4buffer and acetonitrile in the ratio of 75:25 v/v was prepared.
Preparation of standard solution:
About 20mg of Venlafaxine HCl working standard was accurately weighed and transferred into a 200 ml volumetric flask. About 160ml of mobile phase was added and sonicated to dissolve. The volume was diluted with mobile phase and mixed well. Five ml of this solution transferred into 10 ml volumetric flask, diluted to 10ml with mobile phase and mixed well. The resulting solution concentration was found to be 250ppm. Another six different concentrations of working standard were prepared.
3. Chromatographic conditions
|
Column |
Agilent C8, 250 × 4.0 - 4.6mm |
|
Flow rate |
1.2ml/min |
|
Wavelength |
225nm |
|
Temperature |
Ambient |
|
Injection Volume |
20μl |
|
Runtime |
12min |
Determination of amount of Venlafaxine hydrochloride to be used pellets:
Venlafaxine is available as venlafaxine hydrochloride. Labeled claim is to be expressed in terms of the equivalent amount of venlafaxine present in the dosage form. 32.56gm of venlafaxine hydrochloride should be used in unit to get 32gm of venlafaxine.For 3 kg batch size of SR pellets, 0.977 kg of venlafaxine hydrochloride is required.
The total amount of venlafaxine hydrochloride to be used in the formulation can be calculated using the following formula,
Required dose = Label claim × Conversion factor
Conversion factor
100 100
= ---------------------------- × ---------------------
(%w/w assay on anhydrous basis) (100 -%w/v of water by KF)
Formulation development:
Formulation of sustained release pellets of venlafaxine hydrochloride
Sustained release pellets of Venlafaxine hydrochloride with different compositions are prepared by using pan coating method and fluid bed coating methods and evaluated for different properties of the formulations to optimize the best formula with desired characteristics.
4. Formulation of sustained release pellets of Venlafaxine hydrochloride
|
VENLAFAXINE HYDROCHLORIDE PELLETS 32% W/W |
||||||||||||
|
BATCH SIZE (Kg) |
3.000 |
3.000 |
3.000 |
3.000 |
||||||||
|
STAGE –I |
DRUG LOADING |
|||||||||||
|
S.NO |
INGREDIENTS |
D1 |
D2 |
D3 |
D4 |
|||||||
|
|
Crospovidone INF -10 (%) |
1.5% |
3% |
4.5% |
6% |
|||||||
|
1 |
Venlafaxine HCl |
0.977 |
0.977 |
0.977 |
0.977 |
|||||||
|
2 |
Sugar spheres (20#25) (36%) |
1.080 |
1.080 |
1.080 |
1.080 |
|||||||
|
3 |
Crospovidone INF -10 |
0.045 |
0.090 |
0.135 |
0.180 |
|||||||
|
4 |
Sodium Lauryl Sulphate (3%) |
0.030 |
0.030 |
0.030 |
0.030 |
|||||||
|
5 |
Mannitol (7.7%) |
0.231 |
0.231 |
0.231 |
0.231 |
|||||||
|
6 |
MCCP (4.5%) |
0.135 |
0.135 |
0.135 |
0.135 |
|||||||
|
7 |
PVP K 30 (0.4%) |
0.012 |
0.012 |
0.012 |
0.012 |
|||||||
|
8 |
Purified water |
q.s. |
q.s. |
q.s. |
q.s. |
|||||||
|
TOTAL QUANTITY (DRUG PELLETS) |
2.510 |
2.555 |
2.600 |
2.645 |
||||||||
|
STAGE – II |
BARRIER / SUB COATING |
|||||||||||
|
S.NO |
INGREDIENTS |
B1 |
B2 |
B3 |
||||||||
|
|
HPMC E3 (%) |
4% |
6% |
8% |
||||||||
|
1 |
Drug pellets |
2.600 |
2.600 |
2.600 |
||||||||
|
2 |
HPMC E3 |
0.104 |
0.156 |
0.208 |
||||||||
|
3 |
Purified water |
q.s. |
q.s. |
q.s. |
||||||||
|
TOTAL QUANTITY (BARRIER PELLETS) |
2.704 |
2.756 |
2.808 |
|||||||||
|
STAGE-III |
SR COATING |
|||||||||||
|
S.NO |
INGREDIENTS |
S1 |
S2 |
S3 |
S4 |
|||||||
|
|
EC 7cps (%) |
2% |
5% |
6% |
8% |
|||||||
|
1 |
Barrier pellets |
2.756 |
2.756 |
2.756 |
2.756 |
|||||||
|
2 |
Ethyl cellulose 7 cps |
0.055 |
0.138 |
0.165 |
0.220 |
|||||||
|
3 |
HPMC 3 cps |
0.007 |
0.014 |
0.021 |
0.028 |
|||||||
|
4 |
Triethyl citrate |
0.005 |
0.009 |
0.014 |
0.019 |
|||||||
|
5 |
Isopropyl alcohol |
0.531 |
1.062 |
1.593 |
2.124 |
|||||||
|
6 |
Purified water |
q.s. |
q.s. |
q.s. |
q.s. |
|||||||
|
TOTAL QUANTITY (SR PELLETS) |
2.823 |
2.917 |
2.956 |
3.023 |
||||||||
|
ASSAY (theoretical) |
34.61% |
33.50% |
33.05% |
32.31% |
||||||||
Manufacturing process:
In the manufacturing of sustained release Venlafaxine hydrochloride pellets there are 3 stages involved in this process:
1. Drug loading
2. Barrier coating or sub coating
3. Functional coating
The development of present study was mainly based on the process of binding of drug to non-pareil seeds and binding of polymer on to drug coated non-pareil seeds.
1. Drug loading
The drug pellets were prepared by powder layering technique using conventional standard pan coating method. The following steps involved.
Pulverizing and blending:
The required ingredients were weighed and collected from dispensing area and taken for pulverization. PVP was pulverized, collected in a clean polyethylene bag, labeled and kept aside. Venlafaxine HCl was pulverized along with INF 10 and MCCP and Mannitol. They were collected in clean double lined bags and labeled. The pulverized materials were added into the clean the double cone blender one after the other along with SLS and blended for 25 minutes. The blended material /drug powder was then collected in a container and labeled. Samples were withdrawn at different areas and tested for content uniformity and assay.
Preparation of Binding solution:
PVP k30 was dissolved in purified water.
Preparation of drug pellets:
The calculated quantity of sugar spheres were taken into the conventional coating pan. After ensuring the integrity of the apparatus the operation was started by setting the temperature, spray pressure, spray rate etc. Drug loading process was started by spraying the polymer/ binding solution on to the sugar spheres until uniform wetting takes place, then the drug powder was dispersed on to the sugar spheres by rubbing with the hand then the pellets were collected for drying in a tray dryer. Finally they were used for sifting.
Sifting:
The dried pellets were passed through the sieves 14# and 18#. The ups and downs of each sieve were collected separately. Pellets retained on 18# are used for further process. The drug loaded pellets were sent for analysis.
