{ DOWNLOAD AS PDF }
 ABOUT AUHTORS
ABOUT AUHTORS
Naveen Kumar G.S* 1, U. Srinivas 2, Hanumanthchar Joshi 3,
1 Department of Pharmaceutical Analysis,
Sarada Vilas College of pharmacy,
Mysuru, Karnataka, India
2 Department of Pharmacognosy,
Srinivas College of pharmacy, Mangalore, Karnataka India,
3 Department Pharmacognosy,
Sarada Vilas College of pharmacy, Mysuru, Karnataka India
*premukhoja@gmail.com
ABSTRACT
An UV spectrophotometric method using simultaneous equation was developed for the simultaneous determination of meclizine and folic acid in a binary mixture. In the proposed method, the signals were measured at 390.0 nm and 237.0 nm corresponding to absorbance maxima of and folic acid in methanol respectively. Linearity range was observed in the concentration range of 2-12 µg/ml for both the drugs. Concentration of each drug was obtained by using the absorptivity values calculated for both drugs at two wavelengths, 390.0 nm and 237.0 nm and solving the simultaneous equation. The method was validated and proposed method was fast, accurate and precise so it can be used for regular quality control of the drug.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2496
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 5, Issue 6 Received On: 17/02/2017; Accepted On: 06/03/2017; Published On: 01/06/2017 How to cite this article: Naveen KGS, Srinivas U, Joshi H;Development and Validation of Spectrophotometric Method for simultaneous estimation of Meclizine and Folic Acid in Bulk and Pharmaceutical Dosage Forms; PharmaTutor; 2017; 5(6);29-34 |
INTRODUCTION
Meclizine HCl is piperazine 1-[(4-chlorophenyl) phenylmethyl]-4-[(3-methylphenyl) methyl]-dihydrochloride monohydrate is an antiemetic agent used in post-operative vomiting [1, 3]. (Figure 1) a piperazine derivative and antihistamine with anti muscarinic and central sedative properties, mainly used for its antiemetic action and in the prevention and treatment of nausea and vomiting associated with a variety of conditions. Folic acid is chemically 4-(2-amino-4-hydroxypteridin-6-yl) methyl amino benzoyl-l-glutamic acid, part of the vitamin B group (vitamin B9) is a water soluble vitamin (Figure 2). It is one of the most important coenzyme of the haemopoietic systems that control the generation of ferrohaeme[4-5]. Marketed tablet formulations of these agents play an important role in the treatment of persistent nausea and vomiting during pregnancy.

The review of literature reveals that there were analytical methods of two drugs individually in pharmaceutical dosage forms and even in biological samples and few methods reported for either of one drug with combination of another drug. Few analytical methods like RP-HPLC, HPTLC, HPLC, Spectroscopic methods have been reported for the determination of meclizine and its combination with other drugs in pharmaceutical preparations [6-10]. Estimation of folic acid in combination with other drugs by spectrophotometric methods and HPLC has been reported [11-14], but there is no work in the literature reported about the simultaneous determination of meclizine and folic acid in a binary mixture for the analysis of pharmaceutical formulation. Thus there is need to develop a simple rapid and economical method for routine analysis of meclizine and folic acid. The objective of present study was to develop and validate simple, sensitive, accurate, precise, rapid and economical method for estimation of Meclizine and folic acid in bulk and in pharmaceutical formulations.
MATERIALS AND METHODS
Instruments
(a) Spectrophotometer- Double beam UV–Visible spectrophotometer with 1 cm matched quartz cell Model UV-2450 Shimadzu.
(b) Electronic Balance- Shimadzu.
Reagents and Chemicals
Meclizine Hydrochloride and folic acid reference standard were kindly provided by Hetero Pharmaceuticals, Hyderabad for providing gift sample of working standard. Methanol of analytical grade was used as a solvent. All chemicals and reagents used were of analytical reagent grade.
Marketed Preparation
The brand name of marketed combined tablet formulation is PNV PLUS containing Meclizine Hydrochloride 25 mg and Folic acid 2.5 mg manufactured by Yash Pharma. Lab. Pvt. Ltd.
