{ DOWNLOAD AS PDF }
 ABOUT AUTHORS
ABOUT AUTHORS
Shipra Vermaa, Alok Kumar Srivastava*1, O.P. Pandeyb
1Department of Chemistry, Mahatma Gandhi P. G. College, Gorakhpur, U.P., India
2Department of Chemistry, D.D.U. Gorakhpur University, Gorakhpur, U.P., India
*sdevender350@gmail.com
ABSTRACT
Chalcones are a valuable molecule of medicinal importance due to presence of reactive ketoethylenic group –CO–CH=CH–, belonging to the flavonoid family. These reactive α,β-unsatutated keto function in chalcones are responsible for their biological activity. Chalcone can be synthesized by several methods using aldehydes and ketones as starting material. This review is focused about different methods of synthesis and versatile biological activity of chalcones including antimicrobial, anticancer, antioxidant, antimalarial, antituberculosis etc.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2566
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 2 Received On: 22/12/2017; Accepted On: 22/12/2017; Published On: 01/02/2018 How to cite this article: Verma S, Srivastava AK, Pandey OP; A Review on Chalcones Synthesis and their Biological Activity; PharmaTutor; 2018; 6(2); 22-39; http://dx.doi.org/10.29161/PT.v6.i2.2018.22 |
INTRODUCTION
Chalcone is an α,β- unsaturated ketone and central core for a variety of important biological compounds, which are known as chalcones. Benzylideneacetophenone is an important member of the chalcone series. Chemically these are 1,3-diphenyl-2-propene-1-one, in which two aromatic rings are linked by a three carbon keto-ethylinic system. Chalcones are also present in nature and can be obtained from plant species like Angelica, Glycyrrhiza, Humulus and Scutellaria, which are widely used as traditional folk remedies. Chalcones are an important intermediates for the biosynthesis of flavonoids. Besides the biological activity of chalcones like anti-inflammatory, antimitotic, anti-leishmanial, anti-invasive, anti-tuberculosis, anti-fungal, anti-malarial, anti-tumor, and anti-oxidant it is also recognized for its synthetic utility to prepare pharmacologically-interesting heterocyclic systems like pyrazolines, which have also been largely studied owing to their pharmacological activities, includes anti-tumor anti-inflammatory, anti-parasitary, anti-depressive, anticonvulsant, antimicrobial, antinociceptives and nitric oxide synthase inhibitors.
Chalcone as an antimicrobial agent
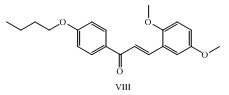
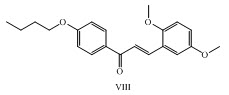
A novel 1-(4-butoxy-2-hydroxy phenyl)-3-(2,5 dimethoxyphenyl) prop-2-en-1-one VIII was synthesized as antimicrobial agent. (Barot et al., 2013)
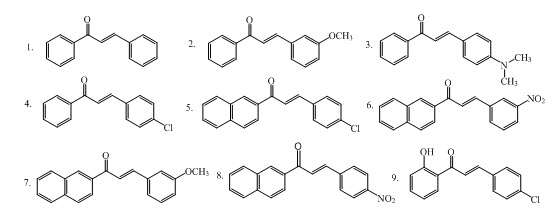
Chalcones V (1-9) were synthesized by Claisen-Schmidt condensation of methyl ketones with several aromatic aldehydes in presence of aqueous solution of sodium hydroxide using microwave irradiations having antibacterial activities. (Bhuiyan et al., 2011)
