About Authors:
Garima Gupta*, Amit Singh
Department of Pharmaceutics,
R.V. Northland Institute,
Greater Noida, G. B. Nagar, U.P.
*Garima189@gmail.com
Abstract:
Technological attempts have been made in the research and development of rate-controlled oral drug delivery systems to overcome physiological adversities, such as short gastric residence times (GRT) and unpredictable gastric emptying times (GET). Conventional oral dosage forms pose low bioavailability problems due to their rapid gastric transition from stomach, especially in case of drugs which are less soluble at alkaline pH of intestine. Similarly, drugs which produce their local action in stomach get rapidly emptied and do not get enough residence time in stomach. So, frequency of dose administration in such cases is increased. To avoid this problem, various efforts have been made to prolong the retention time of drug delivery system. In this review, we will discuss about the various approaches to produce gastro retention of drug delivery system, with current & recent developments of Stomach Specific floating drug delivery system.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1301
INTRODUCTION:-
The oral delivery of drugs is the most preferred route of administration due to ease of administration. Drug bioavailability of pharmaceutical oral dosage forms is influenced by various factors. One important factor is the gastric residence time (GRT) of these dosage forms.1 Indeed, gastric retention has received significant interest in the past few decades as most of the conventional oral delivery systems have shown some limitations related to fast gastric emptying time. A gastro retentive dosage form (GRDF) can overcome this problem and is particularly useful for drugs that are primarily absorbed in the duodenum and upper jejunum segments. The classification of different modes of gastric retention is:2
- High-density (sinking) systems.
- Low-density (floating) systems.
- Expandable systems.
- Superporous hydrogel systems.
- Mucoadhesive systems.
- Magnetic systems.
The oral route is considered as the most promising route of drug delivery. Conventional drug delivery system achieves as well as maintained the drug concentration within the therapeutically effective range needed for treatment, only when taken several times a day.3 Drug that have narrow absorption window in the gastrointestinal tract (GIT) will have poor absorption. For these drugs, gastroretentive drug delivery systems offer the advantages in prolonging the gastric emptying time.4
Several difficulties are faced in designing controlled release systems for better absorption and enhanced bioavailability. One of such difficulties is the inability to confine the dosage form in the desired area of the gastrointestinal tract. Drug absorption from the gastrointestinal tract is a complex procedure and is subject to many variables. It is widely acknowledged that the extent of gastrointestinal tract drug absorption is related to contact time with the small intestinal mucosa.5 Gastroretentive systems can remain in the gastric region for several hours and hence significantly prolong the gastric residence time of drugs. Prolonged gastric retention improves bioavailability, reduces drug waste, and improves solubility for drugs that are less soluble in a high pH environment. To formulate a successful stomach specific or gastroretentive drug delivery system, several techniques are currently used such as hydrodynamically balanced systems (HBS) / floating drug delivery system,6 low density system,7,8 raft systems incorporating alginate gels,9,10 bioadhesive or mucoadhesive systems,11 high density systems,12,13 Superporous hydrogels14 and magnetic systems.15,16
Recent development in technology has provided viable dosage alternatives that can be administered via different routes of administration. Various routes that are used these days include oral, parenteral, topical, nasal, rectal, vaginal, ocular etc. But out of these routes, oral route of drug delivery is considered as the most favoured and practiced way of drug delivery, because of following reasons:17,18
* Ease of administration
* More flexibility in designing
* Ease of production
* Low cost
Most of the drugs given via oral route are subjected to absorption throughout the gastrointestinal tract, with major absorption from stomach and intestine.19,20 Various processes occur after the drug release from the dosage form, which affect the absorption of drugs, e.g. degradation of drug by enzymatic or microbial action, precipitation etc.
Drugs, which get absorb from stomach or show local effect, should spend maximum time in stomach. This however, is found very difficult to occur, in case of conventional dosage forms like tablets and capsules, because of the gastric emptying.
Gastric emptying of a particular dosage form depends on various factors like volume and composition of the meal, temperature and viscosity of the meal, pH of stomach, body posture, emotional state of the individual, diseased state, gastric motility altering drugs etc. Parameters that affect the process of gastric emptying can be studied by various techniques viz. scintigraphy, ultrasonology, endoscopy, radiotelemetry, radiology etc.21,22 Prolonged gastric retention of drug is required in the following conditions:17, 19
* Drug is best absorbed from stomach e.g. aspirin, phenylbutazone etc.
* Gastric fluids facilitate and improve the disintegration and dissolution of the drug,
* Dissolution and absorption of drug is promoted by the food e.g. griseoflvin,
* Slow dissolving drugs,
* Drug show local effect within stomach.
In order to fulfill all these conditions, various approaches of the controlled drug delivery have been developed. One of these types of the approaches, which ensure that a particular drug or dosage form remains within stomach for longer duration of time, is Gastro retentive drug delivery system.23
However, in certain condition gastro retention is considered undesirable:17
* For drugs which are gastro irritant, for e.g. Diclofenac sodium, ibuprofen, acetyl salicylic acid etc.
* For acid labile drugs which are stable at gastric pH, e.g. macrolide antibiotics.
* Drugs which get absorbed throughout the gastrointestinal tract equally.
BASIC GIT PHYSIOLOGY: GASTRIC EMPTYING
The stomach is anatomically divided into three parts:
- fundus
- body
- antrum (pylorus)
The separation between stomach and duodenum is the pylorus. The part made of fundus and body acts as a reservoir for undigested material, whereas the antrum is the main site for mixing motions and act as a pump for gastric emptying by propelling actions. Gastric emptying occurs during fasting as well as fed states. The pattern of motility is however distinct for the two states. During the fasting state an interdigestive series of electrical events take place, which cycle both through stomach and intestine every 2–3 h.24 This is called the interdigestive myloelectric cycle or migrating myloelectric cycle (MMC), which is further divided into four phases.25
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Following four phases as described below:26
* PHASE-I:(basal phase) lasts from 30-60 min with rare contractions.
