{ DOWNLOAD AS PDF }
ABOUT AUHTOR
Narendra M.Petha, J.G.Patil,Mr J.G.Chandorkar
Indofil Industries Ltd, Gujarat
jchandorkar-icc@modi.com
ABSTRCT
Gatifloxacin is a antibacterial agent, Rapid, sensitive and selective analytical method is essential for monitoring the different reactions steps involved in process development of Gatifloxacin. A simple isocratic reverse phase High Performance Liquid Chromatographic [HPLC] method was developed for simultaneous separation of different intermediates and other impurities. The method was utilized successfully in analyzing the reaction streams, related substances in final product and for the assay in drug.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2468
|
PharmaTutor (ISSN: 2347 - 7881) Volume 5, Issue 3 Received On: 08/11/2016; Accepted On: 22/11/2016; Published On: 01/03/2017 How to cite this article: Petha NM, Patil JG, Chandorkar JG;Development and Validation of High Performance Liquid Chromatography method for analysis of Gatifloxacin & its Impurity; PharmaTutor; 2017; 5(3); 37-41 |
INTRODUCTION: -
Impurity profiling is the common name of a group of analytical activities, the aim of which is the detection, identification/structure elucidation and quantitative determination of organic and inorganic impurities, as well as residual solvents in bulk drugs and pharmaceutical formulations. Since this is the best way to characterize the quality and stability of bulk drugs and pharmaceutical formulations, this is the core activity in modern drug analysis. Due to the very rapid development of the analytical methodologies available for this purpose and the similarly rapid increase of the demands as regards the purity of drugs it is an important task to give a summary of the problems and the various possibilities offered by modern analytical chemistry for their solution.
Gatifloxacin level in biological fluids, in different pharmaceutical formulations and as a raw material for related substances have previously been determined by spectrophotometric, gas chromatographic [HPLC] techniques. Literature surveys reveals hardly any method for the analysis of reaction mixture obtained in the preparation of Gatifloxacin. There is an increasing need for rapid and sensitive method for the determination of raw materials, intermediates and finished products in reaction stream during process development of Gatifloxacin. The HPLC system consisted of Jasco make UV/VIS detector model 1575, along with Borwin software (Integrator) were used. Analysis were performed on Stainless steel column containing C-18 packing, 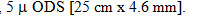
EXPERIMENTAL: -
CHROMATOGRAPHIC CONDITION: -
1) COLUMN: 250 mm x 4.6 mm i.e. - Stainless steel column containing C18, 5 u
2) MOBILE PHASE: BUFFER: ACETONITRILE (80: 20)
3) FLOW RATE: 1.0 ml / minute.
4) DETECTOR WAVELENGTH: 210 nm.
5) SAMPLE SIZE: 20 ul
The approximate Retention Times that should be obtained using these chromatographic conditions are:
- GATIFLOXACIN approximate retention time = 1.85 Minutes
- 2-Methyl Piperazine approximate retention time = 04.46 Minutes.
PREPARATION OF MOBILE PHASE:
Buffer [0.025 gm Ortho Phosphoric acid] 80 v & 20 v of Acetonitrile. Adjust pH 3.0 by TEA.
PREPARATION OF THE TEST SOLUTION FOR ANALYSIS OF GATIFLOXACIN:
Amber glassware must be used when preparing these solutions because GATIFLOXACIN is Photosensitive.
ANALYTICAL STANDARD SOLUTION
[A] Accurately weigh 100 mg [u 5 mg] of Working standard of GATIFLOXACIN & transfer to a 50 ml volumetric flask. Add 10 ml of mobile phase & sonicate until dissolved. Allow to cool to room temperature & dilute to volume with mobile phase.
[B] Accurately weigh 5 mg [u1 mg] of the working standard of 2-Methyl Piperazine & transfer to 50 ml volumetric flask. Add 10 ml of mobile phase & dilute to volume with mobile phase.
[C] Transfer 4.0 ml of solution [B], in solution [A] in volumetric flask. & Dilute to volume with Mobile
phase & mix thoroughly.
PREPARATION OF SAMPLE SOLUTION:
Accurately weigh 100 mg of sample & transfer to 50 ml volumetric flask. Add 10 ml of Mobile phase &
sonicate until dissolved. Allow to cool to room temperature & dilute to volume with mobile phase.
INJECTION PRECISION:
Make duplicate injections of the analytical standard solution. Using a computing Integrator measure the GATIFLOXACIN peak area in the injections made.
The relative Standard deviation must not be greater than + 2.0%.
CALCULATION OF RESULTS:
1. GATIFLOXACIN CONTENT:
ASamp. x WStd.
GATIFLOXACIN Content = ------------------------------- X P
AStd. x WSamp.
ASamp.= Area of GATIFLOXACIN peak in an injection of sample.