MWI = Microwave irradiation
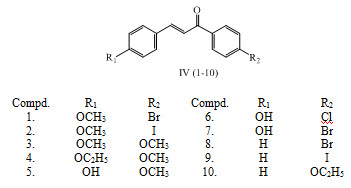
Antimicrobial chalcones IV (1-10) were synthesized by condensing benzaldehyde derivatives with acetophenone derivatives in dilute ethanolic sodium hydroxide solution at room temperature according to Claisen –Schmidt condensation. (Choudhary et al., 2011)
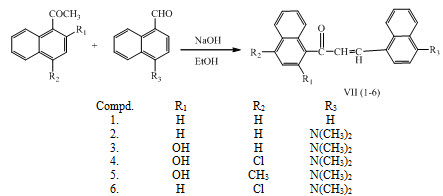
Chalcones VII (1-6) having antimicrobial activity, were synthesized by condensing either 1-acetylnaphthalene or substituted 1-acetylnaphthalenes with 1-naphthaldehyde or 4-dimethylamino-1 naphthaldehyde in ethanolic NaOH solutions. (Davood and Maseud, 2013)
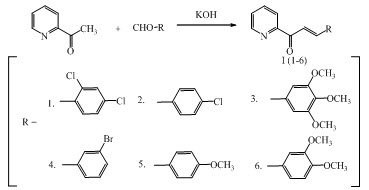
Chalcones I (1-6) were synthesized by condensing 2-acetyl pyridine with aldehyde derivatives in dilute ethanolic potassium hydroxide solution at room temperature according to Claisen-Schmidt condensation. (Prasad et al., 2008)
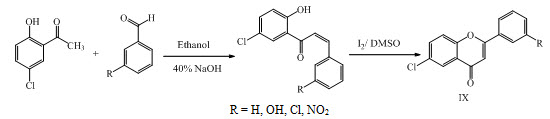
Chalcones IX were synthesized by Claisen-Schimdt condensation of aromatic aldehydes with o-hydroxy acetophenone. Chalcone on reaction with catalytic amount of I2 in DMSO gave Flavones having antimicrobial activity. (Rathore et al., 2015)
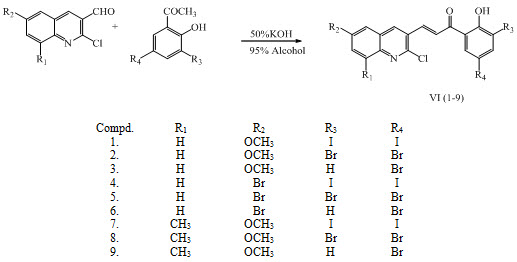
Chalcones VI (1-9) were synthesized by using different substituted hydroxyl acetophenone and quinoline carbaldehyde by Claisen-Schmidt condensatiom to give general 1-[substituted aryl]-3-[substituted hetero aryl]-2-propene-1-ones having antibacterial activity. (Sirsat et al., 2012)
A series of chalcones III (1-11) having antimicrobial activity was prepared by Claisen-Schmidt condensation of acetophenones with aromatic aldehydes in the presence of aqueous solution of potassium hydroxide and ethanol at room temperature. (Tiwari et al., 2010)
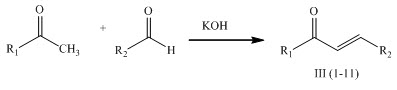

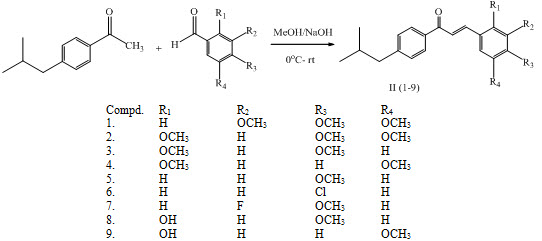
A series of chalcones II (1-9) was synthesized by reacting 1-(4-isobutylphenyl) ethanone with different substituted aldehyde by Clasien-Schimidt condensation having antibacterial and antifungal activity. (Turkar et al., 2010)
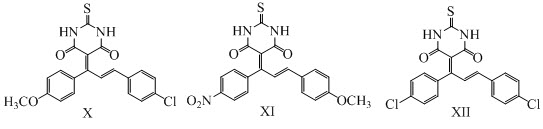
Venkatesh and his co-workers have reported the synthesis of novel series of 5-[1,3-bis (4- substituted phenyl) prop-2-en-1-ylidene]-2-thioxodihydropyrimidine-4,6(1H, 5H)-diones X, XI, XII. The target compounds were synthesized by the Knoevenagel condensation of different chalcones with thiobarbituric acid using acetic acid as a catalyst in ethanol. These compounds have been found to exhibit antimicrobial activity. (Venkatesh et al., 2016)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