* PHASE-II:(preburst phase) lasts for 20-40 min with the intermittent action potential and contractions as the phase progresses, the intensity and the frequency also increases gradually.
* PHASE-III:(burst phase) lasts for 10-20 min it includes intense and regular contractions for short period. It is due to this wave that all the undigested material is swept out of stomach to small intestine.
* PHASE-IV: Lasts for 0-5 min and occurs between phase 3 and 1 of two consecutive cycles.
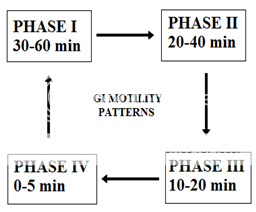
Figure No. 1 patterns of gastroretentive motility
After the ingestion of a mixed meal, the pattern of contractions changes from fasted to that of fed state. This is also known digestive motility pattern and comprises of continuous contractions as in phase II of fasted state. These contractions result in reducing the size of food particles (less than 1mm), which are propelled towards the pylorus in the suspension form. During the fed state onset of mmc, is delayed resulting in slowdown of gastric emptying rate. Scintigraphic studies involving the measurements of gastric emptying rates in healthy human subjects have revealed that an orally administered controlled release dosage form is mainly subject to two physiological adversities:
1. Short GRT
2. Unpredictable GET
Yet another major adversity encountered through the oral route is the first pass effect, which leads to reduced systemic bioavailability of many drugs.26
Gastroretentive drug delivery systems:
Gastroretentive drug delivery systems are the systems which are retained in the stomach for a longer period of time and thereby, improve the bioavailability of drugs. Ifthe drugs are poorly soluble in the intestine due to alkaline pH, gastric retention may increase solubility before they are emptied, resulting in gastrointestinal absorption of drugs with narrow therapeutic absorption window, as well as, controlling release of drugs having site specific?absorption limitation. Drugs that could take advantage of gastric retention include the drugs whose solubility is less in the higher pH of the small intestine than the stomach (e.g. chlordiazepoxide and cinnarizine), the drugs prone for degradation in the intestinal pH (e.g. captopril), and the drugs for local action in the stomach (e.g. misoprostol). Antibiotics, catecholamines, sedatives, analgesics, anticonvulsants, muscle relaxants, anti hypertensives and vitamins can also be administered in HBS dosage form.27
Factors affecting gastro retention:23
a) Physiological factors:
· Size of dosage form: Dosage forms having diameter greater than the diameter of pyloric sphincter escape from gastric emptying and remain within gastric region.
· Shape of dosage form: Round or Ring shaped dosage form are considered better in comparison to other shapes.
· Density: Location of the particular gastro retentive dosage form in gastric region depends on density of the system. Those with low density tend to float on the gastric fluid surface while high density systems sink to bottom of stomach.
b) Biological Factors:
· Age: Geriatric patients show a longer gastric retention time, while the neonates and children have low gastric retention time, in comparison to a normal adult.
· Gender: Gastric retention time in male (3-4 hours) is less than the female (4-6 hours).
· Fed or Unfed state: Gastric motility is higher in fasting conditions which depicts lesser gastric retention time.
· Feed frequency: Higher the frequency of taking food, longer will be the gastro retention time.
· Nature of meal: High amount of fatty acids and other indigestible polymers generally decreases the gastric retention time by altering gastric motility.
· Concomitant drug administration: Administration of certain drugs along with gastric motility enhancers (metoclopramide, cisapride) or depressants (atropine), greatly affect gastric retention time and hence absorption of stomach specific absorbing drugs.
· Disease state: Gastro retentive time is altered during the various gastric diseases like Crohn’s disease etc.
Advantages of Gastro retentive drug delivery system:28,29
* The gastroretensive systems are advantageous for drugs absorbed through the stomach. E.g. Ferrous salts, antacids.
* Acidic substances like aspirin cause irritation on the stomach wall when come in contact with it. Hence HBS formulation may be useful for the administration of aspirin and other similar drugs.
* Administration of prolongs release floating dosage forms, tablet or capsules, will result in dissolution of the drug in the gastric fluid. They dissolve in the gastric fluid would be available for absorption in the small intestine after emptying of the stomach contents. It is therefore expected that a drug will be fully absorbed from floating dosage forms if it remains in the solution form even at the alkaline pH of the intestine.
* The gastroretensive systems are advantageous for drugs meant for local action in the stomach. e.g. antacids.
* When there is a vigorous intestinal movement and a short transit time as might occur in certain type of diarrhoea, poor absorption is expected. Under such circumstances it may be advantageous to keep the drug in floating condition in stomach to get a relatively better response.
Disadvantages of Gastro retentive drug delivery system:
* Floating system is not feasible for those drugs that have solubility or stability problem in G.I. tract.
* These systems require a high level of fluid in the stomach for drug delivery to float and work efficiently-coat, water.
* The drugs that are significantly absorbed through out gastrointestinal tract, which undergo significant first pass metabolism, are only desirable candidate.
* Some drugs present in the floating system causes irritation to gastric mucosa.
