About Authors:
Shah Chirag K.*, Umalkar Deepak, Dr. Rajesh KS.
Parul Institute of Pharmacy, Gujarat University,
Waghodia-391760, Dist. Vadodara,
Gujarat, India.
*cks2484@gmail.com
Abstract:
The present manuscript described the simple, rapid, accurate, sensitive, precise and economical Q- absorption ratio method for simultaneous determination of Cefixime and Moxifloxacin in combined tablet dosage form. Absorption ratio method uses the ratio of absorbances at selected wavelengths, one which is isoabsorptive point and other being the λmax of the one of the components. Cefixime and Moxifloxacin shows isoabsorptive point at 275nm in 0.1N HCl. The second wavelength used is 295nm, which is λmax of Moxifloxacin. The concentrations of the drugs were determined by using ratio of absorances at isoabsorptive point and at λmax of Moxifloxacin. The method successfully applied to the Pharmaceutical formulation because no interference from the tablet formulation excipients was found. The results of analysis have been validated statistically and by recovery studies.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1355
Introduction:
Cefixime Trihydrate (CEF), (6R,7R)-7-{[2-(2-amino-1,3-thiazol-4-yl)-2-(carboxy methoxyimino)acetyl]amino}-3-ethenyl-8-oxo-5-thia 1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic trihydrate (Figure 1), is third generation Cephelosporin antibiotic1. Moxifloxacin (MOX), 1-cyclopropyl-7-[(1S,6S)-2,8-diazabicyclo[4.3.0]non-8-yl]-6-fluoro-8-methoxy-4-oxo-quinoline-3-carboxylic acid(Figure 2), is floroquinolone antibiotic2. This combination is used for treatment of lower Respiration tract infection in adult3. Literature reveals Spectrophotometric4, HPLC8 methods for Cefixime in Pharmaceutical dosage forms and as well as biological fluids. Literature survey also reveals Spectrophotometric14 and HPLC18 methods for Moxifloxacin in Pharmaceutical dosage forms and as well as biological fluids. The combination is not official in any pharmacopeia; hence no official method is available for the estimation of Cefixime and Moxifloxacin in their combined dosage forms. Literature survey does not reveal any simple spectrophotometric or other method for simultaneous estimation of Cefixime and Moxifloxacin in combined dosage forms. The present work describes simple, sensitive, rapid, accurate and economical spectrophotometric method for simultaneous estimation of both the drugs in their combined tablet dosage forms.

[adsense:468x15:2204050025]
Material and Method:
A Shimadzu model 1800 double beam UV/Visible Spectrophotometer with spectral width of 2nm, wavelength accuracy 0.5nm and a pair of 10nm matched quartz cell was used to measure absorbance of all the solutions. Spectra were obtained automatically from UV-Probe ver2.34 software.
Reagents and Materials:
Cefixime powder was gifted by Kaptab Pharmaceuticals, Vadodara, India. Moxifloxacin powder was gifted by BDR Pharmaceutical International Pvt. Ltd., Mumbai, India. HCl AR Grade was procured from SDFCL, Baroda, India.
Preparation of the standard stock solution:
An accurately weighed quantity of CEF (10 mg) and MOX (10 mg) were transferred to a separate 100 ml volumetric flask and dissolve and diluted to the mark with 0.1N HCl. To obtain standard solution having concentration of Cef (100 μg/ml) and Mox (100 μg/ml).
Methodology:
The Absorption Ratio method based on measurements at two wavelengths. Two dissimilar chromophores must necessary have different powers of light absorption at some points or in linear absorption. A set of two simultaneous equations obtained by using mean absorptivity values are given below. Seven working solution having concentration 2, 4, 6, 8, 10, 12 and 14 μg/ml of both the drug Cefixime and Moxifloxacin were prepared respectively in 0.1N HCl and the absorbance at 275nm (Isoabsorptive point) and 295nm(λmax of Moxifloxacin) were measured and absorptivity coefficients were calculated using calibration curve. The absorbance and absorptivity at this wavelength were substituted in following equations to obtain the concentration of both drugs. From overlain spectra Fig3.