3,5-bis-(4-hydroxy-3- methoxybenzylidene-N-phenylpiperidine-2,6-diones XIII (1-10) were synthesized by the subsequent condensation of N-phenyl glutarimides with 4-hydroxy-3-methoxy-benzaldehyde in presence of neutral alumina by an efficient microwave supported solvent free synthesis. The compound exhibited antifungal activity. (Dhivare and Rajput, 2016)

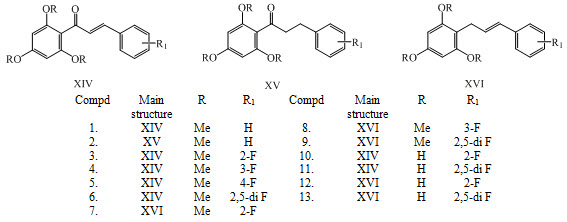
Some new fluorine-substituted chalcones XIV, XV, XVI (1-13) were synthesized and evaluated for their antitubercular activity against Mycobacterium tuberculosis H37Rv and antimicrobial activity against five pathogenic bacteria and three fungi. (Burmaoglu et al., 2017)
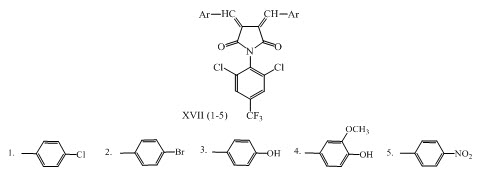
 Chalcones XVII (1-5) were synthesized via reaction between 1-(2,6-dichloro-4-trifluoromethyl-phenyl)-pyrolidine-2, 5-dione, 1-(2, 6-dichloro-4-trifluoromethyl-phenyl)-piperidine-2, 6 dione and substituted aromatic aldehydes in presence acetic acid. The synthesized compounds were evaluated for their antimicrobial activities. (Rajput and Sayyed, 2017)
Chalcones XVII (1-5) were synthesized via reaction between 1-(2,6-dichloro-4-trifluoromethyl-phenyl)-pyrolidine-2, 5-dione, 1-(2, 6-dichloro-4-trifluoromethyl-phenyl)-piperidine-2, 6 dione and substituted aromatic aldehydes in presence acetic acid. The synthesized compounds were evaluated for their antimicrobial activities. (Rajput and Sayyed, 2017)
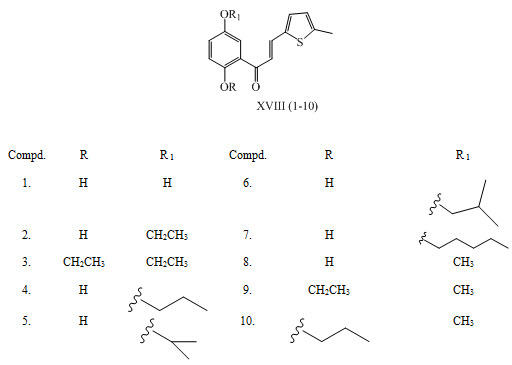
Chalcone as an anticancer agent A series of 2’,5’-dialkoxyl chalcones XVIII (1-10) was prepared by Claisen–Schmidt condensation of appropriate acetophenones with suitable aromatic aldehyde. These synthesized compounds were found to act as anti-tumor and cancer chemopreventive agents. (Cheng et al., 2008)
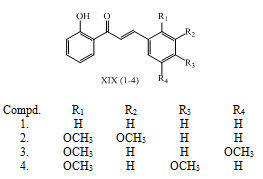
Cesar Echeverria and his co-workers have studied relationships between the structural characteristic of synthetic chalcones XIX (1-4) and their antitumoral activity. (Echeverria et al., 2009)
A new series of quinolinyl chalcone derivatives XX was synthesized by the reaction of quinolinyl and chloroquinolinyl acetophenones with substituted aromatic aldehydes as anticancer and anti-inflammatory agent. (Kotra et al., 2010)
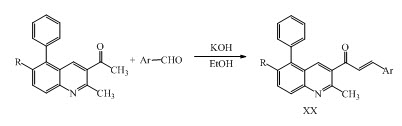
R = H, Cl; Ar = C6H5, p-C6H4NO2, p-C6H4Cl, p- C6H4OH, p- C6H4OCH3, p- C6H4CH3, p- C6H4N(CH3)2, p- C6H4N(C2H5)2, furyl, thiophene
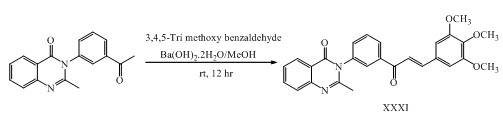
Suvitha Syam and his co-workers have synthesized chalcones XXI, XXII, XXIII, XXIV, XXV, XXVI, XXVII having anticancer activity. (Suvitha et al., 2012)
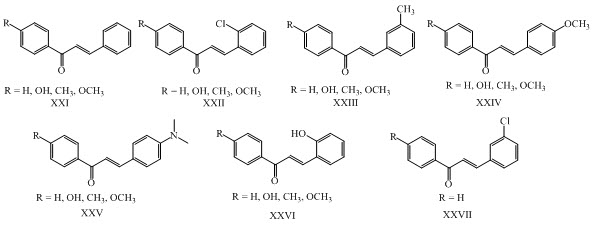
O-allyl chalcones XXVIII (1-8) were synthesized by Claisen Schmidt condensation reaction of O-allylvanillin with appropriate substituted acetophenones having anticancer activity. (Ngameni et al., 2013)
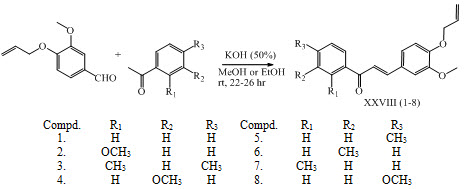