Approaches for Gastric Retention system:
Various approaches that have been adopted to increase the retention of an oral dosage form in the stomach, includes: floating systems, swelling and expanding systems, bioadhesive systems, modified shape systems, high density systems and other delayed gastric emptying devices.30,31 FDDS or HBS have a bulk density lower than the gastric fluids and thus remain buoyant in the stomach without affecting the gastric emptying rate for a prolonged period of time. While the system is floating on the gastric contents, the drug is released slowly at a desired rate from the system. After the drug is released, the residual system is emptied from the stomach.32 swelling type dosage forms are such that after swallowing, these products swell to an extent that prevents their exit from stomach through the pylorus. Bioadhesive systems are used to localize a delivery device within the lumen and cavity of the body to enhance drug absorption process. These bioadhesive polymers adhere to the epithelial surface of the GIT. High density formulations include coated pellets, which have density greater than that of stomach contents. Here the drug is coated by inert heavy materials like zinc oxide, barium sulphate and iron powder. Modified shapes molded from silastic polymer or elastomer also extend the GRT.33
Different approaches, that have been developed, for formulating dosage form to produce adequate gastric retention and release within gastric region, are as follows:34,35
* High-density drug delivery system
* Floating drug delivery system
- Hydrodynamically balanced drug delivery system
- Gas-generating drug delivery system
- Raft-forming drug delivery system
- Low-density drug delivery system
* Expandable drug delivery system
* Super porous hydrogels
* Mucoadhesive or bioadhesive drug delivery system
* Magnetic drug delivery system
High Density Gastro-retentive Drug Delivery System:It is a well known fact, that the density of the gastric content is approximately similar to water. So, when the density of the dosage form is higher than water, it tends to deposit or sink at the bottom of the stomach, near pyloric region. These sinked dosage forms, there, withstand against peristaltic contractions and do not get emptied from the stomach. Retarded gastrointestinal transit, in case of such dosage form has been reported to extend gastric retention time up to 6 to 24 hours. Commonly used excipients in such dosage forms are barium sulphate, titanium oxide etc., which raises the density of system up to 1.4 to 2.5 gram per cubic centimeter.
Such type of system has shown promising results in the animals, but no system has been in the market for human consumption.36
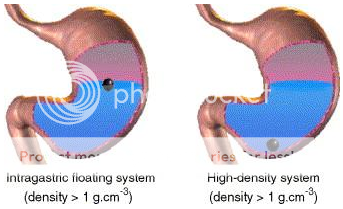
Figure No. 2 Diagramof Gastroretentive drug delivery system (low density and high density systems)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Floating Delivery System: Floating drug delivery systems meant for gastric retention, float on the surface of the gastric fluids, due to their low density and produce prolonged effect by showing the release, while being buoyant on gastric fluid surface.37 This type of delivery system is of great value for drugs which get absorbed from upper part of the stomach i.e. their absorption window resides in upper part of stomach.35
Though, immediate floating of the delivery system can only be achieved if the density of the delivery system is on lower side.38 Delivery system with higher density, initially settle down in stomach and then tend to float with decrease in the density of the system. But, with such system, there may be a possibility of gastric emptying of system, before the floating starts. Low density of system, which leads to floating, rendered either by incorporation of low density excipients or by providing a mechanism which leads to air entrapment within the system.39,40
Different types of floating systems have been developed, which may involve generation of gas (effervescent) or non effervescent. Approaches for producing floating systems are as follows:
· Hydro-dynamically balanced system: These systems consists of hydrophilic gel forming polymers like HPMC, hydroxy ethyl cellulose, hydroxy propyl cellulose, agar, alginic acid etc. and are generally designed for single unit dosage.41 In this approach, hydrophilic polymer is mixed with drug along with other excipients and encapsulated in gelatin shell. Gelatin, being hygroscopic in nature dissolves rapidly in the gastric fluid and exposes the hydrophilic polymer and drug content to the fluids.23 Polymer fraction present on the surface then undergoes hydration and swelling, to produce a floating mass.
· Gas generating systems (Effervescent systems): Floating to a system can also be produced by the gas bubble generation. For this, carbon dioxide is generated within the system by incorporating bicarbonates and carbonates, which on reaction with gastric content (gastric acid) produce gas.23 System utilizes the presence of swellable polymers like methocel, chitosan etc. along with effervescent components like bicarbonates with citric or tartaric acid for gas generation.18 General approach for preparation of such system involves preparation of core with drug, swellable polymer along with effervescent system and coating with hydrophobic polymer like ethyl cellulose, which acts as semi-permeable membrane to regulate the inflow of the gastric content and keep the system intact within the polymer coating for complete gastro retention period.
· Raft forming system:18,42In this type of system, a gel forming solution is prepared that swells on coming in contact with gastric contents and form viscous gel like layer which resemble same as a raft in river. This, raft like layer of gel, has a very low bulk density due to the generation of carbon dioxide within system, which makes the layer to float on the surface of gastric content. Raft forming system consists of a gel forming polymeric agent and carbon dioxide producing agents like bicarbonates and carbonates.43 Gel forming agents that are being widely utilized for the formation of the raft like consistency are from the alginate category e.g. alginic acid. Such formulations also include antacids such as aluminium hydroxide, calcium carbonate etc. for reduction of the gastric acidity. As the solution after gel formation floats on the surface of the gastric fluids, such systems are used for the treatment of the gastro-esophageal reflux.35
· Low density systems: Major limitation in case of effervescent delivery system is the time lag before floating on the gastric contents. In this time period, it may be possible that the delivery system may get evacuated, before floating and drug release. So, in order to overcome this limitation, low density systems (lesser than 1000 mg per cubic centimeter) have been developed, which show immediate floating and release of drug on the gastric content surface. System is basically consists of low density materials which entrap oil or air.35
Expandable Systems:35,44 A dosage form, which is bigger in size than the diameter of the pyloric sphincter can withstand with the gastric transit and escape the evacuation from the gastric region. But while designing such system, it should be kept in mind that the dosage form should be of adequate size, so that it can be easily swallowed and should not cause gastric obstruction. It should also be considered, that after complete drug release from system, it can be evacuated easily from the gastric system. The concept of designing such expandable system is to prepare a carrier (e.g. capsule) and to incorporate in it, a compressed system which expands, as it comes in contact with the gastric contents. As, the size of the system increases and reaches to diameter or dimensions, greater than the size of the pyloric sphincter, it cannot leave the stomach while gastric emptying process.