Study of Overlain Spectra and Selection of Wavelength
Solutions of Meclizine Hydrochloride and Folic acid were scanned between 200and 400nm. From the overlay spectra (Figure 3) twowave lengths, 390.0nm and 237.0nm were selectedand absorptivity values E (1%, 1cm) of both the drugsat both wavelengths were determined for formation of simultaneous equation.
Cx = (A2ay1-A1ay2)/ (ax2ay1-ax1ay2) (1)
Cy = (A1ax2-A2ax1)/ (ax2ay1-ax1ay2) (2)
Where, A1 and A2 are absorbances of mixture at390nm and 237nm respectively, ax1 and ax2 are
absorptivities of Meclizine Hydrochloride at λ1and λ2 respectively and ay1 and ay2 are absorptivities of Folic acid at λ1and λ2respectively. Cx and Cy are concentrations of Meclizine Hydrochloride and Folic acid respectively.
Preparation of Solutions
Meclizine Hydrochloride Standard Stock Solution
Accurately weighed Meclizine Hydrochloride (10mg) was transferred in100 ml volumetric flask. The drug was dissolve in methanol with sonication and final volume was adjusted with methanol up to mark to prepare a100μg/ml stock solution (Figure 3).
Meclizine Hydrochloride Working Standard Solution
From the above stock solution (100μg/ml), an accurately measured 0.2, 0.4, 0.6, 0.8, 1.0 and 1.2 ml was transfer into separate 10 ml volumetric flask and final volume was adjusted with methanol upto mark to prepare 2-12μg/ml solutions.
Folic acid Standard Stock Solution
Accurately weighed Folic acid (10mg) was transferred in100 ml volumetric flask. The drug was dissolve in methanol with sonication and final volume was adjusted with methanol up to mark to prepare a100μg/ml stock solution.
Folic acid Working Standard Solution
From the above stock solution (100μg/ml), an accurately measured 0.2, 0.4, 0.6, 0.8, 1.0 and 1.2 ml was transfer into separate 10 ml volumetric flask and final volume was adjusted with methanol up to mark to prepare 2-12μg/ml solutions.
Sample Solution
Transfer powder exactly equivalent to laboratory mixture containing 25 mg of Meclizine Hydrochloride and 2.5mg of Folic acid to a 100 ml volumetric flask. Add about 60 ml of methanol and sonicate for 15 minutes solution was filtered through Whatmann No.1 filter paper. The residue was washed with 10ml methanol three times and make up volume with methanol. From this solution take 1ml and dilute up to 50ml with methanol. Again take 1ml and dilute up to 10ml with methanol.
Method Validation
Linearity
The linearity was evaluated by linear regression analysis. The calibration curve was obtained with concentrations of pure Meclizine Hydrochloride and Folic acid solution ranging from 2-12μg/ml and 2-12μg/ml respectively for the chromatographic method.
Precision
The precision of the procedure was determined by repeatability (intraday). Intraday precision was evaluated by assaying same concentration and during the same day. Repeatability of sample measurement was carried out in six different sample preparations from same homogenous blend of sample. Another replicate determination on three different days to estimate inter day precision.
Accuracy
Recovery studies were performed to validate the accuracy of developed method. To a pre analysed sample solution, a definite concentration of standard drug was added and recovery was studied. A80%,100% and 120% of pure drug solutions were added to the pre analyzed samples.
Limit of Detection and Limit of Quantification
The limits of detection (LOD) and limit of quantification (LOQ) were calculated based on the standard deviation of the response and the slope by using calibration curves.
Ruggedness
Ruggedness of the method was determined by analysis of aliquots from homogeneous slot by two analyst using same operational and environmental conditions.
Analysis of Laboratory Mixture
The absorbances of solutions were recorded at 390.0nm and 237.0nm against blank. Concentration of each drug was obtained by solving the simultaneous equation. (Table5).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULT AND DISCUSSION In this simultaneous equation method, the overlain spectra of drugs showed the λ max of 390.0nm and 237.0nm for Meclizine Hydrochloride and Folic acid respectively. Both the drugs obeyed linearity range 2-12μg/ml and correlation coefficient (r2) were found to be <1 in both cases. The data of regression analysis of the calibration curves is shown in Table 1.