Ketabforoosh and his co-workers have synthesized series of chalcones XXIX and flavanones XXX as anti-cancer agents. (Ketabforoosh et al., 2014)
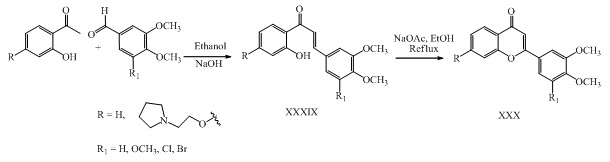
A novel quinazolinone-chalcone derivative XXXI was synthesized by the reaction of benzaldehyde and acetophenone in presence of barium hydroxide and evaluated for their anticancer potential. (Wani et al., 2015)
A new series of 3-aryl thiophene-2-aryl and hetero aryl chalcones XXXII were synthesized and evaluated for their invitro antiproliferative activity against human colon cancer cell lines. (Venkatarami reddy et al., 2016)
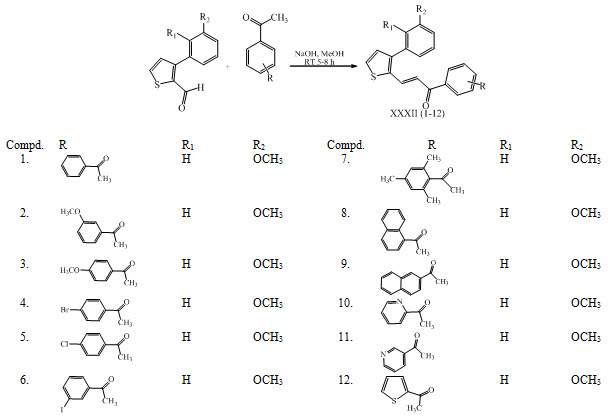
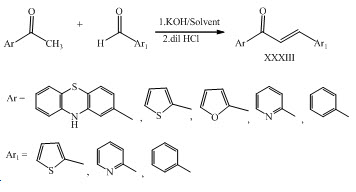
Tuong-Ha Do and his co-workers have prepared some heterocyclic chalcones XXXIII having cytotoxic activity. (Do et al., 2016)
Chalcone as antituberculosis agent
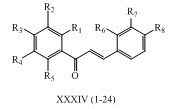
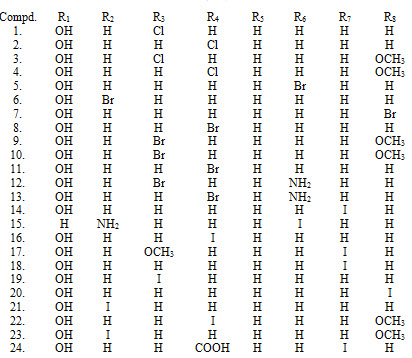
Umaa K. and his co-workers have studied anti-mycobacterial potential of number of chalcone derivatives XXXIV (1-24). (Umaa et al., 2013)
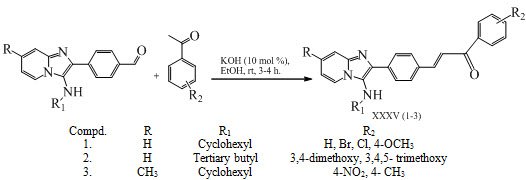
Anand Kumar Pandeya and his co-workers have synthesized a series of novel pyrido[1,2-a]imidazo-chalcones XXXV (1-3) and evaluated their anti-tubercular activity against Mycobacterium tuberculosis. (Pandeya et al., 2016)
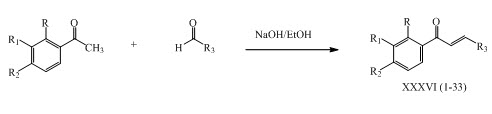
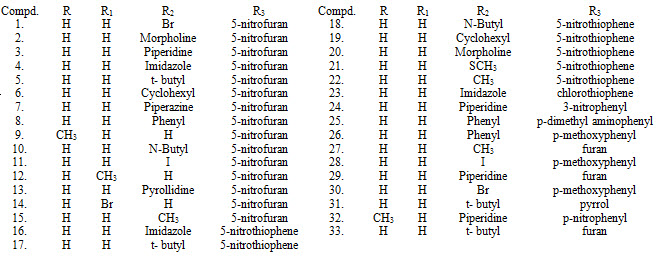
Heteroaryl chalcones XXXVI (1-33) were synthesized by Claisen-Schmidt condensation and evaluated their antituberculosis activity. (Gomes et al., 2017)
Chalcone as Anti-inflammatory agent
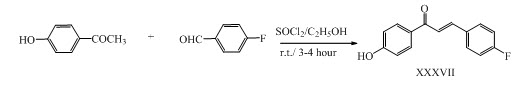
Fluorinated chalcone derivatives XXXVII with potent anti-inflammatory activity were synthesized by Claisen-Schmidt condensation method followed by reaction with SOCl2/ETOH. (Hussain et al., 2012)
Yau-Hung Chen and his co-workers have synthesized novel chalcones XXXVIII, XXXIX with potent anti-inflammatory activities. (Chen et al., 2013)
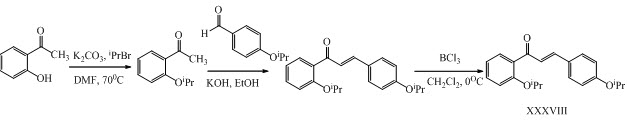
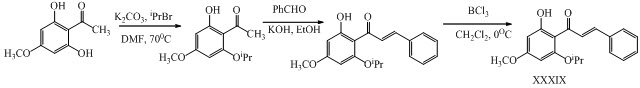
ipr = isopropyl
Chalcone as Antioxidant agent
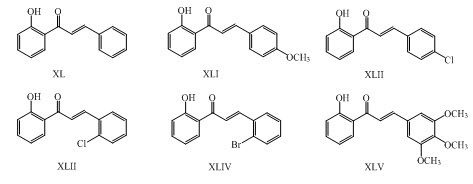
Kankate and his coworkers have synthesized chalcones (XL-XLV) having antioxidant activity. ( Kankate et al.,2010)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
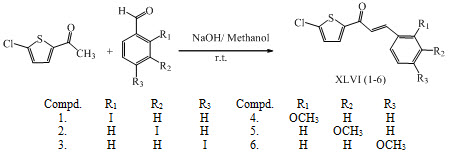
A series of novel heterocyclic chalcones XLVI (1-6) were synthesized by condensing 2-acetyl-5-chlorothiophene with benzaldehyde derivatives in methanol at room temperature using a catalytic amount of sodium hydroxide and reported as antioxidant agent. (Chidan et al., 2013)
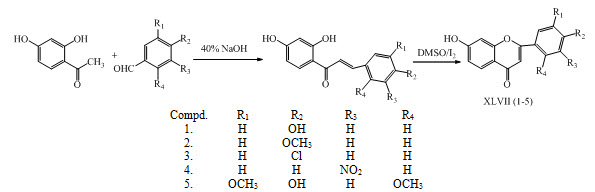
Flavonoids XLVII (1-5) of chalcones were synthesized by reaction of 2,4-dihydroxy acetophenone with different substituted aromatic aldehyde to form 2,4-dihydroxy chalcones having antioxidant activity. (Murti et al., 2013)