AStd. = Mean Area of GATIFLOXACIN Peak in injection of analytical standard solution.
WSamp. = Weight of the sample taken to prepare relevant sample solution. (in mg)
WStd. = Weight of GATIFLOXACIN reference standard taken to prepare analytical standard Solution. (in mg)
P = Known Purity of GATIFLOXACIN Reference standard.
2. 2-Methyl Piperazine CONTENT:
ASamp. x WStd.
2-Methyl Piperazine Content = ---------------------------- X P
AStd. x WSamp.
ASamp. = Area of 2-Methyl Piperazine peak in an injection of sample.
AStd. = Mean Area of 2-Methyl Piperazine peak in injections of analytical standard solution.
WSamp. = Weight of the sample taken to prepare relevant sample solution. (in mg)
WStd. = Weight of 2-Methyl Piperazine reference standard taken to prepare analytical standard solution.(in mg)
P = Known Purity of 2-Methyl Piperazine reference standard
RESULTS AND DISCUSSIONS: -
SYSTEM SUITABILITY:
System suitability data as shown in Table No. 1 shows method is accurate.
Table No. 1: -
|
Compound |
Standard Deviation |
RSD |
Theoretical Plates |
Resolution Factor |
Tailing Factor |
|---|---|---|---|---|---|
|
2-METHYL PIPERAZIN
|
5.04619 |
0.6621 |
5370 |
1.6 |
1.1 |
|
GATIFLOXACIN
|
302.6325 |
1.624284 |
7271 |
- |
- |
2.SUPPORTING DATA FOR TEST PROCEDURE REPRODUCIBILITY IN ASSAY TEST
Data in Table No. 2 shows method is rugged & reproducible
|
GATIFLOXACIN
|
|
|
|
|
2-Methyl Piperazine |
|
||
|
DAY |
WEIGHT |
|
X |
DAY |
WEIGHT |
|
X |
|
|
|
[mg/ml] |
|
[%] |
|
[mg/ml] |
|
[%] |
|
|
28/09/2004 |
1 |
|
99.32 |
28/09/2004 |
0.01 |
|
98.73 |
|
|
|
|
|
98.84 |
|
|
|
99.12 |
|
|
|
|
|
99.5 |
|
|
|
99.36 |
|
|
29/09/2004 |
1 |
|
|
29/09/2004 |
0.01 |
|
|
|
|
|
|
|
99.57 |
|
|
|
98.83 |
|
|
|
|
|
99.24 |
|
|
|
99.21 |
|
|
|
|
|
99.14 |
|
|
|
99.08 |
|
|
30/09/2004 |
1 |
|
|
30/09/2004 |
0.01 |
|
|
|
|
|
|
|
99.38 |
|
|
|
99.33 |
|
|
|
|
|
99.72 |
|
|
|
98.54 |
|
|
|
|
|
99.71 |
|
|
|
99.34 |
|
|
|
|
|
|
|
|
|
|
|
|
|
AVG. [%] |
|
99.38 |
|
|
|
99.06 |
|
|
|
S.D. |
|
0.2844732 |
|
|
|
0.29580399 |
|
|
|
RSD |
|
0.2862479 |
|
|
|
0.29861093 |
|
|
|
2V |
|
0.5724959 |
|
|
|
0.59722186 |
|
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
3.Recovery-
Data in Table No. 3 shows method has recovery more than 99% so method is rugged and accurate.
Table No. 3
|
CONTENT OF GATTIFLOXACINE |
|
|
|
|
SUPPORTING DATA |
|
||
|
|
|
|
|
|
|
FOR TEST PROCEDURE |
|
|
|
|
|
|
|
|
|
ACCURACY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Concentration [STD] |
|
A (mg/ml) |
|
B (mg/ml) |
|
X |
|
|
|
1.0 [mg/ml] |
|
1 |
|
0.9778 |
|
0.9778 |
|
|
|
|
|
1 |
|
0.9847 |
|
0.9847 |
|
|
|
|
|
1 |
|
0.9846 |
|
0.9846 |
|
|
|
|
|
1 |
|
1.0031 |
|
1.0031 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
_ |
|
|
|
|
|
|
|
|
|
X = |
0.98755 |
|
|
|
|
|
|
|
|
S = |
0.01086 |
|
|
|
|
|
|
|
|
CV = |
1.09949 |
|
|
|
|
100% of Theoretical Concentration 95% Confidence Limits = |
|
0.98755 |
0.01726426 |
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CONTENT OF 2-METHYL PIPERAZIN |
|
|
|
SUPPORTING DATA |
|
|||
|
|
|
|
|
|
|
FOR TEST PROCEDURE |
|
|
|
|
|
|
|
|
|
ACCURACY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Concentration [STD] |
|
A (mg/ml) |
|
B (mg/ml) |
|
X |
|
|
|
0.01 [mg/ml] |
|
0.01 |
|
0.00993 |
|
0.9925 |
|
|
|
|
|
0.01 |
|
0.00995 |
|
0.9946 |
|
|
|
|
|
0.01 |
|
0.00991 |
|
0.9905 |
|
|
|
|
|
0.0096 |
|
0.00966 |
|
1.00614583 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
_ |
|
|
|
|
|
|
|
|
|
X = |
0.99594 |
|
|
|
|
|
|
|
|
S = |
0.00701 |
|
|
|
|
|
|
|
|
CV = |
0.70377 |
|
|
|
|
100% of Theoretical Concentration 95% Confidence Limits = |
|
0.99594 |
0.01114444 |
|
|
|||
A- Actual Concentration Taken.