5. Critical process parameters during drug loading
|
S.NO |
Name of the parameter |
Operation parameter |
|
1 |
Pan RPM |
15±5 |
|
2 |
Atomization air pressure |
1±.0.5 kg/cm2 |
|
3 |
Drying Time |
8-10 hrs |
2. Sub coating / barrier coating
Preparation of Barrier coating solution:
Barrier coating solution was prepared by using HPMC E3 in heated purified water.
Purified water was taken and kept for heating until it reached 60°C-70°C. Required quantity of HPMC E3 was taken and dissolved in purified water with continuous stirring for 30 minutes (or) till clear solution was formed. The resulting solution was cooled to room temperature and filtered through nylon mesh, labeled and used for coating purpose.
Barrier coating of drug pellets:
Drug loaded pellets were loaded into FBP and the pellets were warmed till the product temperature of 40±2°C was obtained. The sub coating dispersion prepared was sprayed with following parameters. The dispersion was kept under continuous stirring during the coating process. The coating was continued till target weight build up was obtained. The fluidization air flow was reduced to suitable level and the sub coated pellets were dried at the product temperature of 33°C -35°C for 15-20 minutes.
6. Process parameters for sub coating in fluidized bed coater
|
S.NO |
Process Parameter |
Range |
|
1 |
Inlet temperature(oC) |
60±10 |
|
2 |
Product / Bed temperature(oC) |
45±5 |
|
3 |
Exhaust temperature(oC) |
30-45 |
|
4 |
Atomization air pressure (barr) / (kg/cm2) |
1.5 ± 1 |
|
5 |
Spray rate (g/min) |
60-120 |
|
6 |
Wurster height (mm) |
20-50 |
|
7 |
Pump RPM |
5±3 |
|
8 |
Drying time |
15-20 min |
Note: If lumps formation was observed, unload the pellets and sifting was done
Sifting:
The dried pellets were passed through the sieves 14# and 20#. The ups and downs of each sieve were collected separately. Pellets retained on 20# are used for further process. Samples were taken from each batch and subjected for assay and dissolution test.
3. Functional coating/ SR coating
After drug and seal coating this step plays most important role in sustaining the drug release.
This is also called as polymer coating, where a coating solution containing appropriate concentration of polymer was prepared and sprayed on seal coated pellets.
Preparation of SR coating solution:
Required quantity of IPA was taken into a beaker and to this calculated quantity of Ethyl cellulose was added under continuous stirring. In another beaker water was taken, to this initially half quantity of HPMC 3cps was added with continuous agitation and then remaining quantity of HPMC 3cps was added with constant stirring. The resulting solution was added to above dispersed polymeric solution under continuous stirring and then calculated quantity of Triethyl citrate was added under slow stirring .Finally the solution was filtered and used for coating purpose.
SR coating of sub coated pellets:
Now the sub coated pellets were loaded into fluidized bed processer and the pellets were warmed till product temperature 40±2°C. The functional coating dispersion was kept under continuous stirring, during the coating process. The coating was continued till target weight build up was obtained. The fluidization air flow was reduced to suitable level and the pellets were warmed at the product temperature 40±2°C for 30 minutes. The functional coated pellets were sifted through mesh 20# and passed pellets were collected into a container.
7. Process parameters for functional coating in fluidized bed coater
|
S.NO |
Process Parameters |
Range |
|
1 |
Inlet temperature(oC) |
60±10 |
|
2 |
Product / Bed temperature(oC) |
45±5 |
|
3 |
Exhaust temperature(oC) |
30-45 |
|
4 |
Atomization air pressure (barr) /( kg/cm2) |
1.5 ± 1 |
|
5 |
Spray rate (g/min) |
60-120 |
|
6 |
Wurster height (mm) |
20-50 |
|
7 |
Pump RPM |
5±3 |
|
8 |
Drying time |
15-20 min |
In vitro characterization of pellets:
A) Physical Evaluation:
Percentage (%) yield: All the batches of sustained release Venlafaxine hydrochloride pellets prepared by both pan coating and fluid bed coating were evaluated for percentage yield of the pellets. The actual percentage yields of pellets were calculated by using the following formula.
Practical yield of pellets
Percentage yield of pellets(%)= ------------------------- × 100
Theoretical yield of pellets
Sieve analysis:
The average particle size of the pellets was analyzed by simple sieve analysis method. The sample collector and sieves arranged as per specification. Hundred gm of the pellets are shifted in to sieve shaker where a series of sieves was placed (14 #, 16 #, 18 #, 20 # and 25 #). The machine was run for 5 minutes, all the meshes/sieves were taken out and retained pellets were collected by respective mesh and the % retention of pellets by that mesh was calculated. The retains collected on the larger dia sieve (A) and from the sample collector separately passings passes through the smaller dia sieve (B)
Particle size distribution and determination:
This practice was done for the pellets obtained after functional coating to check average size of the pellets. Hundred gm of the pellets are shifted in to sieve shaker where a series of sieves was placed (14 #, 16 #, 18#, 20 # and 25 #). The machine was run for 5 minutes, all the meshes were taken out and retained pellets were collected by respective mesh and the % retention of pellets by that mesh was calculated. Average particle size was determined. A graph was plotted taking mean size opening on X- axis and percent weight retained on smaller sieve on Y - axis.
Flow Properties of Venlafaxine hydrochloride SR pellets:
The flow properties of pellets were evaluated for bulk density, tapped density angle of repose, carr’s index and haussner’s ratio.
Angle of repose:
The pellets were allowed to flow through the funnel freely onto the surface. The diameter of the powder cone was measured and angle of repose was calculated using the following equation.
θ = tan-1(h/r)
Where, h and r are the height and radius of the pellets cone, respectively.
Determination of bulk density and tapped density:
An accurately weighed quantity of the pellets (W), was carefully poured into the graduated cylinder and the unsettled apparent volume (V0) to the nearest graduated unit occupied by the pellets was measured. The measuring cylinder containing a weighed quantity of pellets (after measurement of bulk density) was closed with a lid and subjected to 500 taps until the pellets volume has reached a minimum volume in tapped density tester. The final volume (Vf) was measured and continued operation till the two consecutive readings were equal. The tapped density was calculated by using the formula,
Bulk density = Mass of the pellets/ Bulk volume of pellets
Tapped density = Mass of the pellets / Tapped volume of pellets
Friability:
About 6.5g pellets were weighed collectively and placed in the chamber of the friabilator rotated at 25rpm for 4min. In the friabilator, the pellets were exposed to rolling, resulting from free fall of pellets within the chamber of the friabilator. After 100 rotations (4 minutes), the pellets were taken out from the friabilator and intact pellets were again weighed collectively after removing fines using sieve # 44 sieve. Friability values below 0.8% are generally acceptable. The percentage friability was calculated according to the following formula.
% F = W1-W2/W1 × 100
Where, W1 = weight of the pellets before test, W2 = weight of the pellets after test.
Water content by KF method:
Around 50ml of methanol was taken in titration vessel of Karl Fischer titrator and titrated with Karl Fischer reagent to end point. In a dry mortar the pellets grinded to fine powder. Accurately about 0.5 g of the sample, weighed and transferred quickly to the titration vessel, stirred to dissolve and titrated with Karl Fischer reagent to end point.