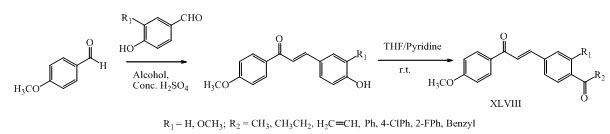
Jian-Zhang Wu and his co-workers have synthesized a series of chalcone derivatives XLVIII having antioxidant activity. (Jian-Zhang et al., 2014)
Jih-Jung Chen and his co-workers have isolated a new chalcone, glycyglabrone from the roots of Glycyrrhiza glabra, together with three known compounds, licoagrochalcone, licochalcone and kanzonol. The isolated chalcones XLIX, L, LI were evaluated for their antioxidant activity. (Chen et al., 2017)

Chalcone as Antimalarial agent
Chalcone derivatives LII (1-27) were synthesized using Claisen-Schmidt condensation and evaluate their antimalarial activity against sexual blood stages of Plasmodium falciparum. (Yadav et al., 2012)
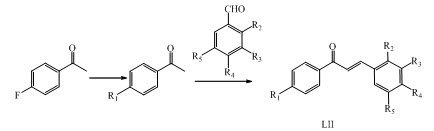
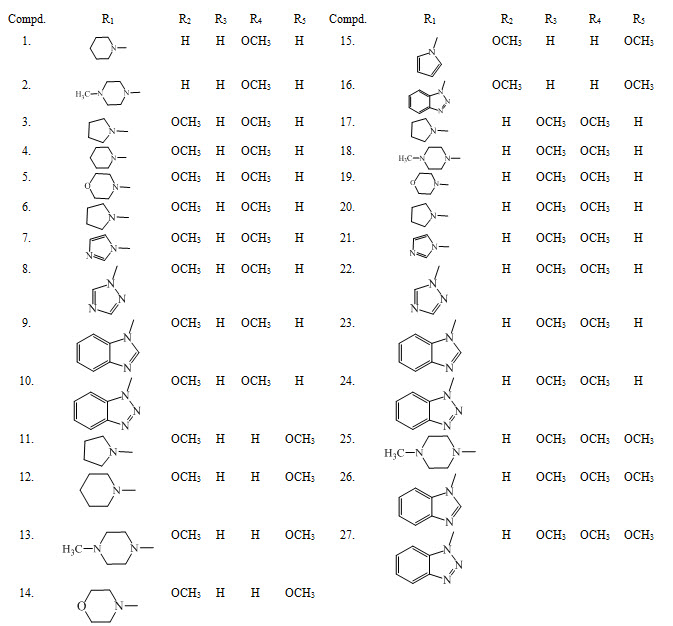
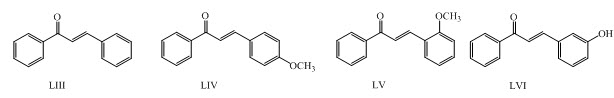
Chalcones LIII, LIV, LV, LVI were synthesized by Claisen-Schmidt condensation and reported them as potent antimalarial. (Sulistyowaty et al., 2014)
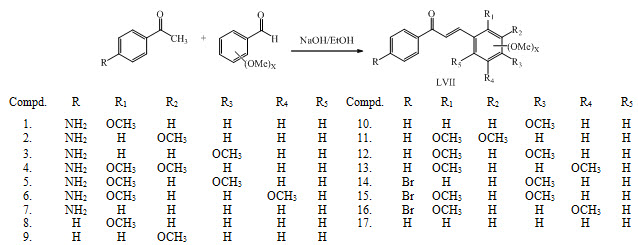
Chalcones LVII (1-17) were synthesized by Claisen-Schmidt reactions. These compounds have been reported to exhibit antimalarial activity. (Suwito et al., 2014)
Chalcone as Anticonvulsant agent
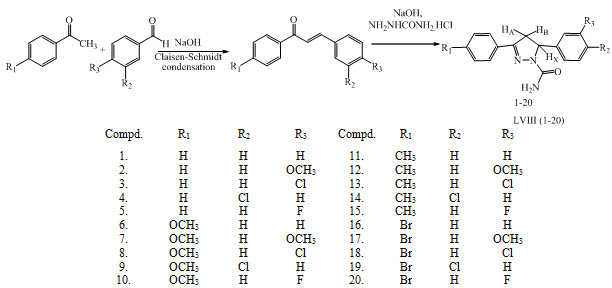
Some substituted 3,5-diphenyl-2-pyrazoline-1-carboxamide derivatives LVIII (1-20) were synthesized by reacting substituted 1,3-diphenylprop-2-en-1-one (chalcone) with semicarbazide hydrochloride. These compounds have been reported as anticonvulsant agent. (Siddiqui et al., 2010)
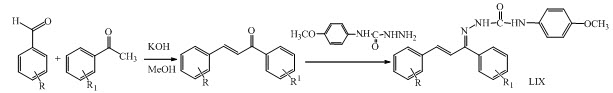
A new series of chalcone LIX was synthesized by Claisen- Schmidt reaction having anticovulsant activity. (Sharma et al., 2013)
Neeraj Kumar and Lalit Singh Chauhan have synthesized a series of novel chalcone LX (1-10) incorporated hydrazide derivatives as anticonvulsants agent. (Kumar and Chauhan, 2015)
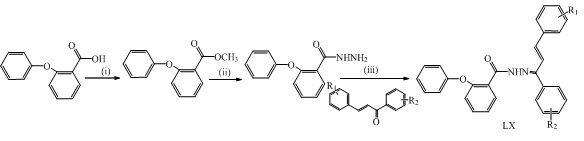
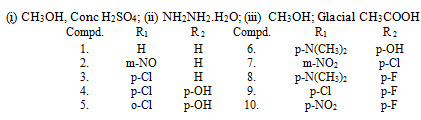
Chalcone as Antidiabetic agent
Chalcones LXI and their corresponding 2-pyrazoline derivatives LXII, LXIII, LXIV, LXV, LXVI were synthesized and evaluated for their anti-diabetic activity. (Emayavaramban et al., 2013)
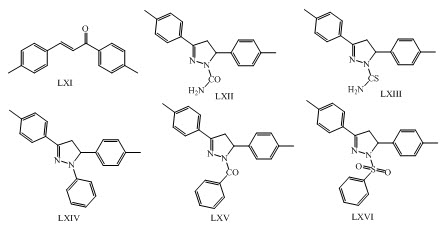
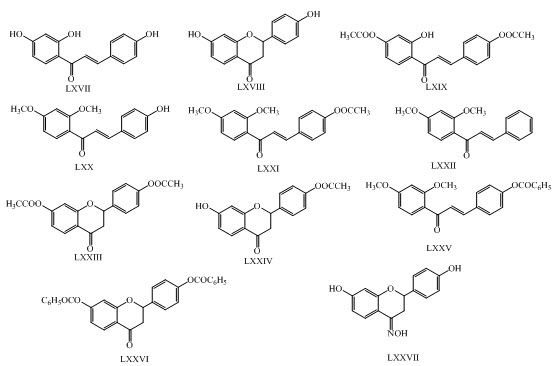
Rashmi Gaur and her co-workers have synthesized anti-diabetic derivatives of isoliquiritigenin and liquiritigenin having chalcones LXVII, LXVIII, LXIX, LXX, LXXI, LXXII, LXXIII, LXXXIV,LXXV, LXXVI, LXXVII and flavonoid moiety.(Gaur et al., 2014)
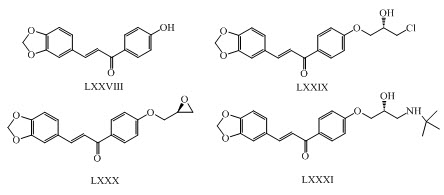
Chalcone-based aryl oxypropanolamine LXXVIII, LXXIX, LXXX, LXXXI were synthesized as a potential antidiabetic and antidyslipidaemic agent. (Shukla et al., 2017)
Chalcone as Antihypertensive agent
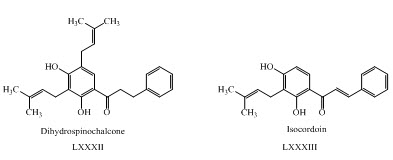