Super Porous Hydrogels: These also come under the category of expandable or swellable system. Super porous hydrogels tends to swell and enlarge in size within a minute, due to rapid water uptake from gastric contents by capillary action via numerous interconnected pores and have sufficient mechanical strength to withstand pressure during gastric contractions. This can be achieved, by incorporating hydrophilic particulate materials, e.g. croscarmellose sodium.35
Mucoadhesive and bioadhesive systems:44 Mucoadhesive systems adhere to gastric mucosa and remain in the gastric region for prolong time. Adherence occurs due to hydration and swelling of the adhesive polymer on contact with gastric contents. Materials that are commonly used for imparting the mucoadhesive or bioadhesive property to the whole system include carbopol, chitosan, tragacanth, PEG, HPMC etc. However, it is very difficult to maintain the system adhered within the gastric region, due to rapid turnover rate of the gastric mucus. Such systems also pose another problem that it is very difficult to control the specific adhesivity of system to gastric mucosa and it may also adhere to other surfaces, e.g. esophagus.
Magnetic Systems: Magnetic systems involves the incorporation of the small magnet inside the core or matrix of the system and application of the another magnet on the abdomen region, externally. This system, however, provide satisfactory results, there is a problem of placing the magnet externally at the right position with great accuracy and precision.35
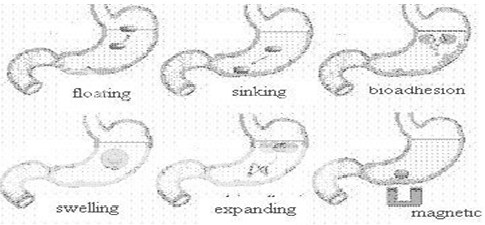
Figure No. 3 Various gastroretentive System33
Stomach Specific Floating Drug Delivery
Stomach Specific floating drug delivery system (FDDS) have a bulk density less than gastric fluids and so remain buoyant in the stomach without affecting the gastric emptying rate for a prolonged period of time. While the system is floating on the gastric contents, the drug is released slowly at the desired rate from the system. After release of drug, the residual system is emptied from the stomach. This results in an increased GRT and a better control of fluctuations in plasma drug concentration. The floating sustained release dosage forms present most of the characteristics of hydrophilic matrices and are known as ‘hydrodynamically balanced systems’ (‘HBS’) since they are able to maintain their low apparent density, while the polymer hydrates and builds a gelled barrier at the outer surface. The drug is released progressively from the swollen matrix, as in the case of conventional hydrophilic matrices. These forms are expected to remain buoyant (3- 4 hours) on the gastric contents without affecting the intrinsic rate of emptying because their bulk density is lower than that of the gastric contents. Among the different hydrocolloids recommended for floating form formulations, cellulose ether polymers are most popular, especially hydroxy propyl methyl cellulose (HPMC). Fatty material with a bulk density lower than one may be added to the formulation to decrease the water intake rate and increase buoyancy.45
Parallel to formulation studies, investigations have been undertaken in animals and humans to evaluate the intragastric retention performances of floating forms. These assessments were realised either indirectly through pharmacokinetic studies with a drug tracer, or directly by means of X-ray and gamma scintigraphic monitoring of the form transit in the GI tract.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Mechanism of floating systems
Various attempts have been made to retain the dosage form in the stomach as a way of increasing the retention time. These attempts include introducing floating dosage forms (gas-generating systems and swelling or expanding systems), mucoadhesive systems, high-density systems, modified shape systems, gastric-emptying delaying devices and co-administration of gastric-emptying delaying drugs. Among these, the floating dosage forms have been most commonly used. Floating drug delivery systems (FDDS) have a bulk density less than gastric fluids and so remain buoyant in the stomach without affecting the gastric emptying rate for a prolonged period of time. While the system is floating on the gastric contents (given in the Figure 1 (a)), the drug is released slowly at the desired rate from the system. After release of drug, the residual system is emptied from the stomach. This results in an increased GRT and a better control of the fluctuations in plasma drug concentration. However, besides a minimal gastric content needed to allow the proper achievement of the buoyancy retention principle, a minimal level of floating force (F) is also required to keep the dosage form reliably buoyant on the surface of the meal. To measure the floating force kinetics, a novel apparatus for determination of resultant weight has been reported in the literature. The apparatus operates by measuring continuously the force equivalent to F (as a function of time) that is required to maintain the submerged object. The object floats better if F is on the higher positive side (Figure 1(b)). This apparatus helps in optimizing FDDS with respect to stability and durability of floating forces produced in order to prevent the drawbacks of unforeseeable intragastric buoyancy capability variations.46
F = F buoyancy - F gravity
= (Df - Ds) gv--- (1)
Where, F = total vertical force, Df = fluid density,
Ds = object density, v = volume and
g = acceleration due to gravity.
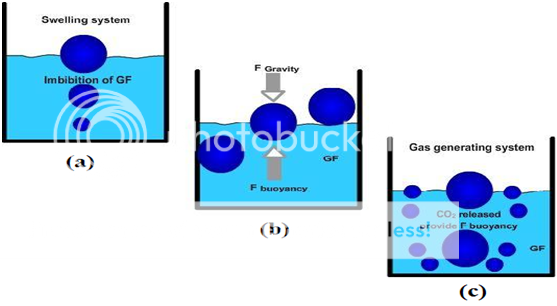
Figure No. 4 Mechanism of floating system, Gastric Fluid.
Based on the mechanism of buoyancy FDDS can be classified into:
A. Single Unit Floating Dosage Systems
a) Effervescent Systems (Gas-generating Systems)
b) Non-effervescent Systems
B. Multiple Unit Floating Dosage Systems
a) Non-effervescent Systems
b) Effervescent Systems (Gas-generating Systems)
c) Hollow Microspheres
C. Raft Forming Systems
A. Single Unit Floating Dosage Systems:
a) Effervescent Systems (Gas-generating Systems):
These buoyant systems utilized matrices prepared with swellable polymers like HPMC, polysaccharides like chitosan, effervescent components like sodium bicarbonate, citric acid and tartaric acid or chambers containing a liquid that gasifies at body temperature. The optimal stoichiometric ratio of citric acid and sodium bicarbonate for gas generation is reported to be 0.76:1. The common approach for preparing these systems involves resin beads loaded with bicarbonate and coated with ethylcellulose. The coating, which is insoluble but permeable, allows permeation of water. Thus, carbon dioxide is released, causing the beads to float in the stomach.47
Excipients used most commonly in these systems include HPMC, polyacrylate polymers, polyvinyl acetate, Carbopol®, agar, sodium alginate, calcium chloride, polyethylene oxide and polycarbonates.