Cx = Qm - Qy X (A1/Ax1) ……………(1)
Qx - Qy
Cy = Qm - Qx X (A1/Ay1) ……………(2)
Qy - Qx
QM, QX, and QY were obtained as below:
QM = A2/A1, QX = ax2/ax1, QY = ay2/ay1
Where, A1 and A2 were absorbance of sample at 275 nm and 295 nm respectively, ax1 and ax2 are absorptivity of Cefixime at 275 nm and 295 nm, ay1 and ay2 are absorptivity of Moxifloxacin at 275 nm and 295 nm. Validity of above framed equation was checked by using mixed standard of pure drug sample of two drugs, measuring their absorbance at respective wavelength and calculating concentration of two components. Results of which are reported in Table.3
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Validation of the method
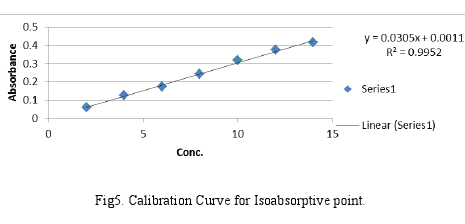
1. Linearity and Range: The calibration curves were plotted over a concentration range 2-14 μg/ml for each Cef and Mox. Accurately measured standard stock solution of each Cef and Mox (0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4 ml) were transferred to a series of 10 ml volumetric flask separately and diluted upto the mark with 0.1N HCl. The absorbances of the solutions were then measured at 275 nm and 295 nm. The calibration curves were constructed by plotting absorbances V/S concentration and the regression equations were calculated which are shown in Fig4 and Fig5 respectively.
2. Precision (Repeatability): Precision and accuracy was studied by covering three different concentrations {low (80%), medium (100%) and high (120%)} viz. 8, 10 and 12 μg/ml. Absorbance of the solutions was measured for three replicate samples of each concentration. Intra-day precision studies were run in triplicate in the same day and inter-day on three consecutive days. Precision data is shown in Table 1
Table 1: Precision Data
|
Cefixime |
%RSD |
||
|
1st day |
2nd day |
3rd day |
|
|
80% |
0.501212 |
0.668989 |
1.692747 |
|
100% |
0.428249 |
1.14518 |
0.825769 |
|
120% |
0.555842 |
1.009958 |
1.337086 |
|
Moxifloxacin |
%RSD |
||
|
1st day |
2nd day |
3rd day |
|
|
80% |
0.809896 |
0.900747 |
0.441269 |
|
100% |
0.468351 |
0.924781 |
1.013452 |
|
120% |
0.417267 |
0.85742 |
0.711877 |
3. Accuracy (% Recovery):
It is the measure of closeness between the actual value and the analytical value that is calculated by applying the test procedure for a number of times. Recovery was done at three different levels viz. 80%, 100% and 120%, within the beer’s limit for both the drugs. The previously analyzed sample of concentration 5 μg/ml was spiked with known concentrations of the pure samples and then reanalyzed using the proposed methods. Percentage recovery was calculated using the equations for both the methods. Percentage recovery is given in Table 2.
|
Conc. |
% Recovery |
|
|
Cefixime |
Moxifloxacin |
|
|
80% |
95.32% |
92.40% |
|
100% |
99.33% |
94.44% |
|
120% |
102.21% |
98.57% |
4. Limit of detection and quantification:
Limit of detection (LOD) is the minimum concentration of the analyte in the sample which can be analyzed by the instrument. Limit of quantification (LOQ) is the minimum concentration of the analyte that can be reliably quantified. Equations for the LOD and LOQ were listed below. The values of LOD and LOQ are given in Table 3.
LOD = 3 SD / SLOP ………… (3)
LOQ= 10 SD / SLOP ………… (4)
5. APPLICATION OF THE DEVELOPED METHOD ON TABLET DOSAGE FORM:
20 tablets were weighed, crushed and an accurately weighed sample equivalent to 20mg of Cefixime and 20mg of Moxifloxacin, and the stock solution of this was prepared in 0.1N HCl, sonicated for 10 min, was then filtered through Whatman filter paper and then volume was made up to 50 ml with methanol. This stock solution contains 400 mg/ml of each drug. Then the appropriate dilutions were made using 0.1N HCl, in the linearity range as previously mentioned. All determinations were carried out three times. The absorbance of the prepared solutions was observed at 275 nm and 295 nm and then the concentration of both the drugs was calculated using equation 1 and 2.