Figure 3: Overlay UV Spectra of Meclizine and Folic acid
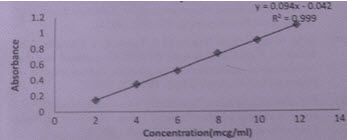
Figure 4: Calibration Curve for Meclizine Hydrochloride
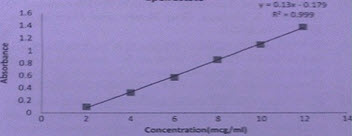
Figure 5: Calibration Curve for Folic acid
Table 1 Data of Regression Analysis and Calibration Curve
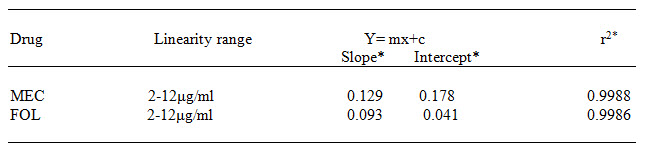
*=Average result of six replicate samples (Meclizine and Folic acid)
The absorptivity values were calculated and along with absorbances, these values were submitted in equation (1) and (2) to obtain concentration of drugs. The percentage purity of drugs in laboratory mixture was found to be 99.77±0.667% for Meclizine and 99.76±0.207 % for Folic acid. The accuracy of the method was determined by performing recovery study by standard addition method. The results were summarized in Table 2. The experiment was repeated three times in a day for intra-day and on three different days for inter-day precision. The results were summarized in Table 3. The LOD for Meclizine and Folic acid were found to be 0.067μg/ml and 0.069μg/ml respectively, while LOQ were 0.201μg/ml and 0.209μg/ml respectively. The method was found to be rugged as the percentage purity of the drugs determined by two different analysts and results were shown in Table 4. Marketed formulation was analyzed with the proposed method and the percentage purity of meclizine was found to be 99.72% w/w and for folic acid it was found to be 99.95% w/w in Table 6.
Table 2 Results of Recovery Studies
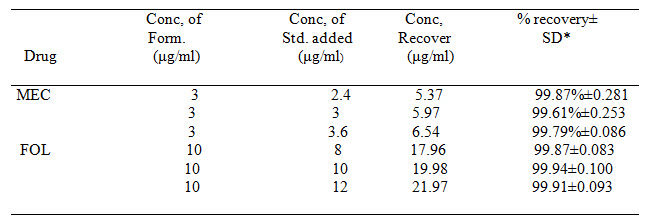
*=Average result of six replicate samples (Meclizine and Folic acid)
Table 3 Results of Intra Day & Inter Day Precision
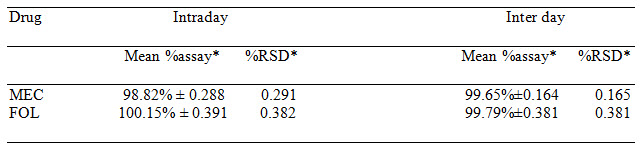
*= Average result of six replicate samples (Meclizine and Folic acid)
Table 4 Results of Ruggedness Study
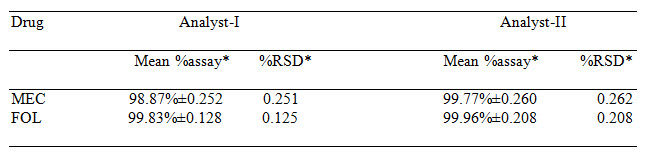
*= Average result of six replicate samples (Meclizine and Folic acid)
Table 5 Analysis of Laboratory Mixture

*= Average result of six replicate samples (Meclizine and Folic acid)
Table 6 Assay of Meclizine and Folic acid
|
Drug substance |
Label claim |
Percentage purity (% w/w) |
|
|
Taken |
Found |
||
|
Meclizine |
25 |
24.982 |
99.72 |
|
Folic acid |
2.5 |
2.478 |
99.95 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
ACKNOWLEDGEMENT
The authors are thankful to the Management, Sarada Vilas College of Pharmacy for their kind help and providing necessary facilities
REFERENCES
1. The Merck index; An Encyclopedia of Chemical; Drugs and Biologicals; Merck Research Laboratories; Division of Merck & Co Inc; White house Station NJ; 2001,Edn13. 1032
2. Martindale; The extra pharmacopoeia; Royal Pharmaceutical society of Great Britain; 1Lambeth High street London SE17 Jn England; 1996, Vol 31; 435–436.