Ozdemir et al 48 prepared floating bilayer tablets with controlled release for furosemide. The low solubility of the drug could be enhanced by using the kneading method, preparing a solid dispersion with betacyclodextrin mixed in a 1:1 ratio. One layer contained the polymers HPMC 4000, HPMC 100, and CMC (for the control of the drug delivery) and the drug. The second layer contained the effervescent mixture of sodium bicarbonate and citric acid. Radiographic studies on 6 healthy male volunteers showed that floating tablets were retained in stomach for 6 hours and further blood analysis studies showed that bioavailability of these tablets was 1.8 times that of the conventional tablets. On measuring the volume of urine the peak diuretic effect seen in the conventional tablets was decreased and prolonged in the case of floating dosage form.
Penners et al 49 prepared an expandable tablet containing mixture of polyvinyl lactams and polyacrylates that swell rapidly in an aqueous environment and thus stays in stomach over an extended period of time. In addition to this, gas-forming agents were also incorporated so as soon as the gas formed, the density of the system was reduced and thus the system tended to float on the gastric environment.
Talwaret al 50 prepared a once-daily formulation for oral administration of ciprofloxacin. The formulation was composed of 69.9% ciprofloxacin base, 0.34% sodium alginate, 1.03% xanthum gum, 13.7% sodium bicarbonate, and 12.1% cross-linked poly vinyl pyrrolidine. The cross linked PVP initially and the gel-forming polymers later formed a hydrated gel matrix that entrapped the gas, causing the tablet to float and be retained in the stomach The hydrated gel matrix created a diffusion path for the drug, resulting in sustained release of the drug.
b) Non-effervescent Systems:
This type of system, after swallowing, swells unrestrained via imbibitions of gastric fluid to an extent that it prevents their exit from the stomach. Thesesystems may be referred to as the ‘plug-type systems’ since they have a tendency to remain lodged near the pyloric sphincter. One of the formulation methods of such dosage forms involves the mixing of drug with a gel, which swells in contact with gastric fluid after oral administration and maintains a relative integrity of shape and a bulk density of less than one within the outer gelatinous barrier. The air trapped by the swollen polymer confers buoyancy to these dosage forms. Examples of this type of FDDS include colloidal gel barrier,51 micro porous compartment system,52 alginate beads,53 and hollow microspheres.54
Another type is a Fluid-filled floating chamber55 which includes incorporation of a gas-filled floatation chamber into a micro porous component that houses a drug reservoir. Apertures or openings are present along the top and bottom walls through which the gastrointestinal tract fluid enters to dissolve the drug. The other two walls in contact with the fluid are sealed so that the undissolved drug remains therein. The fluid present could be air, under partial vacuum or any other suitable gas, liquid, or solid having an appropriate specific gravity and an inert behaviour. The device is of swallowable size, remains afloat within the stomach for a prolonged time, and after the complete release the shell disintegrates, passes off to the intestine, and is eliminated.
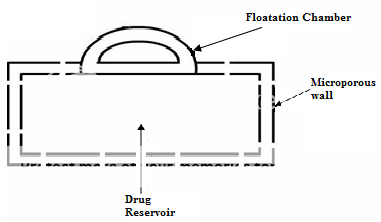
Figure No. 5 Gas filled floatation chamber
A newer Self-correcting floatable asymmetric configuration drug delivery system56 has a 3-layer matrix to control the drug release. This 3-layer principle has been improved by development of an asymmetric configuration drug delivery system in order to modulate the release extent and achieve zero-order release kinetics by initially maintaining a constant area at the diffusing front with subsequent dissolution/erosion toward the completion of the release process. The system was designed in such a manner that it floated to prolong gastric residence time in vivo, resulting in longer total transit time within the gastrointestinal tract environment with maximum absorptive capacity and consequently greater bioavailability. This particular characteristic would be applicable to drugs that have pH-dependent solubility, a narrow window of absorption, and areabsorbed by active transport from either the proximal or distal portion of the small intestine.
Yang et al 57 developed a swellable asymmetric triple-layer tablet with floating ability to prolong the gastric residence time of triple drug regimen (tetracycline, metronidazole, and clarithromycin) in Helicobacter pylori–associated peptic ulcers using HPMC and poly (ethylene oxide) (PEO) as the rate-controlling polymeric membrane excipients. The design of the delivery system was based on the swellable asymmetric triple-layer tablet approach. HPMC and poly (ethylene oxide) were the major rate-controlling polymeric excipients. Tetracycline and metronidazole were incorporated into the core layer of the triple-layer matrix for controlled delivery, while bismuth salt was included in one of the outer layers for instant release. The floatation was accomplished by incorporating a gas-generating layer consisting of sodium bicarbonate and calcium carbonate with swellable polymers. Over 6-8 hours of sustained delivery of tetracycline and metronidazole was achieved with this dosage form which was still floating.
Streubel et al 58 prepared single-unit floating tablets based on polypropylene foam powder (Accurel MP 1000®) and matrix-forming polymer. Highly porous foam powder in matrix tablets provided density much lower than the density of the release medium. It was concluded that varying the ratios of matrix-forming polymers and the foam powder could alter the drug release patterns effectively.
Wu et al 59 prepared floating sustained release tablets of nimodipine by using HPMC and PEG 6000. Prior to formulation of floating tablets, nimodipine was incorporated into poloxamer-188 solid dispersion after which it was directly compressed into floating tablets. It was observed that by increasing the HPMC and decreasing the PEG 6000 content a decline in invitro release of nimodipine was observed.