V x F
% Water content= --------------------- x 100
Weight of the sample
Where,
F= factor of Karl Fischer reagent.
V= volume in ml of Karl Fischer reagent consumed for sample titration.
Shape and surface roughness by scanning electron microscopy (SEM):
The samples were coated with a thin gold layer by sputter coater unit (SPI, Sputter, USA). Then, the SEM photographs were taken by JEOL, JSM-6610LL, Scanning electron microscope, Japan operated at an accelerated voltage of 20000 Volt.
B) Chemical Evaluation
- Assay
- Dissolution (acid stage followed by buffer stage)
Assay /drug content: (by HPLC)
Chemicals and reagents:
- Ammonium phosphate monobasic: AR Grade
- Acetonitrile: AR Grade
- Phosphoric acid: AR Grade
- Water: Milli-Q- grade.
Preparation of mobile phase:
A degassed mixture of pH 7.4buffer and acetonitrile in the ratio of 75:25 v/v was prepared.
8. Chromatographic conditions
|
Column |
Agilent C8, 250 × 4.0 - 4.6mm |
|
Flow rate |
1.2ml/min |
|
Wavelength |
225nm |
|
Temperature |
Ambient |
|
Injection Volume |
20μl |
|
Runtime |
12min |
Preparation of standard solution:
About 20mg of venlafaxine HCl working standard was accurately weighed and transferred into a 200 ml volumetric flask. About 160ml of mobile phase was added and sonicated to dissolve. The volume was diluted with mobile phase and mixed well. About 5ml of this solution transferred into 10 ml volumetric flask, diluted to 10ml with mobile phase and mixed well.
Preparation of sample Solution:
About 10g of pellets grinded to fine powder in a dry mortar and accurately weighed the quantity of powder equivalent to 20mg of Venlafaxine HCl into a 500ml volumetric flask. About 300 ml of mobile phase was added ,sonicated for 20 minutes with intermittent shaking until powder completely dissolved (sonicator bath temperature to be maintained between 20-25oC). Cooled, diluted to 500ml with mobile phase and mixed well. A portion of sample solution centrifuged at about 3000 rpm for 15 minutes. 5ml of the clear supernatant solution was transferred into 10 ml volumetric flask, diluted to volume with mobile phase and mixed well. The obtained solution was filtered through 0.45μm membrane filter.
Procedure:
The HPLC column was equilibrated with mobile phase for 30 minutes at a flow rate of 1.2ml/min. 20μl of mobile phase as blank, standard preparation (5 times) and the sample preparation was injected separately into the liquid chromatographic system and the area due to major peaks recorded.
Evaluation of system suitability parameters:
The column efficiency as determined for the Venlafaxine hydrochloride peak from standard solution was not less than 2000 theoretical plates and the tailing factor for the same peak was not more than 2.0. The % RSD of peak areas from five replicate injection of standard solution was not more than 2.0%. The retention time for Venlafaxine hydrochloride peak was about 4.073minutes.
Calculation: Calculate the amount of Venlafaxine hydrochloride present in pellets, in % using the following formula:
% venlafaxine HCl
AT WS VT M1
= ------ × ---- × DS × ----- × DT × ------- × P
AS VS WT M2
AT 20 5 500 10 277.4
= ----- × ------ ×----- × ------ × ---- × ------- × 99.4
AS 200 10 152.2 5 313.86
Where,
AT = Peak area due to Sample preparation
DS = Dilution of the standard
AS = Peak area due to working standard preparation
DT = Dilution of the sample
WS = Weight of Working standard taken in mg
M1 = Molecular weight of Venlafaxine
WT = Weight of Sample taken in mg
M2 = Molecular weight of Venlafaxine HCl
VS = Volume of mobile phase to dissolve working standard
VT = Volume of mobile phase to dissolve sample
P = (%) Purity of working standard used
Dissolution (by HPLC):
Dissolution Study:
Venlafaxine hydrochloride sustained release pellets were evaluated for in vitro drug release.
In vitrodissolution/drug release rate studies in acidic medium:
Dissolution rate testing conditions profile:
- Apparatus :USP apparatus II- paddle
- Medium :0.1N HCl up to two hours
- Volume :900 ml
- Sampling time :120 minutes (at the end of 2nd h)
- Rpm :100
- Temperature :37ºC± 0.5ºC
Preparation of sample Solution
Accurately weighed the quantity of pellets equivalent to 20mg of Venlafaxine HCland transferred individually into in each of the 6 dissolution jars, containing 900 ml of 0.1 N Hydrochloric acid, kept in a thermostatically controlled water bath, maintained at temperature 37 ± 0.5ºC and rpm of 100 throughout the experiment. Care must be taken to exclude air bubbles. The apparatus started immediately and operated for 2 hours. At the end of 2nd h, samples measuring 10 ml were withdrawn from a zone midway between the surface of the medium and top of the rotating blade and not less than 1cm from the each vessel wall and filtered through 0.45μm filter. First few ml of the filtrate was discarded. 5ml of each filtered sample, transferred into each 10 ml volumetric flasks, diluted to 10ml with mobile phase and mixed well. These samples were analyzed by HPLC by preparing the standard at the same time and results were reported. After sampling the baskets lifted. The medium drained completely without losing any pellet.
In vitro dissolution/drug release rate studies in acidic medium:
Calculation: Calculate the amount of Venlafaxine hydrochloride released in 0.1N hydrochloric acid, in % using the following formula
% Venlafaxine HCl released
AT WS VT 100 M1
= ----- × ----- × DS × ----- × DT× ------× ----- × P
AS VS WT LC M2
AT 20 5 900 10 100 277.4
= ----- × ----- ×----- × ----- × ----- × ----- × ------ × 99.4
AS 200 10 152.2 5 32 313.86
Where,
AT = Peak area due to Sample preparation
DS = Dilution of the standard
AS = Peak area due to working standard preparation
DT = Dilution of the sample
WS = Weight of Working standard taken in mg
M1 = Molecular weight of Venlafaxine
WT = Weight of Sample taken in mg
M2 = Molecular weight of Venlafaxine HCl
VS = Volume of mobile phase to dissolve working standard
VT = Volume of dissolution medium (acid medium)
P = (%) Purity of working standard used
LC = Label claim
In vitrodissolution/drug release rate studies in basic medium:
Dissolution rate testing conditions profile:
- Apparatus :USP apparatus II- paddle
- Medium :pH 6.8 Phosphate buffer
- Volume :900 ml
- Samplinginterval : 1,2,4,8,12 ,16, 20,24 (hr)
- Rpm :100
- Temperature :37ºC± 0.5ºC
Preparation of sample Solution:
Accurately weighed the quantity of pellets equivalent to 20mg of Venlafaxine HCland transferred individually into in each of the 6 dissolution jars, containing 900 ml of 0.1 N Hydrochloric acid, kept in a thermostatically controlled water bath, maintained at temperature 37 ± 0.5ºC and rpm of 100 throughout the experiment. Care must be taken to exclude air bubbles. The apparatus started immediately and operated for 2 hours. At the end of 2nd h, after sampling, the baskets lifted. The medium drained from vessels completely without losing any pellet. The dissolution medium, 900ml of phosphate buffer pH 6.8 with temperature equilibrated to 37°C ± 0.5°C was placed into each of six dissolution flasks. The apparatus was continued to run for 24 hours. Samples measuring 10 ml were withdrawn from a zone midway between the surface of the medium and top of the rotating blade and not less than 1cm from the each vessel wall at regular intervals upto 24hrs using auto sampler and equal volume of fresh dissolution medium was replaced to maintain the constant volume throughout the experiment. Then samples were filtered through 0.45μm filter, which was in inline with auto sampler. First few ml of the filtrate was discarded. 5ml of each filtered sample, transferred into each 10 ml volumetric flasks, diluted to 10ml with mobile phase and mixed well. These samples were analyzed by HPLC by preparing the standard at the same time and results were reported.