B- B Actual CONCENTRATION Recover.
C- X—Recovery.
4. SUPPORTING DATA FOR TEST PROCEDURE ACCURACY
Data in Table No 4.1 and 4.2 show the linearity curve (Slope) & regression data for the product and its impurity, which confirms method, is accurate & reproducible
|
ASSAY OF GATIFLOXACIN |
SUPPORTING DATA FOR TEST PROCEDURE ACCURACY |
CRITERIA MEASURED |
DRUG SUBSTANCE ACCEPTANCE VALUE |
RESULTS |
|
Concentration Range
|
40 – 150% |
- |
|
Graphic Plot R R2 |
24 Points |
0.99837 0.996743 |
|
Average Fractional Recovery [X] _ (Average of X X 100) i.e. = ------------------------------ 6 |
99 – 101% |
100.06% |
|
Slope (A Vs B)
|
1.00 ideal |
1.014 |
|
Cv= (i.e. Average of all Cv)
|
- |
0.7730688 |
CV = Coefficient of Variation
Table No. 4.2
|
CONTENT OF RELATED SUBSTANCE – 2-Methyl Piperazine |
SUPPORTING DATA FOR TEST PROCEDURE ACCURACY |
|
CRITERIA MEASURED |
DRUG SUBSTANCE ACCEPTANCE VALUE |
RESULTS |
|
Concentration Range
|
40 – 150% |
- |
|
Graphic Plot R R2 |
24 Points |
0.99994 0.99988 |
|
Average Fractional Recovery [X] _ (Average of X X 100) i.e = --------------------------------- 6 |
95 – 105% |
99.20% |
|
Slope (A vs B)
|
1.00 ideal |
0.989 |
|
Cv= (i.e. Average of all Cv)
|
- |
0.72090 |
RESULTS AND DISCUSSSIONSS
As per USP XXVII, system suitability was carried out freshly prepared reference solution B to check various parameters such as efficiency, resolution and peak tailing which found to comply with USP requirements. (Refer Table No1)
The content of an impurity in Gatifloxacin by proposed method. The lower values of reproducibility indicate that the method is precise and accurate. The mean recoveries of Impurity were in the range of 99.3% to 100%, which shows that there is no interference from the mobile phase, which also confirm the reproducibility and reliability of the method.
CONCLUSION
i)The proposed method is simple rapid and selective.
ii) Percent Relative standard Deviation was very slow, below 2.0% which indicate that method id highly precise &reproducible.
iii)Short Analysis time (less than 10Min)coupled with simplicity and ease of operation warrants use of the method for analysis of Gatifloxacin along with its impurity stated above in Bulk and well as in Formulated dosages for Assays and for said Related Substance by HPLC.
iv)Therefore, method can be useful in routine quality Control analysis in bulk
ACKNOWLEDGMENT: - The Authors are thankful to Management of Aarti Drugs Ltd. for encouraging the work by providing the facility and working standard.
REFERENCES: -
1. Barragan, F.J., Callejon, M, (2005) Spectrofluorimetric and micelle-enhanced spectrofluorimetric determination of gatifloxacin in human urine and serum, Journal of Pharmaceutical and Biomedical Analysis, Vol. 37, No. 2, pages 327-332.
2. Borner K, Hartwig H, Lode H (2000) Determination of gatifloxacin in human serum by HPLC. Chromatographia 52 (Supplement): 105-107
3. Lubasch A, Keller I, Borner K, Koeppe P, Lode H (2000) Comparative pharmacokinetics of ciprofloxacin, gatifloxacin, grepafloxacin, levofloxacin, trovafloxacin, and moxifloxacin after a single oral administration in healthy volunteers. Antimicrob Agents Chemother 44: 2600-2603
4. “Spectrophotometric methods for the estimation of gatifloxacin and moxifloxacin in bulk and in the pharmaceutical formulations. P.U.Patel, B.N.Suhaghia, M.M.Patel, C.N.Patel, S.A.Patel and F.A.Mehta. [At 7th Asian analytical conference, Hong Kong]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