3. British Pharmacopoeia; The Stationary office under license from the controller of Her Majesty’s stationary office for the department of health on behalf of the health Ministers; 2003, 1595.
4. Indian Pharmacopoeia; Ministry of health and family welfare, Government of India, New Delhi; 2011, Vol 2;1012-1013, 1510-1511.
5. Nafisa Y, Raju K.V and Sindu J., Development and Validation of UV-Spectrophotometric Method for Simultaneous Estimation of Meclizine Hydrochloride. World Journal of Pharmacy and Pharmaceutical Research, 2014, 3(10), 450-457.
6. Kumar M.S, Bairam R and Mahesh P., Formulation and Evaluation of Oral Disintegrating Release Dosage Form containing Meclizine HCl. International Journal of Pharmacy, 2012, 2(4), 717-26.
7. Hamid M.Y and Omera H.A., Spectrophotometric Micro Determination of Meclizine HCl in their Pharmaceutical Formulations and Spiked Plasma. World Journal of Pharmacy, 2014, 3(6), 102-112.
8.Reddy S, Karthik K.P and Kumar V., Simultaneous Estimation of Meclizine HCl and Nicotinic acid in Pharmaceutical Dosage Form by RP-HPLC Method. Applied Journal of Pharmaceutical Research, 2010, 5(2), 73-80.
9. Bari S.B and Kaskhedikar S.G., Simultaneous estimation of meclizine hydrochloride and caffeine in solid dosage form by employing multicomponent and derivative spectrophotometry. Indian Drugs, 1997, 2(34), 85–88.
10. Shinde S.A, Sayyed Z.M, Chaudhari B.P, Chaware V.J and Biyani K.R., Development and Validation of Spectrophotometric Method for Simultaneous Estimation of Meclizine Hydrochloride and Pyridoxine Hydrochloride in Tablet Dosage Form. Journal of Pharm Sci Bioscientific Res, 2016, 6(1), 137-143.
11. Throat D.B, Kalyani Pawar and Paunekar Ashwini., Method development and validation of folic acid by uv-visible spectroscopy. IJPSR, 2015, 6(7), 3088-3090.
12. Abera-Sturi FJ, Jime AI, Arias JJ and Jime F., Simultaneous spectrophotometric determination of folic acid, pyridoxine, riboflaavin, and thiamine by partial least-squares regression. Anal Lett 2002, 35(1), 1677-1691.
13. Rucha K. Patel, Nilam M. Patel, Samir K and Shah.,Development and validation of analytical methods for simultaneous estimation of ferrous ascorbate and folic acid in their combined dosage form.AsianJ.Pharm.Ana,2015,5(3),126-132.
14. Shivani K, Tulja G and Rani., Method development and validation for simultaneous estimation of atorvastatin, fenofibrate and folic acid in pharmaceutical dosage form. Int.J.Pharm Drug Anal, 2015,3 (4),140-149.
15. Pathak A and Rajput S.J., Simultaneous derivative spectrophotometric analysis of doxylamine succinate, pyridoxine hydrochloride and folic acid in combined dosage forms. Indian J Pharm Sci, 2008, 70(4), 513–517.
16.Sreeram V, Basaveswara Rao M.V and Srinivasa rao karumuri., A validated ultra high pressure liquid chromatographic method for qualification and quantification of folic acid in pharmaceutical preparations. International Journal of Chemical Studies, 2013,1(1), 17-27.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