Results and Discussion:
In absorption ratio method (Q-Analysis), the primary requirement for developing a method for analysis is that the entire spectra should follow the Beer’s law at all the wavelength, which was fulfilled by both these drugs. The two wavelengths were used for analysis of the drugs were 275 nm (iso-absorptive point) and 295 nm (λmax of Moxifloxacin) at which the calibration curves were prepared for both the drugs. The UV overlay spectra of Cef (285 nm) and Mox (295 nm) showing isoabsorptive point (275 nm) in 0.1N HCl is shown in figure 3.
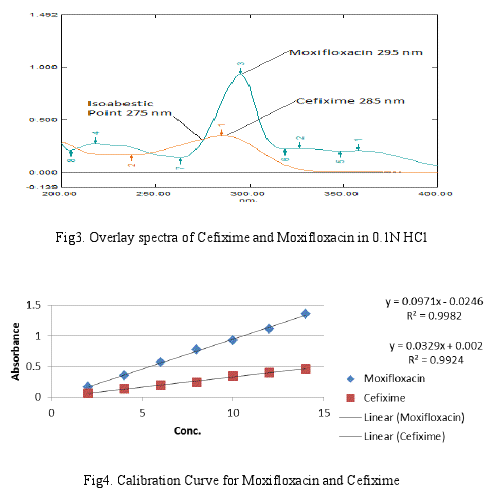
The validation parameters were studied at all the wavelengths for proposed method. Accuracy was determined by calculating recovery, and the mean was determined (Table 2.). The method successfully used to determine the amounts of Cef and Mox in present tablet dosage forms. The results obtained were in good agreement with the corresponding labeled amount (Table 3). Precision was calculated as repeatability and intra and inter day variation (%RSD) for both the drugs. Optical characteristics and summary of the validation parameters for the method is given in Table 3. By observing the validation parameters, the method was found to be simple, sensitive, accurate, precise and economical. Hence the method can be employed for the routine analysis of these two drugs in combined dosage form.
Table3. Result Table
|
Parameters |
Cefixime |
Moxifloxacin |
Isoabsorptive Point |
|
Wavelength |
285nm |
295nm |
275nm |
|
Beer’s law limit |
2-14 (μg/ml) |
2-14 (μg/ml) |
2-14 (μg/ml) |
|
Regression equation Slope Intercept |
y = 0.0329x + 0.002 |
y = 0.0971x - 0.0246 |
y = 0.0305x + 0.0011 |
|
0.0329 |
0.0971 |
0.0305 |
|
|
0.002 |
0.0246 |
0.0011 |
|
|
Correlation Coefficient (R2) |
0.9924 |
0.9982 |
0.9952 |
|
Precision 80% 100% 120% |
0.501212 |
0.809896 |
1.147078 |
|
0.428249 |
0.468351 |
1.283468 |
|
|
0.555842 |
0.417267 |
1.717539 |
|
|
Interday 80% 100% 120% |
0.668989 |
0.900747 |
1.033497 |
|
1.14518 |
0.924781 |
0.888957 |
|
|
1.009958 |
0.85742 |
0.997174 |
|
|
Intraday 80% 100% 120% |
1.692747 |
0.441269 |
0.620105 |
|
0.825769 |
1.013452 |
1.401399 |
|
|
1.337086 |
0.711877 |
0.584191 |
|
|
% Recovery 80% 100% 120% |
102.32% |
108.40% |
- |
|
105.12% |
107.27% |
- |
|
|
100.21% |
98.57% |
- |
|
|
LOD (μg/ml) |
0.209 |
0.282 |
0.303 |
|
LOQ (μg/ml) |
0.695 |
0.848 |
1.023 |
|
Assay+SD |
99.33 + 0.5% |
94.44 + 0.5% |
- |
LOD= Limit of Detection, LOQ= Limit of Qualification, CEF= Cefixime, MOX= Moxifloxacin
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Acknowledgment:
The author is thankful to Kaptab Pharmaceutical, Vadodara, India. for providing the Cefixime and also to BDR Pharmaceutical International Pvt. Ltd., Mumbai, India. for providing the Moxifloxacin for research work. The authors are highly thankful to Parul Institute of Pharmacy, Gujarat University, Waghodiya, Dist. Vadodara, Gujarat, India. for providing all the faculties to carry out the work. The author also thankful to the Mr. Stavan Master, lecturer, Baroda College of Pharmacy, Gujarat University, Waghodiya, Dist. Vadodara, Gujarat, India. sharing his knowledge with me.