Nur and Zhang 60 prepared floating tablets of captopril using HPMC (4000 and 15 000 cps) and carbopol 934P. It was concluded that the buoyancy of the tablet is governed by both the swelling of the hydrocolloid particles on the tablet surface when it contacts the gastric fluids and the presence of internal voids in the centre of the tablet (porosity). A prolonged release from these floating tablets was observed as compared with the conventional tablets and a 24-hour controlled release from the dosage form of captopril was achieved.
Single-unit formulations are associated with problems such as sticking together or being obstructed in the gastrointestinal tract, which may have a potential danger of producing irritation. The main drawback of such system is “all or none” phenomenon. In such cases there is a danger of passing of the dosage form to intestinal partat the time of house-keeper waves. To overcome this difficulty multiple unit dosage forms are designed.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
B. Multiple Unit Floating Systems:
In spite of extensive research and development in the area of HBS and other floating tablets, these systems suffer from an important drawback of high variability of gastrointestinal transit time, when orally administered, because of their all-or-nothing gastric emptying nature. In order to overcome the above problem, multiple unit floating systems were developed, which reduce the inter-subject variability in absorption and lower the probability of dose-dumping. Reports have been found on the development of both non-effervescent and effervescent multiple unit systems.61 Much research has been focused and the scientists are still exploring the field of hollow microspheres, capable of floating on the gastric fluid and having improved gastric retention properties.
a) Non-effervescent Systems:
No much report was found in the literature on non-effervescent multiple unit systems, as compared to the effervescent systems. However, few workers have reported the possibility of developing such system containing indomethacin, using chitosan as the polymeric excipient. A multiple unit HBS containing indomethacin as a model drug prepared by extrusion process is reported.62 A mixture of drug, chitosan and acetic acid is extruded through a needle, and the extrudate is cut and dried. Chitosan hydrates and floats in the acidic media, and the required drug release could be obtained by modifying the drug-polymer ratio.
b) Effervescent Systems (Gas-generating Systems):
Ikura et al 63 reported sustained release floating granules containing tetracycline hydrochloride. The granules are a mixture of drug granulates of two stages A and B, of which A contains 60 parts of HPMC, 40 parts of polyacrylic acid and 20 parts of drug and B contains 70 parts of sodium bicarbonate and 30 parts of tartaric acid. 60 parts by weight of granules of stage A and 30 parts by weight of granules of stage B are mixed along with a lubricant and filled into capsule. In dissolution media, the capsule shell dissolves and liberates the granules, which showed a floating time of more than 8 h and sustained drug release of 80% in about 6.5 h. Floating minicapsules of pepstatin having a diameter of 0.1-0.2 mm has been reported by Umezawa.64
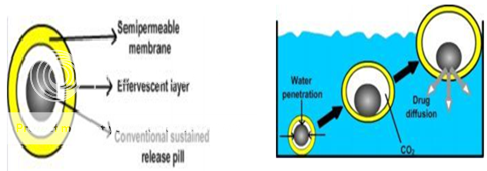
Figure No. 6 Different layers i) Semi-permeable membrane, ii) Effervescent Layer iii) Core pill layer b) Mechanism offloatation via CO2 generation.
These mini capsules contain a central core and a coating. The central core consists of a granule composed of sodium bicarbonate, lactose and a binder, which is coated with HPMC. Pepstatin is coated on the top of the HPMC layer. The system floats because of the CO2 release in gastric fluid and the pepstatin resides in the stomach for prolonged period. Alginates have received much attention in the development of multiple unit systems. Alginates are non-toxic, biodegradable linear copolymers composed of L-glucuronic and L-mannuronic acid residues. A multiple unit system prepared by Iannuccelli et al 65 comprises of calcium alginate core and calcium alginate/PVA membrane, both separated by an air compartment. In presence of water, the PVA leaches out and increases the membrane permeability, maintaining the integrity of the air compartment. Increase in molecular weight and concentration of PVA, resulted in enhancement of the floating properties of the system. Freeze-drying technique is also reported for the preparation of floating calcium alginate beads 66. Sodium alginate solution is added drop wise into the aqueous solution of calcium chloride, causing the instant gelation of the droplet surface, due to the formation of calcium alginate. The obtained beads are freeze-dried resulting in a porous structure, which aid in floating. The authors studied the behaviour of radiolabeled floating beads and compared with non floating beads in human volunteers using gamma scintigraphy. Prolonged gastric residence time of more than 5.5 h was observed for floating beads. The non floating beads had a shorter residence time with a mean onset emptying time of 1 h.
Ichikawa et al 67 developed a new multiple type of floating dosage system having a pill in the core, composed of effervescent layers and swellable membrane layers coated on sustained release pills (shown in figure 3). The inner layer of effervescent agents containing sodium bicarbonate and tartaric acid was divided into 2 sub layers to avoid direct contact between the 2 agents. These sub layers were surrounded by a swellable polymer membrane containing polyvinyl acetate and purified shellac. When this system was immersed in the buffer at 37ºC, it settled down and the solution permeated into the effervescent layer through the outer swellable membrane. CO2 was generated by the neutralization reaction between the 2 effervescent agents, producing swollen pills (like balloons) with a density less than 1.0 g/ml.
c) Hollow Microspheres:
Hollow microspheres are considered as one of the most promising buoyant systems, as they possess the unique advantages of multiple unit systems as well as better floating properties, because of central hollow space inside the microsphere. The general techniques involved in their preparation include simple solvent evaporation, and solvent diffusion and evaporation. The drug release and better floating properties mainly depend on the type of polymer, plasticizer and the solvents employed for the preparation. Polymers such as polycarbonate, Eudragit® S and cellulose acetate were used in the preparation of hollow microspheres, and the drug release can be modulated by optimizing the polymer quantity and the polymer-plasticizer ratio.