Calculation: Calculate the amount of Venlafaxine hydrochloride retained in 0.1N hydrochloric acid, in % using the following formula
% Venlafaxine HCl dissolved
AT WS VT 100 M1
= ------ × ------ × DS × ---- × DT× ------ × ------ × P
AS VS WT LC M2
AT 20 5 900 10 100 277.4
= ---- × ------ ×---- × ---- × ----- × ----- × ------- × 99.4
AS 200 10 152.2 5 32 313.86
Where,
AT = Peak area due to Sample preparation
DS = Dilution of the standard
AS = Peak area due to working standard preparation
DT = Dilution of the sample
WS = Weight of Working standard taken in mg
M1 = Molecular weight of Venlafaxine
WT = Weight of Sample taken in mg
M2 = Molecular weight of Venlafaxine HCl
VS = Volume of mobile phase to dissolve working standard
VT = Volume of dissolution medium (basic buffer)
P = (%) Purity of working standard used
LC = Label claim
Kinetic study [11]
Mathematical modeling and comparison of dissolution profiles:
Release kinetic studies of all SR formulations S1-S4 was studied using mathematical models zero order, first order, higuchi, korsmeyer- peppas. The model which best fits the dissolution profile of various formulations was chosen.
Similarity factor and dissimilarity factor:
Moore & Flanner proposed a model independent mathematical approach to compare the dissolution profile using two factors f1, f2.
Dissimilarity factor f1 = {[Σt=1 n|Rt - Tt|] / [Σt=1 n Rt]}. 100
Similarity factor f2 = 50. log {1 + (1/n) [Σt=1 n (Rt - Tt) 2] – 0.5}. 100
Where Rt, Tt are the cumulative percentage dissolved at each of the selected n time point of the reference & test product respectively.
9. Similarity factor f2 and its significance
|
S.No |
Similarity factor |
Significance |
|
1 |
<50 |
Test and reference profiles are dissimilar |
|
2 |
50 - 100 |
Test and reference profiles are similar |
|
3 |
100 |
Test and reference profiles are identical |
|
4 |
>100 |
The equation yields a negative value |
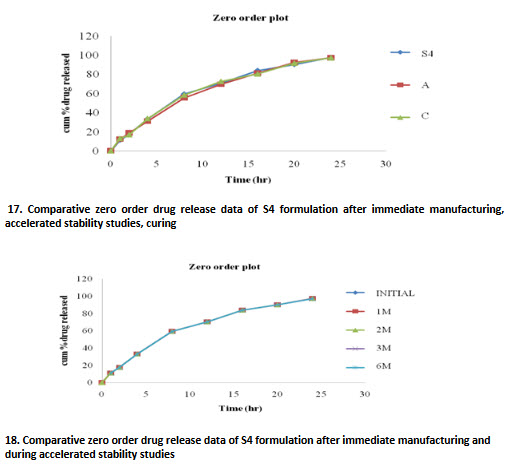
Curing effect: According to Polymer chemistry and process engineering, curing refers to the toughening or hardening of a polymer material by cross-linking of polymer chains, brought about by chemical additives, ultraviolet radiation or heat. The pellets of S4 were cured in a hot air oven for 16 hours at 55 ºC to check the effect of curing on drug release; the cured pellets are subjected for in vitro release studies.
Stability conditions according to ICH guidelines: [12]
The stability of pharmaceutical preparation should be evaluated by accelerated stability studies. The optimized formulation S4 of Venlafaxine hydrochloride SR pellets was selected for the stability studies. The pellets were evaluated for physical appearance, assay, in vitro dissolution studies and compared with pellets which were evaluated immediately after manufacturing.
10. Long term, accelerated, and, intermediate storage conditions for drug substances
|
Study |
Storage condition |
Minimum time period covered by data at submission |
|
Long term |
25°C ± 2°C/60% RH ± 5% RH or 30°C ± 2°C/65% RH ± 5% RH |
12 months |
|
Intermediate |
30°C ± 2°C/65% RH ± 5% RH |
6 months |
|
Accelerated |
40°C ± 2°C/75% RH ± 5% RH |
6 months |
Results & discussion:
Analytical methods:
Calibration table of venlafaxine HCl working standard
|
Concentration in ppm |
Mean Peak Area (N=3) |
|
0 |
0 |
|
50 |
51043.8±0.3 |
|
100 |
103463.4±0.5 |
|
150 |
149327.2±0.4 |
|
200 |
204588.9±0.6 |
|
250 |
256138.2±0.8 |
|
300 |
316965.6±0.7 |
|
350 |
364387.4±0.6 |
All values are expressed as mean ±S.D, n=3
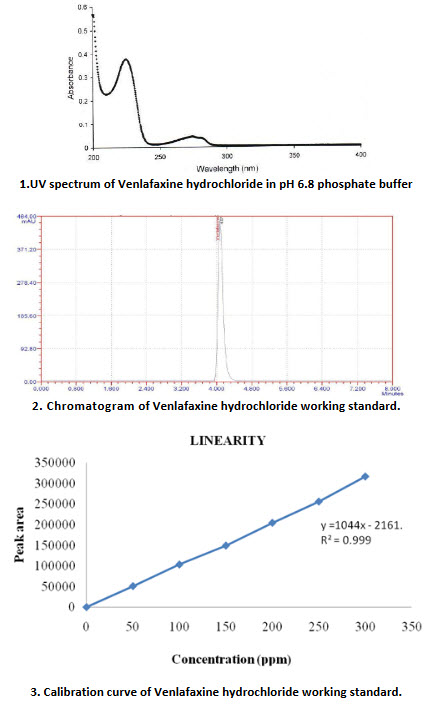
12.Venlafaxine hydrochloride characterization
|
S.No |
Test |
Specification |
Result |
|
1 |
Description |
White to off - White crystalline powder |
White crystalline powder |
|
2 |
Solubility |
Freely soluble in water |
Complies |
|
3 |
Water content ( by Karl Fischer) |
< 1.5% |
0.83% |
|
4 |
Bulk density |
|
0.25 g/cc |
|
5 |
Tapped density |
|
0.33 g/cc |
|
6 |
Hausner’s ratio |
1.26 -1.34 |
1.32 |
|
7 |
Compressibility Index (%) |
21 – 25 |
24.2 % |
|
8 |
Melting point |
215°C -217°C |
215°C |
|
9 |
Loss on drying (LOD) |
? 0.5% w/w |
0.2 %w/w |
|
10 |
Assay |
98% w/w -102% w/w |
99.1% |
|
11 |
Particle size analysis |
10 -40 µm |
20 µm |
|
12 |
Purity |
≥ 99.4% |
99.4% |
13. Physical observation of drug-excipient compatibility studies
|
S.No. |
Composition details |
Observations |