G. Avila-Villarreal and his co-workers have studied antihypertensive and vasorelaxant effects of dihydrospinochalcone and Isocordoinisolated LXXXII, LXXXIII from Lonchocarpus xuul. (Avila-Villarreal et al., 2013)
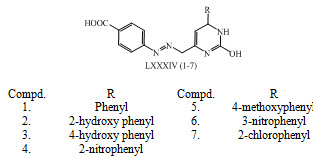
Pyrimidine based chalcones LXXXIV (1-7) were synthesized and evaluated for their antihypertensive activity. (Bukhari et al., 2013)
Quinoline based chalcone LXXXV were synthesized and evaluated their antihypertensive activity. (Kumar et al., 2015)

CONCLUSION
In this review we have discussed about different biological activity of chalcones and their synthetic methods. It is clear from above discussion that chalcones is a precursor of different heterocyclic moiety of valuable medicinal compounds.
REFERENCES
1. Avila-Villarreal, G.; Hernández-Abreu, O.; Hidalgo-Figueroa, S.; Navarrete-Vázquez, G.; Escalante-Erosa, F.; Pe˜na-Rodríguez, L.M.; Villalobos-Molina, R.; Estrada-Soto, S. (2013), “Antihypertensive and vasorelaxant effects of dihydrospinochalcone-Aisolated from Lonchocarpus xuul Lundell by NO production:Computational and ex vivo approaches”, Phytomedicine, (20), 1241-1246.
2. Barot, V.M.; Gandhi, S. A.; Mahato, A.; Mehta, N.B. (2013), “Synthesis, X-ray diffraction study and Antimicrobial study of1-(4-butoxy-2-hydroxyphenyl)-3-(2,5-dimethoxyphenyl) prop-2-en-1-one”, International Journal of Scientific and Research Publications, (3).
3. Belsare, D.P.; Pal, S.C.; Kazi, A.A.; Kankate, R.S.V.; Anjari S.S. (2010), “Evaluation of Antioxidant Activity of Chalcones and Flavonoids” International Journal of ChemTech Research, (2), 1080-1089.
4. Bhuiyan, M.M.H.; Hossain, M.I.; Mahmud, M.M.; Mohammad, A.A. (2011) “Microwave-assisted Efficient Synthesis of Chalcones as Probes for Antimicrobial Activities”, Chemistry Journal, (01), 21-28.
5. Bukhari, S.N.A.; Butt, A.M.; Amjad, M.W.B. (2013), “Synthesis and evaluation of chalcone analogues based pyrimidines as angiotensinconverting enzyme inhibitors”, Pakistan Journal of Biological Science, 16(21), 1368-1372.
6. Burmaoglu, S.; Algul, O.; Gobek, A.; Aktas, D.A.; Ulger, M.; Erturk, B.G.; Kaplan, E.; Dogen, A.; Aslan, G.(2017), “Design of potent fluoro-substituted chalcones as antimicrobial agents”, Journal Of Enzyme Inhibition And Medicinal Chemistry, 32(1), 490–495.
7. Chaudhary, N.A.; Juyal V. (2011), “Synthesis of chalcone and their derivatives as antimicrobial agents”, International Journal of Pharmacy and Pharmaceutical Sciences; (3).
8. Chen, J.J.; Cheng, M.J.; Shu, C.W.; Sung, P.J. (2017), “A New Chalcone and Antioxidant Constituents of Glycyrrhiza glabra”, Chemistry of Natural Compounds, 53(4), 632-634.
9. Chen, Y.H.; Wang, W.H.; Wang, Y.H.; Lin, Z.Y.; Wen, C.C.; Chern, C.Y. (2013), “Evaluation of the Anti-Inflammatory Effect of Chalcone and Chalcone Analogues in a Zebrafish Model”, Molecules, (18), 2052-2060.