Sustained release floating microspheres using polycarbonate were developed by Thanoo et al,68 employing solvent evaporation technique. Aspirin, griseofulvin and p-nitroaniline were used as model drugs. Dispersed phase containing polycarbonate solution in dichloromethane, and micronized drug, was added to the dispersion medium containing sodium chloride, polyvinyl alcohol and methanol. The dispersion was stirred for 3-4 h to assure the complete solvent evaporation, and the microspheres obtained were filtered, washed with cold water and dried. The spherical and hollow nature of the microspheres was confirmed by Scanning electron microscopic studies. The microspheres showed a drug payload of more than 50%, and the amount of drug incorporated is found to influence the particle size distribution and drug release. The larger proportion of bigger particles was seen at high drug loading, which can be attributed to the increased viscosity of the dispersed phase.
Kawashima et al 69 described hollow microspheres (microballoons) with drug in their outer polymer shells, prepared by a novel emulsion solvent diffusion method. A solution of drug and enteric acrylic polymer (Eudragit®S) in a mixture of ethanol and dichloromethane is added to the aqueous phase containing polyvinyl alcohol (0.75% w/v) and stirred continuously to obtain o/w emulsion. The microspheres obtained are filtered, water washed and dried. The diffusion and evaporation profiles of ethanol and dichloromethane, suggested a rapid diffusion of ethanol from the droplets into the aqueous phase, which might reduce the polymer solubility in the droplet because of insoluble property of Eudragit® S in dichloromethane. Hence, the polymer precipitation occurs instantly at the droplet surface, forming a film-like shell enclosing dichloromethane and drug. The microspheres showed good flow and packing properties, and a floating time of more than 12 h on acidic medium containing surfactant.
Josephet al 55 developed a floating dosage form of piroxicam based on hollow polycarbonate microspheres. The microspheres were prepared by the solvent evaporation technique. Encapsulation efficiency of ~95% was achieved. In vivo studies were performed in healthy male albino rabbits. Pharmacokinetic analysiswas derived from plasma concentration vs time plot and revealed that the bioavailability from the piroxicam microspheres alone was 1.4 times that of the free drug and 4.8 times that of a dosage form consisting of microspheres plus the loading dose and was capable of sustained delivery of the drug over a prolonged period.
C. Raft Forming Systems:
Raft forming systems have received much attention for the delivery of antacids and drug delivery for gastrointestinal infections and disorders. The mechanism involved in the raft formation includes the formation of viscous cohesive gel in contact with gastric fluids, wherein each portion of the liquid swells forming a continuous layer called a raft. This raft floats on gastric fluids because of low bulk density created by the formation of CO2.Usually, the system contains a gel forming agent and alkaline bicarbonates or carbonates responsible for the formation of CO2 to make the system less dense and float on the gastric fluids.70 Jorgen et al71,72 described an antacid raft forming floating system. The system contains a gel forming agent (e.g. alginic acid), sodium bicarbonate and acid neutralizer, which forms a foaming sodium alginate gel (raft) when in contact with gastric fluids. The raft thus formed floats on the gastric fluids and prevents the reflux of the gastric contents (i.e. gastric acid) into the esophagus by acting as a barrier between the stomach and esophagus. A patent assigned to Reckitt and Colman Products Ltd., describes a raft forming formulation for the treatment of helicobacter pylori (H. Pylori) infections in the GIT. The composition contained drug, alginic acid, sodium bicarbonate, calcium carbonate, mannitol and a sweetener. These ingredients were granulated, and citric acid was added to the granules. The formulation produces effervescence and aerates the raft formed, making it float.
Drugs Used In the Formulations of Stomach Specific Floating Dosage Forms
* Floating microspheres– Aspirin, Griseofulvin, p-nitroaniline, Ibuprofen, Ketoprofen,73 Piroxicam, Verapamil, Cholestyramine, Theophylline, Nifedipine, Nicardipine, Dipyridamol, Tranilast74 and Terfinadine.75
* Floatinggranules – Diclofenac sodium, Indomethacin and Prednisolone.
* Films – Cinnarizine,76 Albendazole.
* Floating tablets and Pills- Acetaminophen, Acetylsalicylic acid, Ampicillin, Amoxycillin trihydrate, Atenolol, Fluorouracil, Isosorbide mononitrate,77 Para - aminobenzoic acid, Piretanide,78 Theophylline, Verapamil hydrochloride, Chlorpheniramine maleate, Aspirin, Calcium Carbonate, Fluorouracil, Prednisolone, Sotalol,79 pentoxyfilline and Diltiazem HCl.
* Floating Capsules- Chlordiazepoxide hydrogen chloride, Diazepam,80 Furosemide, Misoprostol, L-Dopa, Benserazide Ursodeoxycholic acid81 and Pepstatin, and Propranolol.
Polymers and other ingredients
Following types of ingredients can be incorporated into HBS dosage form in addition to the drugs:
* Hydrocolloids (20%-75%): They can be synthetics, anionic or non-ionic like hydrophilic gums, modified cellulose derivatives. Eg. Acacia, pectin, Chitosan, agar, casein, bentonite, veegum, HPMC (K4M, K100M and K15M), Gellan gum (Gelrite®), Sodium CMC, MC, HPC.
* Inert fatty materials (5%-75%): Edible, inert fatty materials having a specific gravity of less than one can be used to decrease the hydrophilic property of formulation and hence increase buoyancy. Examples Beeswax, fatty acids, long chain fatty alcohols, Gelucires® 39/01 and 43/01.
* Effervescent agents: Sodium bicarbonate, citric acid, tartaric acid, Di-SGC (Di-Sodium Glycine Carbonate, CG (Citroglycine).
* Release rate accelerants (5%-60%): eg lactose, mannitol.
* Release rate retardants (5%-60%): eg Dicalcium phosphate, talc, magnesium stearate.
* Buoyancy increasing agents (upto80%): eg. Ethyl cellulose.