|||
|
Storage condition / Duration |
|||||
|
Initial |
40°C/ 75%RH |
||||
|
1st M |
2nd M |
3rd M |
|||
|
1 |
Venlafaxine HCl |
White to off - white powder |
NCC |
NCC |
NCC |
|
2 |
Venlafaxine HCl + Sugar spheres |
White to off - white powder |
NCC |
NCC |
NCC |
|
3 |
Venlafaxine HCl + Crospovidone INF-10 |
White to off - white powder |
NCC |
NCC |
NCC |
|
4 |
Venlafaxine HCl + Mannitol |
White to off - white powder |
NCC |
NCC |
NCC |
|
5 |
Venlafaxine HCl + TEC |
White to off - white powder |
NCC |
NCC |
NCC |
|
6 |
Venlafaxine HCl + PVPK30 |
White to off - white powder |
NCC |
NCC |
NCC |
|
7 |
Venlafaxine HCl + HPMC E3 |
White to off - white powder |
NCC |
NCC |
NCC |
|
8 |
Venlafaxine HCl + SLS |
White to off - white powder |
NCC |
NCC |
NCC |
|
9 |
Venlafaxine HCl + EC 7 cps |
White to off - white powder |
NCC |
NCC |
NCC |
|
10 |
Venlafaxine HCl + MCCP |
White to off - white powder |
NCC |
NCC |
NCC |
|
11 |
Venlafaxine HCl + IPA |
White to off - white powder |
NCC |
NCC |
NCC |
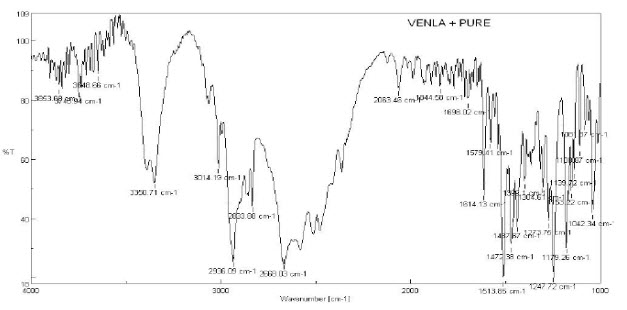
4. FT-IR spectrum of pure Venlafaxine hydrochloride
14. Interpretation of Venlafaxine hydrochloride
|
Functional Group |
Peaks of functional groups (cm-1) |
|
CH- stretching (Aromatic) |
3014.19 |
|
CH- stretching (Aliphatic) |
2936.09 |
|
C=C stretching |
1614.13 |
|
C-N stretching |
1513.85 |
|
C-O stretching |
1437.67 |
|
OH stretching |
3350.71 |
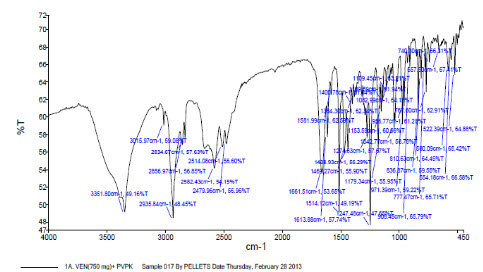
5. FT-IR spectrum of physical mixture of PVPK30 and Venlafaxine hydrochloride
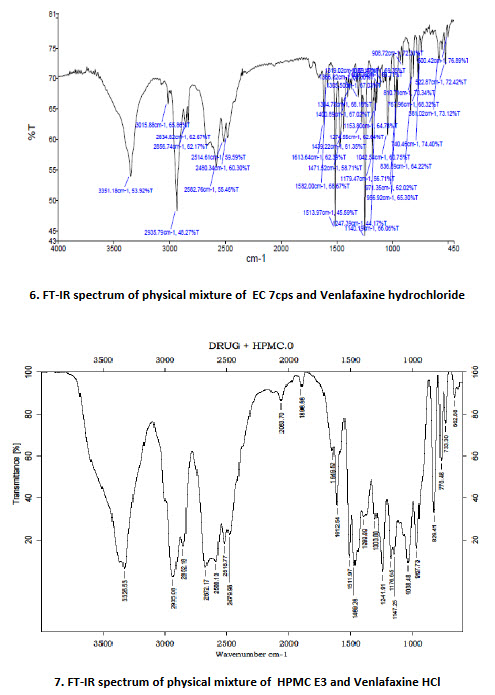
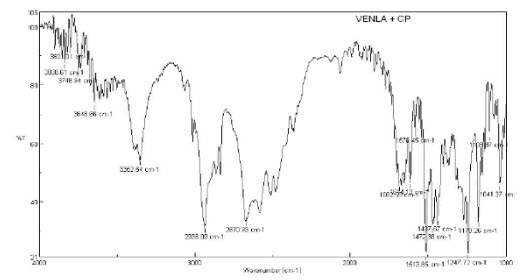
8.FT-IR spectrum of physical mixture of CP (INF-10)and Venlafaxine HCl
15. Interpretation of CP (INF-10) and Venlafaxine HCl
|
Functional Group |
Peaks of functional groups (cm-1) |
|
CH- stretching (Aromatic) |
3012.01 |
|
CH- stretching (Aliphatic) |
2936.09 |
|
C=C stretching |
1614.13 |
|
C-N stretching |
1513.85 |
|
C-O stretching |
1437.01 |
|
OH stretching |
3352.64 |
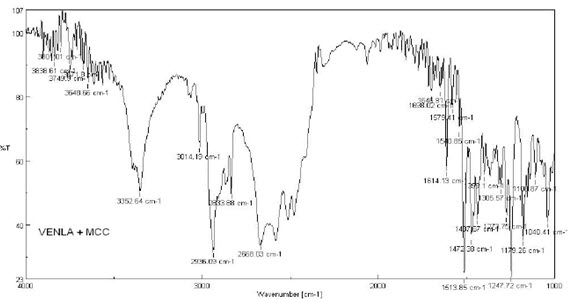
9. FT-IR spectrum of physical mixture of MCCP and Venlafaxine HCl
16. Interpretation of MCCP and Venlafaxine HCl
|
Functional Group |
Peaks of functional groups (cm-1) |
|
CH- stretching (Aromatic) |
3014.19 |
|
CH- stretching (Aliphatic) |
2936.09 |
|
C=C stretching |
1614.13 |
|
C-N stretching |
1513.85 |
|
C-O stretching |
1437.67 |
|
OH stretching |
3352.64 |
17. Percentage (%) yield values
|
Drug loaded pellets (INF -10)% |
D1 (1.5%) |
D2 (3%) |
D3 (4.5%) |
D4 (6%) |
Optimized formulation |
|
Percentage (%) yield |
87.2 ± 0.2 |
92.7 ± 0.2 |
94.8 ± 0.2 |
85.5 ± 0.2 |
D3 |
|
Barrier pellets HPMC E3 % |
B1 (4%) |
B2 (6%) |
B3 |
|
|
|
Percentage (%) yield |
89.1 ± 0.3 |
93.9 ± 0.3 |
87.7 ± 0.3 |
|
B2 |
|
SR pellets EC 7cps % |
S1 (2%) |
S2 (5%) |
S3 (6%) |
S4 (8%) |
|
|
Percentage (%) yield |
81.3± 0.3 |
85.9± 0.2 |
89.3± 0.4 |
93.1± 0.2 |
S4 |
All values are expressed as mean ±S.D, n=3
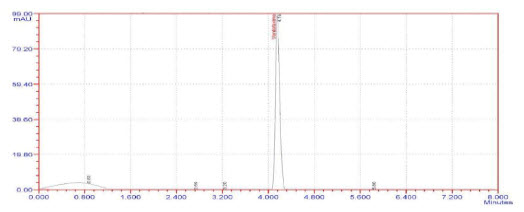
10. Chromatogram of Venlafaxine HCl SR pellets (S4)
18. Results of assay (%) values
|
Drug loaded pellets (INF -10)% |
D1 (1.5%) |
D2 (3%) |
D3 (4.5%) |
D4 (6%) |
Optimized formulation |
|
Assay (%) |
27.07±0.52 |
30.74±0.63 |
32.82±0.71 |
28.71±0.67 |
D3 |
|
Barrier pellets HPMC E3 % |
B1 (4%) |
B2 (6%) |
B3 (8%) |
|
|
|
Assay (%) |
30.03±0.22 |
32.45±0.17 |
28.24±0.21 |
|
B2 |
|
SR pellets EC 7cps % |
S1 (2%) |
S2 (5%) |
S3 (6%) |
S4 (8%) |
|
|
Assay (%) |
29.35±0.17 |
30.14±0.23 |
30.74±0.34 |
32.19±0.25 |
S4 |
All values are expressed as mean ±S.D, n=3
Optimization studies of pellets:
Optimization of drug coating:
Four batches (D1-D4) of drug coated pellets were formulated by varying concentration of crospovidone INF – 10 (1.5%, 3%, 4.5%, 6%) through powder layering technique. Then the drug coated pellets were analyzed for the amount of drug i.e. assay (%), Percentage (%) yield. D1 showed less practical yield and drug content. In D1 formulation breakage of pellets and process problems observed during coating leads to insufficient coating. In D4 formulation drug loss observed due to lumps formation during coating. D2 and D3 formulations show optimum values of percentage yield and assay values. Among them D3 formulation was finalized for further coating stages, i.e. barrier coating.
Optimization of barrier coating:
Barrier coating was given to D3 drug coated pellets by using fluidized bed coating. Three batches (B1-B3) were developed with D3 drug coated pellets. Main aim of Barrier/sub coating was given to pellets to protect the drug coated pellets from SR coating and environmental conditions. It also increases the shelf life of product. Sub coating was done with different polymer concentration of HPMC E3 (4%, 6%, 8%) to get enough mechanical strength and weight gain to the pellets during coating process. In B1 and B3 formulations, yield was found to be low. Hence these formulations don’t show better protection for drug coated pellets. In B3 formulation, process problems like lumps formation were observed. In B1 formulation the concentration of HPMC was found to be insufficient for barrier coating. So optimum percentage of sub coating i.e., B2 formulation was finalized for further coating stages, i.e. SR coating
Optimization of SR coating:
SR coating was given to B2 barrier coated pellets by using fluidized bed coating. Four batches (S1-S4) were developed with B2 barrier coated pellets.In S4 formulation EC, HPMC and Triethyl citrate concentrations were increased for better film formation there by better protection was obtained to drug coated pellets. Further these SR formulations subjected to evaluation tests like flow properties, friability, sieve analysis, and in vitro drug release studies. S4 showed good percentage yield and its release profile compiles with the marketed product which is the main aim of the present study. During coating process lumps were not observed in S4 formulation.
From the above trails it was concluded that S4 formulation was optimized for SR coating.
Sieve analysis:
19. Sieve analysis of Venlafaxine hydrochloride SR pellets
|
SR pellets EC 7cps % |
S1 (2%) |
S2 (5%) |
S3 (6%) |
S4 (8%) |
|
% ofpellets retained through sieve 18# |
4.3± 0.2 |
3.7± 0.3 |
2.4± 0.4 |
1.5± 0.2 |
|
%ofpellets passed through sieve 18# |
95.7± 0.2 |
96.3± 0.3 |
97.6± 0.4 |
98.5± 0.2 |
|
Percentageofpellets retained through sieve 20# (%) |
8.7± 0.4 |
7.5± 0.2 |
6.3± 0.2 |
5.4± 0.3 |
|
Percentageofpellets passed through sieve 20# (%) |
91.3± 0.4 |
92.5± 0.2 |
93.7± 0.2 |
94.6± 0.3 |
|
Percentageofpellets retained through sieve 25# (%) |
92.7± 0.3 |
93.5± 0.2 |
94.3± 0.4 |
95.6± 0.2 |
All values are expressed as mean ±S.D, n=3
Particle size distribution and determination:
20. Weight distribution of Venlafaxine hydrochloride SR pellets
|
# No. |
Nominal # mesh Aperture Size, μm |
Aperture size (passed/ Retained), μm |
Mean size Open- ing d, μm |
% weight retained on |
Weight size n x d |
||||||
|
S1 |
S2 |
S3 |
S4 |
S1 (2%) |
S2 (5%) |
S3 (6%) |
S4 (8%) |
||||
|
FBC |
_ |
_ |
_ |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
14 |
1400 |
1400/FBC |
1400 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
16 |
1180 |
1180/1400 |
1290 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
18 |
1000 |
1000/1180 |
1090 |
4.3 |
3.7 |
2.4 |
1.5 |
4687 |
4033 |
2616 |
1635 |
|
20 |
850 |
850 /1000 |
925 |
8.7 |
7.5 |
6.3 |
5.4 |
8047.5 |
6937.5 |
5827.5 |
4995 |
|
25 |
710 |
710 /850 |
780 |
92.7 |
93.5 |
94.3 |
95.6 |
72306 |
72930 |
73554 |
74568 |
|
|
|
|
|
Σ(n) = 105.7 |
Σ(n) = 104.7 |
Σ(n) = 103 |
Σ(n) = 102.5 |
Σ(nd) = 85040.5 |
Σ(nd) = 83900.5 |
Σ(nd) = 81997.5 |
Σ(nd) = 81198 |
|
Average particle size μm |
804.5 μm |
801.34 μm |
796 μm |
792.17 μm |
|||||||
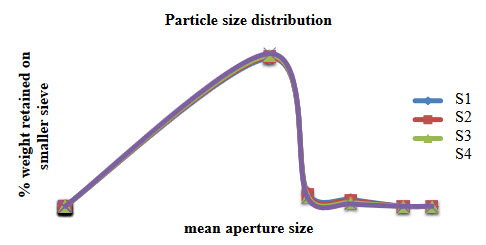
11.Particle size distribution of Venlafaxine hydrochloride SR pellets
Flow Properties of Venlafaxine hydrochloride SR pellets:
21. Results of flow properties of SR pellets
|
Formulation code |
Bulk density ±SD (g/cc) |
Tapped density±SD (g/cc) |
Angle of repose ±SD (q) |
Compressibility index (%) ±SD |
Haussner’s ratio ±SD |
|
S1 |
0.628±0.009 |
0.712 ±0.002 |
32.6±0.01 |
11.797±0.001 |
1.133±0.001 |
|
S2 |
0.655±0.001 |
0.742 ±0.001 |
31.8±0.02 |
11.725±0.009 |
1.132±0.004 |
|
S3 |
0.686±0.002 |
0.775 ±0.006 |
29.87±0.030 |
11.483±0.003 |
1.129±0.003 |
|
S4 |
0.702±0.004 |
0.793±0.007 |
27.8±0.03 |
11.475±0.002 |
1.129±0.006 |
All values are expressed as mean ±S.D, n=3
|
Formulation code |
Friability ±SD (%) |
%water content ±SD |
|
S1 |
0.563±0.033 |
2.05±0.04 |
|
S2 |
0.459±0.052 |
1.97±0.02 |
|
S3 |
0.326±0.131 |
1.85±0.03 |
|
S4 |
0.179±0.064 |
1.70±0.02 |
22. Results of physicochemical properties of the pellets
All values are expressed as mean ±S.D, n=3