10. Cheng, J.H.; Hung, C.F.; Yang, S.C.; Wangc, J.P.; Wond, S.J.; Lin, C.N. (2008), “Synthesis and cytotoxic, anti-inflammatory, and anti-oxidant activities of 2’,5’ dialkoxylchalcones as cancer chemopreventive agents”, Bioorganic & Medicinal Chemistry, (16), 7270–7276.
11. Chidan Kumar, C. S.; Loh, W.S.; Ooi ,C.W.; Quah, C.K.; Fun, H.K. (2013), Structural Correlation of Some Heterocyclic Chalcone Analogues and Evaluation of Their Antioxidant Potential”, Molecules, (18), 11996-12011.
12. Davood, A.; Maseud, S. (2013), “Synthesis and Glucosylation of Chalcone-3'-Carboxylic Acids using Glucosyl Donor”, Quest Journal of Research in Pharmaceutical Science, (1), 01-08.
13. Dhivare, R.S.; Rajput, S.S. (2016), “Microwave assisted solvent free synthesis and antifungal evaluation of3, 5-bis-(4-hydroxy-3-methoxybenzylidene)-Nphenylpiperidine- 2, 6-dionederived from N-phenyl glutarimides”, International Journal of ChemTech Research, 9(3), 325-331.
14. Do, T.H.; Nguyen, D.M.; Truong, V.D.; Do, T.H.T.; Le, M.T.; Pham, T.Q.; Thai, K.M.; Tran, T.D. (2016), “Synthesis and Selective Cytotoxic Activities on Rhabdomyosarcoma and Noncancerous Cells of Some Heterocyclic Chalcones”, Molecules, (21), 1-10.
15. Echeverria, C.; Santibanez, J.F.; Donoso-Tauda, O.; Escobar, C.A.; Ramirez-Tagle, R. (2009), “Structural Antitumoral Activity Relationships of Synthetic Chalcones”, International Journal of Molecular Sciences, (10), 221-231.
16. Emayavaramban, M.; Santhi, N.; Gopi, C.; Manivannan, C.; Raguraman, A. (2013), “Synthesis, Characterization and Anti-diabetic activity of 1,3,5-triaryl-2-pyrazolines in acetic acid under Ultrasound Irradiation, International Letters of Chemistry”, Physics and Astronomy, (14), 172-185.
17. Gaur, R.; Yadav, K.S.; Verma, R.K.; Yadav, N.P.; Bhakuni, R.S. (2014), “In Vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin”, Phytomedicine, (21), 415-422.
18. Hasan, S.A.; Elias, A.N.; Jwaied, A.H.; Khuodaer, A.R.; Hussain, S.A. (2012), “Synthesis of new fluorinated chalcone derivative with anti-inflammatoryActivity”, International Journal of Pharmacy and Pharmaceutical Sciences, 4(5), 430-434.
19. Jian-Zhang, W.; Chan-Chan, C.; Lai-Lai, S.; Zhan-Kun W.; Shou-Biao, W.; Wu-Lan, Li.; Su-Hua, C.; Rong-Ping, Z.; Pei-Hong, Q. (2014), “Synthetic Chalcones with Potent Antioxidant Ability on H2O2-Induced Apoptosis in PC12 Cells”. International Journal of Molecular Science, (15), 18525-18539.
20. Ketabforoosh, S. H. M. E.; Kheirollahi, A.; Safavi, M.; Esmati, N.; Ardestani, S.K.; Emami, S., Firoozpour, L.; Shafiee, A.; Foroumadi, A. (2014), “Synthesis and Anti-Cancer Activity Evaluation of New Dimethoxylated Chalcone and Flavanone Analogs”, Arch. Pharm. Chem. Life Sci, (347), 1–8.
21. Kotra, V.; Ganapaty, S.; Adapa, S.R. (2010), “Synthesis of a new series of quinolinyl chalcones as anticancer and anti- inflammatory agents”, Indian Journal of Chemistry, (49B), 1109-1116.
22. Kumar, H.; Devaraji, V.; Joshi, R.; Jadhao, M.K.; Ahirkar, P.; Prasath, R.; Bhavanac, P.; Ghosha, S.K. (2015), “Antihypertensive Activity of a Quinoline Appended Chalcone Derivative and its Site Specific Binding Interaction with Relevant Target Carrier Protein”, RSC Advances.
23. Kumar, N.; Chauhan, L.S. (2015), “Synthesis and anticonvulsant activity of some chalcones incorporated hydrazide derivatives”, Journal of Chemical and Pharmaceutical Research, 7(6), 775-780.
24. Marcelo, N.; Gomes, Rodolpho, C.; Braga, Edyta, M.; Grzelak, Bruno, J.; Neves, Muratov, E.; Ma,R. (2017) “QSAR-driven design, synthesis and discovery of potent chalcone derivatives with antitubercular activity”, Medicinal Chemistry.
25. Murti, Y.; Goswami, A.; Mishra, P. (2013), “Synthesis and Antioxidant Activity of Some Chalcones and Flavanoids”, International Journal of PharmTech Research, 5(2): 811-818.
26. Ngameni, B.; Kuete, V.; Ambassa, P.; Justin, K.; Marlyse, M.L.; Tchoukoua, A.; Roy, R.; Ngadjui, B.T.; Tetsuya, M. (2013), “Synthesis and Evaluation of Anticancer Activity of O-allylchalcone Derivatives”, medicinal chemistry, 3(3): 233-237.