References
1.Maryadele. J. O’ Neil. The Merck Index: Encyclopedia of Chemicals, drugs and biological, 14th Ed. New Jersey: Published by Merck Research Laboratories, Division of Merck and Co., Inc. Whitehouse station 2006. p. 1924.
2.S. Budavari, Eds, In; The Merck Index: Encyclopedia of Chemicals, drugs and biological, 13th Ed. New Jersey: Published by Merck Research Laboratories, Division of Merck and Co., Inc. Whitehouse station 2001, p. 1097 and 1125.
3.meherpharmainnovation.blogspot.com/2010/07/moxifloxacincefixime.html
4.Dhoka Madhura V. et al. Simultaneous Estimation of Cefixime Trihydrate and Erdosteine in Pharmaceutical Dosage form by RP-HPLC method. IJCTR 2010; 2: 79-87.
5.Gandhi Santosh V. et al. A simple and sensitive RP-HPLC method for simultaneous estimation of Cefixime and Ofloxacin in combined tablet dosage form. IJPPS 2011; 3: 46-8.
6.Kumar Rajnish. et al. Development of colorimetric method for the analysis of pharmaceutical formulation containing both Ofloxacin and Cefixime. IJPPS 2011; 3 (2): 178-9.
7.Patel Satish. et al. Simultaneous Spectrophotometric Determination of Cefixime Trihydrate and Ofloxacin in Tablet Dosage form IRJPS 2011; 105-8.
8.Pareek V. et al. Role of Different Hydrotopic Agents in Spectrophotometric and Chromatographic estimation of Cefixime. IJPBS 2010.
9.Kumar Hemanth A.K. et al. Simple and rapid liquid chromatography method for determination of moxifloxacin in saliva. Journal of Chromatography B 2011; 3663-7.
10.Rathinavel G. et al. A Validated RP – HPLC Method for Simultaneous Estimation of Cefixime and Cloxacillin in Tablets. Journal of Chemistry 2008; 5: 648-51.
11.Kumudhavalli M.V. et al. Development And Validation Of Rp-Hplc Method For Simultaneous Determination Of Cefixime And Potassium Clavulanate In Tablet Dosage Form. IJPRR 2010; 2: 57-60.
12.Motwani Sanjay K. et al. Validated spectrophotometric methods for the estimation of moxifloxacin in bulk and pharmaceutical formulations. SpectrochimicaActa Part A: Molecular and Biomolecular Spectroscopy, 2007; 250-6.
13.Gandhi L.R. et al. Absorption Ratio method for estimation of Moxifloxacin HCl & Ketorolac Tromethamine in their combined dosage form by UV-Visible Spectroscopy. IJPRD 2011; 21-6.
14.Mishra M. Simple and Validated UV-Spectroscopic method for estimation of Moxifloxacin HCl in bulk and formulation. JGPT 2010.
15.Sahu. et al. Spectrophotometric Estimation of Moxifloxacin in Bulk and its Pharmaceutical Formulations. Pharmacologyonline 2011; 491-502.
16.Nimmagadda Srinivas. et al. Development and validation of a HPLC method for simultaneous quantitation of gatifloxacin, sparfloxacin and moxifloxacin using levofloxacin as internal standard in human plasma. Wiley 2008; 1288-95.
17.Kumar Hemanth A.K. Simple and rapid liquid chromatography method for determination of moxifloxacin in plasma. Journal of Chromatography B 2009; 1205-08.
18.Rama Subbaiah. et al. Method Development and Validation for estimation of Moxifloxacin HCl in tablet dosage form by RP-HPLC method. PAA 2010.
19.ICH, Q2 (R1): Validation of Analytical Procedures, Text and Methodology, Geneva, 2005, p. 8-17.
20.Robert AN, Alfred HW. Pharmaceutical Process Validation. 3rd ed. Informa Publishers, 2003, p. 693-99.
21.Beckett AH, Stenlake JB. Practical pharmaceutical chemistry, 4th ed., Delhi: CBS publishers and distributors; 1997. p. 293-6.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