Scanning Electron Microscopy (SEM)
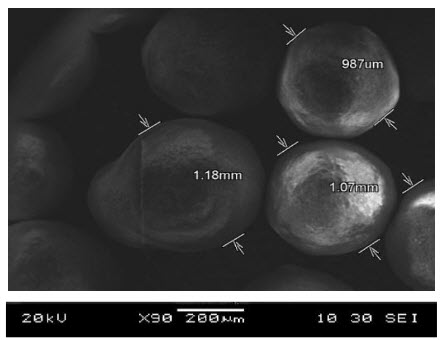
12. SEM photographs of SR pellets (S4) of Venlafaxine hydrochloride
In vitro dissolution studies:
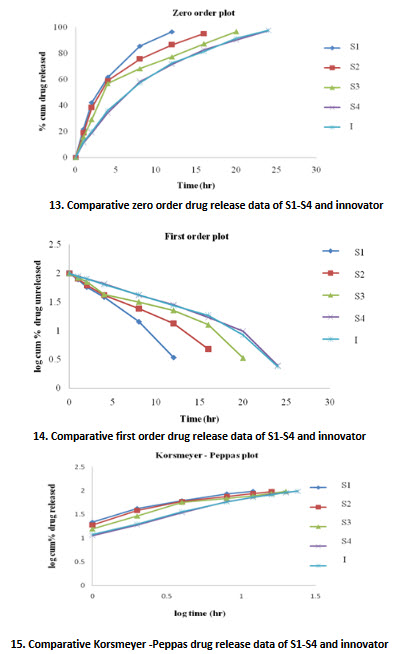
Dissolution data of Venlafaxine hydrochloride pellets
23. Dissolution values of SR pellets of Venlafaxine hydrochloride
|
Time (hr) |
S1 |
S2 |
S3 |
S4 |
Innovator |
|
0 |
0 |
0 |
0 |
0 |
0 |
|
1 |
21.83±0.03 |
18.79±0.02 |
15.61±0.07 |
11.19±0.09 |
12.05±0.02 |
|
2 |
42.16±0.02 |
38.26±0.03 |
29.15±0.03 |
18.73±0.02 |
19.85±0.08 |
|
4 |
61.43±0.04 |
58.98±0.05 |
56.79±0.05 |
34.15±0.03 |
35.93±0.09 |
|
8 |
85.62±0.07 |
75.61±0.03 |
68.23±0.04 |
58.29±0.08 |
57.32±0.03 |
|
12 |
96.58±0.05 |
86.59±0.04 |
77.32±0.03 |
71.46±0.07 |
72.55±0.06 |
|
16 |
|
95.23±0.06 |
87.29±0.08 |
83.86±0.03 |
81.29±0.03 |
|
20 |
|
|
96.65±0.06 |
90.12±0.04 |
91.53±0.05 |
|
24 |
|
|
|
97.53±0.02 |
97.61±0.02 |
All values are expressed as mean ±S.D, n=3
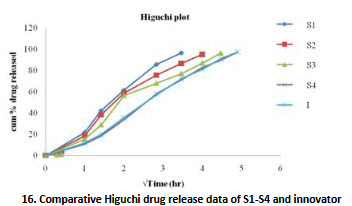
16. Comparative Higuchi drug release data of S1-S4 and innovator
24. Kinetic data of venlafaxine HCl SR pellets at different graphical plots
|
Formulation Code |
Zero order |
First order |
Higuchi |
Korsmeyer Peppas |
||||
|
K0 |
r |
K1 |
r |
KH |
r |
n |
R |
|
|
S1 |
9.588 |
0.764 |
0.2717 |
0.990 |
28.74 |
0.983 |
1.395 |
0.965 |
|
S2 |
7.214 |
0.694 |
0.1773 |
0.989 |
25.15 |
0.981 |
1.358 |
0.945 |
|
S3 |
5.767 |
0.715 |
0.1473 |
0.952 |
22.34 |
0.977 |
1.277 |
0.944 |
|
S4 |
4.776 |
0.876 |
0.1358 |
0.952 |
19.89 |
0.979 |
1.080 |
0.988 |
|
Innovator |
4.790 |
0.870 |
0.1381 |
0.952 |
19.98 |
0.984 |
1.112 |
0.989 |
25. Determination of dissimilarity factor(f1) and similarity factor(f2)
|
DIFFERENCE FACTOR (f1) & SIMILARITY FACTOR (f2) |
|||||
|
Time(t) [in Hours] |
Reference ® |
Test (T) |
Rt-Tt |
(Rt-Tt)² |
|Rt-Tt| |
|
Innovator |
S4 |
||||
|
0 |
0 |
0 |
0 |
0 |
0 |
|
1 |
12.05 |
11.19 |
0.86 |
0.7396 |
0.86 |
|
2 |
19.85 |
18.73 |
1.12 |
1.2544 |
1.12 |
|
4 |
35.93 |
34.15 |
1.78 |
3.1684 |
1.78 |
|
8 |
57.32 |
58.29 |
-0.97 |
0.9409 |
0.97 |
|
12 |
72.55 |
71.46 |
1.09 |
1.1881 |
1.09 |
|
16 |
81.29 |
82.86 |
-1.57 |
2.4649 |
1.57 |
|
20 |
91.53 |
90.12 |
1.41 |
1.9881 |
1.41 |
|
24 |
97.61 |
97.53 |
0.08 |
0.0064 |
0.08 |
|
Sum |
468.13 |
|
|
11.7508 |
8.88 |
|
Number of Time points or intervals (Excluding Zero) |
8 |
||||
|
Difference Factor - f1 [ Acceptance Criteria : 0 - 15] |
1.896 |
||||
|
Similarity Factor - f2 [ Acceptance Criteria : 50 - 100] |
77.773 |
||||
26. Results of optimized formulation S4 during stability studies
|
Parameter |
Temperature 40ºC / 75% RH |
||||
|
Time |
Initial |
1st month |
2nd month |
3rd month |
6th month |
|
Physical appearance |
- |
No Change |
No Change |
No Change |
No Change |
|
In-vitro drug release (%) |
97.53 |
97.52 |
97.50 |
97.48 |
97.46 |
26. Optimized formulation
|
OPTIMIZED FORMULATION FOR VENLAFAXINE HYDROCHLORIDE PELLETS 32%W/W |
|||||
|
DRUG LOADING |
|||||
|
S.NO |
INGREDIENTS |
FORMULATION |
|||
|
|
Crospovidone INF -10 (%) |
D3 (4.5%) |
|||
|
|
BATCH SIZE (kg) |
3.000 |
|||
|
1 |
Venlafaxine HCl |
0.977 |
|||
|
2 |
Sugar spheres (20#25) |
1.080 |
|||
|
3 |
Crospovidone INF -10 |
0.135 |
|||
|
4 |
Sodium Lauryl Sulphate |
0.030 |
|||
|
5 |
Mannitol |
0.231 |
|||
|
6 |
MCCP |
0.135 |
|||
|
7 |
PVP K 30 |
0.012 |
|||
|
8 |
Purified water |
q.s. |
|||
|
TOTAL QUANTITY (DRUG PELLETS) |
2.600 |
|
|||
|
BARRIER COATING |
|
||||
|
S.NO |
INGREDIENTS |
FORMULATION |
|
||
|
|
HPMC E3 (%) |
B2 (6 %) |
|
||
|
1 |
Drug pellets |
2.600 |
|
||
|
2 |
HPMC E3 |
0.156 |
|
||
|
3 |
Purified water |
2.229 |
|
||
|
TOTAL QUANTITY (BARRIER PELLETS) |
2.765kg |
|
|||
|
SR COATING |
|
||||
|
S.NO |
INGREDIENTS |
FORMULATION |
|
||
|
|
EC 7cps (%) |
S4 (8%) |
|
||
|
1 |
Barrier pellets |
2.756 |
|
||
|
2 |
Ethyl cellulose 7 cps |
0.220 |
|
||
|
3 |
HPMC 3 cps |
0.028 |
|
||
|
4 |
Triethyl citrate |
0.019 |
|
||
|
5 |
Isopropyl alcohol |
2.124 |
|
||
|
6 |
Purified water |
q.s. |
|
||
|
TOTAL QUANTITY (SR PELLETS) |
3.023 |
|
|||
Conclusion:
The aim of the present study was to formulate and evaluate a stable Venlafaxine hydrochloride sustained release pellets which are pharmaceutically equivalent equivalent to innovator Effexor XR® (f2 > 50).The formulation process was carried out in FBP by wurster technique. The work was carried out to extend/prolong the release of Venlafaxine hydrochloride by using different polymers such as EC, HPMC. The study includes preformulation study of drug and excipients, formulation and evaluation, release kinetics and stability studies of pellets.