27. Pandey, A.K.; Sharma, R.; Purohit, P.; Dwivedi, R.; Chaturvedi, V.; Chauhan, P.M.S. (2016), “Synthesis of pyrido[1,2-a]imidazo-chalcone via 3-component Groebke-Blackburn-Bienayme reaction and their bioevaluation as potent Antituberculosis Agents”, Chemistry & Biology Interface, (6), 290-299.
28. Prasad, Y.R.; Kumar, P.P.; Kumar, P.R.; Rao, A.S. (2008) “Synthesis and Antimicrobial Activity of Some New Chalcones of 2-Acetyl Pyridine”, E-Journal of Chemistry, (5), 144-148.
29. Rajput, S.S.; Sayyed, R.A.M.A. (2017), “Synthesis And Evaluation of Antimicrobial Activity of Some Novel Chalcones of 2, 6-Dichloro-4-Trifluoro Methyl Aniline”, Heterocyclic Letters, 7(2), 333-339.
30. Rathore, M.M.; Rajput, P. R.; Parhate, V.V. (2015), “Synthesis and Antimicrobial Activity of Some Chalcones and Flavones”, International Journal of Chemical and Physical Sciences, (4), 473-477.
31. Sharma,C. S.; Shekhawat, K. S.; Chauhan, C. S.; Kumar, N. (2013), “Synthesis and anticonvulsant activity of some chalcone derivatives”, Journal of Chemical and Pharmaceutical Research, 5(10): 450-454.
32. Shukla, P.; Satyanarayana, M.; Verma, P.C.; Tiwari, J.; Dwivedi, A.P. (2017), “Chalcone-based aryloxypropanolamine as a potential antidiabetic and antidyslipidaemic agent”,Current Science, (112), 1675-1689.
33. Siddiqui, A.A.; Rahman, M.A.; Shaharyar, M.; Mishra, R. (2010), “Synthesis And Anticonvulsant Activity Of Some Substituted 3,5-Diphenyl-2-Pyrazoline-1-Carboxamide Derivatives”, Chemical Sciences Journal, (2010) 1-10.
34. Sirsat, S.B.; Halikar, N.K.; Pund, M.M.; Vartale, S.P. (2012), “Synthesis and biological screening of some novel hetero aryl chalcone and their complexes”, Research journal of pharmaceutical,biological and chemical sciences, (3), 242.
35. Sulistyowaty, M.I.; Nofianti, K.A.; Suzana, B. T. (2014), “In Vitro Antimalarial Activity Of Chalcone And Its Derivatives”, International Journal of Pharmacy and Pharmaceutical Sciences, (6), 669-671.
36. Suvitha, S.; Ibrahim, S.; Abdelwahab., Mohammed Ali, A.M.; Syam, M. (2012), “Synthesis of Chalcones with Anticancer Activities”, Molecules, (17), 6179-6195.
37. Suwito, H.; Jumina, Mustofa, Pudjiastuti P.; Fanani, M.Z.; Kimata-Ariga, Y.; Katahira, R.; Kawakami, T.; Fujiwara, T.; Hase, T.; Sirat, H.M.; Puspaningsih, N.N.T. (2014) “Design and Synthesis of Chalcone Derivatives as Inhibitors of the Ferredoxin — Ferredoxin-NADP+ Reductase”, Interaction of Plasmodium falciparum: Pursuing New Antimalarial Agents”, Molecules, (19), 21473-21488.
38. Tiwari, B.; Pratapwar, A.R.; Tapas, S.R.; Butle Vatkar, B.S. (2010), “Synthesis and Antimicrobial Activity of Some Chalcone Derivatives”, International Journal of ChemTech Research, (2), 499-503.
39. Turkar, S.S.; Rodge, A.H.; Hatnapure, G.D.; Keche, A.P.; Gaikwad, G.S. (2010), “Synthesis and anti-bacterial, anti-fungal activity of some novel chalcone derivatives”, Journal of Chemical and Pharmaceutical Research, 2(5), 348-355.
40. Umaa, K.; Kavithamani, A.; Maida Engels, S.E.; Geetha, G. (2013), “Quantitative structure activity studies on the anti-mycobacterial potentials of certain chalcone derivatives”, International Journal of Research in Organic Chemistry, 3(2), 6-10.
41. Venkataramireddy,V.; Shankaraiah, V.; Rao, A.T.; Kalyani, C.; Narasu, M.L.; Varala, R.; Jayashree, A. (2016) “Synthesis and anti-cancer activity of novel 3- aryl thiophene-2-carbaldehydes and their aryl/heteroaryl chalcone derivatives”, Rasayan journal of Chemistry, 9(1), 31 – 39.
42. Venkatesh, T.; Bodke, Y.D.; Kenchappa, R.; Telkar, S. (2016), “Synthesis, Antimicrobial and Antioxidant Activity of Chalcone Derivatives Containing Thiobarbitone Nucleus”, Medicinal Chemistry, 6(7), 440-448.
43. Wani, Z.A.; Pathania, A.S.; Mahajan, G.; Behl, A.; Mintoo, M.J.; Guru, S.K.; Viswanath, A.; Malik, F.; Kamal, A.; Mondhe, D.M. (2015), “Anticancer activity of a novel quinazolinone-chalcone derivative through cell cycle arrest in pancreatic cancer cell line”, Journal of Solid Tumors, (5), 73-85.
44. Yadav, N.; Dixit, S.K.; Bhattacharya, A.; Mishra, L.C.; Sharma, M.; Awasthi, S.K.; Bhasin V.K. (2012), “Antimalarial Activity of Newly Synthesized Chalcone Derivatives In Vitro”, Chem Biol Drug Des, (80), 340–347.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