Preformulation studies were performed on the drug and excipients used in the formulations were found to be compatible. No drug and excipient reactions were observed.Drug-excipient interaction studies were carried out by FT-IR in order to indicate the compatibility of drug with the polymers. The results revealed that the drug and excipients were satisfactorily compatible, without any significant changes in the chemical nature of the drug.
Flow properties evaluated showed that the optimized formulation has good flow properties. Drug content and content uniformity of the optimized formulation was found to be good and gave reproducible results. Based on the in vitro release studies, S4 was considered as optimized formulation which extends the drug release upto 24hrs and showed 97.53% drug release. Different kinetic models were applied to optimized formulation S4 and observed that it follows first order release kinetics and mechanism of drug release is by Higuchi model (n > 1), indicated that the drug transport mechanism by super case - II transport. The optimized S4 formulation was found as pharmaceutically equivalent to innovatordue to similarity (f2 =77.77) in drug release profile.
Stability studies were conducted on the optimized formulation S4 at 40°C/ 75% RH (accelerated stability testing) for 6 months according to ICH guidelines. Dissolution release profile and physical appearance of optimized formulation S4 showed that there was no significant difference in physicochemical parameters (p < 0.05) during the stability study.
It was concluded that the order of extending the release of the drug increase with the increase in the coating concentration of the polymer. The dissolution data revealed that the level of coating and the ratio of polymers are very important to achieve optimum formulation.
References:
1. Follonier N., Doelker E. Biopharmaceutical comparasion of oral multiple-unit and single unit sustained release dosage forms, STP Pharma Sciences.1992; 2: 141-145.
2. Vial-Bernasconi A. C., Doelker E., Buri P. Prolonged release capsules divided and monolithic forms, STP Pharma Sciences.1988; 4: 397-409.
3. NS Dey, S Majumdar, MEB Rao. Multiparticulate Drug Delivery Systems for Controlled Release, Tropical J. Pharm. Res. September 2008; 7 (3): 1067-1075.
4. Lachman Leon, Liberman H.A and Kanig J.L. The Theory and Practice of Industrial Pharmacy, 3rd ed., Varghese publishing house, 1991.p. 293-294.
5. Bechgaard. H., Nielsen.G. Sustained Release multiple units and single unit doses, Drugs. Dev. Ind. Pharm.; 1978; 4:53-57.
6. Isaac Ghebre-Sellassie., Axel knoch. Pelletization Techniques, In: James Swarbrick editor. Encyclopedia of Pharmaceutical Technology, 3rd ed.; Informa healthcare: New York; 2007. p. 2651-2663.
7. Jagan Mohan Kandukuri., Venkatesham Allenki., et al. Pelletization techniques for oral drug delivery, Int. J. Pharm. Sci. and Drug Res. 2009, 1: 63-70.
8. Smith D, Dempster C, Glanville J, Freemantle N, Anderson I. Efficacy and tolerability of venlafaxine compared with selective serotonin reuptake inhibitors and other antidepressants: A meta-analysis, Br J Psychiatry. 2002, 180: 396-404.
9. Tripathi KD. Essential of medical pharmacology, 5th ed. Jaypee Brothers Medical Publishers (P) Ltd: New Delhi; 2004.
10. Morriton R.C. Excipient interaction, In: K.Katdare A, Chaubal M.V. Excipient Development for Pharmaceutical Biotechnology and Drug Delivery System. New York: Informa Healthcare USA, INC, 2006, p: 101-104
11. Raveendra Pai. Extended release matrix pellets: preparation and compression into disintegrating tablet, IJDD. 2011:329-339.
12. ICH Harmonised Tripartite Guideline, Stability Testing of New drug substances and products Q1A (R2) 4th Version dated February 2003.
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 1 Received On: 19/012/2014; Accepted On: 23/12/2014; Published On: 15/01/2014 How to cite this article: A Nidadavolu, Formulation Development and In Vitro Characterization of Sustained Release Pellets of Venlafaxine HCl, PharmaTutor, 2014, 2(1), 129-156 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