* Low density material: Polypropylene foam powder (Accurel MP 1000®).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Evaluation parameters of stomach specific FDDS
However, it has to be pointed out that good in vitro floating behaviour alone is not sufficient proof for efficient gastric retention in vivo. The effects of the simultaneous presence of food and of the complexmotility of the stomach are difficult to estimate. Obviously, only in vivo studies can provide definite proof that prolonged gastric residence is obtained.
1) Measurement of buoyancy capabilities of the FDDS.82The floating behaviour was evaluated with resultant weight measurements. The experiment was carried out in two different media, deionised water and simulated meal, in order to monitor possible difference. The results showed that higher molecular weight polymers with slower rate of hydration had enhanced floating behaviour and it was observed more in simulated meal medium compared to deionised water.
2) Floating time and dissolution: The test for floating time measurement is usually performed in stimulated gastric fluid or 0.1 mole.lit-1 HCl maintained at 370o C. It is determined by using USP dissolution apparatus containing 900 ml of 0.1 mole.lit-1 HCl as the dissolution medium at 370o C. The time taken by the dosage form to float is termed as floating lag time and the time for which the dosage form floats is termed as the floating or flotation time.83
Recently Gohel et al 84 proposed a more relevant in vitro dissolution method to evaluate a floating drug delivery system (for tablet dosage form). A 100-mL glass beaker was modified by adding a side arm at the bottom of the beaker so that the beaker can hold 70 ml of 0.1 mole.lit-1 HCl dissolution medium and allow collection of samples. A burette was mounted above the beaker to deliver the dissolution medium at a flow rate of 2 ml/min to mimic gastric acid secretion rate. The performance of the modified dissolution apparatus was compared with USP dissolution.
Apparatus 2 (Paddle) The problem of adherence of the tablet to the shaft of the paddle was observed with the USP dissolution apparatus. The tablet did not stickto the agitating device in the proposed dissolution method. The drug release followed zero-order kinetics in the proposed method. Similarity of dissolution curves was observed between the USP method and the proposed method at 10% differencelevel (f2=57). The proposed test may show good in vitro-in vivo correlation since an attempt is made to mimic the in vivo conditions such as gastric volume, gastric emptying, and gastric acid secretion rate.
3) Drug release: Dissolution tests are performed using the dissolution apparatus. Samples are withdrawn periodically from the dissolution medium with replacement and then analyzed for their drug content after an appropriate dilution.
4) Content uniformity, Hardness, Friability (Tablets).
5) Drug loading, drug entrapment efficiency, particle size analysis, surface characterization(for floating microspheres and beads) Drug loading is assessed by crushing accurately weighed sample of beads or microspheres in a mortar and added to the appropriate dissolution medium which is then centrifuged, filtered and analyzed by various analytical methods like spectrophotometry. The percentage drug loading is calculated by dividing the amount of drug in the sample by the weight total beads or microspheres. The particle size and the size distribution of beads or microspheres are determined in the dry state using the optical microscopy method. The external and cross-sectional morphology (surface characterization) is done by scanning electron microscope (SEM).85
6) X-Ray/Gamma Scintigraphy:X-Ray/Gamma Scintigraphy is a very popular evaluation parameter for floating dosage form now a day.86 It helps to locate dosage form in the GIT and by which one can predict and correlate the gastric emptying time and the passage of dosage form in the GIT. Here the inclusion of a radio-opaque material into a solid dosage form enables it to be visualized by X- rays. Similarly, the inclusion of a γ-emitting radionuclide in a formulation allows indirect external observation using a γ-camera or scintiscanner.87 In case of γ-scintigraphy, the γ-rays emitted by the radionuclide are focused on a camera, which helps to monitor the location of the dosage form in the GI tract.88
7) Pharmacokinetic studies:Pharmacokinetic studies are the integral part of the in vivo studies and several works has been on that. Sawicki89 studied the pharmacokinetics of verapamil, from the floating pellets containing drug, filled into a capsule, and compared with the conventional verapamil tablets of similar dose (40 mg). The tmax and AUC (0-infinity) values (3.75 h and 364.65ng.ml-1h respectively) for floating pellets were comparatively higher than those obtained for the conventional verapamil tablets (tmax value 1.21 h, and AUC value 224.22ng.ml-1h). No much difference was found between the Cmax values of both the formulations, suggesting the improved bioavailability of the floating pellets compared to the conventional tablets. An improvement in bioavailability has also been observed with piroxicam in hollow polycarbonate microspheres administered in rabbits. The microspheres showed about 1.4 times more bioavailability, and the elimination half-life was increased by about three times than the free drug.
Recent advances in stomach specific floating dosage forms
Ninan Ma et al 90 developed a type of multi-unit floating alginate (Alg) microspheres by the ionotropic gelation method with calcium carbonate (CaCO3) being used as gas-forming agent. Attempts were made to enhance the drug encapsulation efficiency and delay the drug release by adding chitosan (Cs) into the gelation medium and by coating with Eudragit, respectively. The gastrointestinal transit of optimized floating sustained-release microspheres was compared with that of the non-floating system manufactured from identical material using the technique of gamma-scintigraphy in healthy human volunteers. It was found that the drug encapsulation efficiency of Cs–Alg microspheres was much higher than that of the Ca–Alg microspheres, andcoating the microspheres with Eudragit RS could extend the drug release significantly. Both uncoating and coating microspheres were able to continuously float over the simulated gastric fluid (SGF) for 24 h in vitro. Prolonged gastricretention time (GRT) of over 5 h was achieved in the volunteer for the optimized coating floating microspheres (FM). In contrast, non-floating system (NFM) could be emptied within 2.5 h.
Strübing et al 91 investigated the mechanism of floating and drug release behaviour of poly (vinyl acetate)-based floating tablets with membrane controlled drug delivery. Propranolol HCl containing tablets with Kollidon® SR as an excipient for direct compression and different Kollicoat® SR 30 D/Kollicoat® IR coats varying from 10 to 20 mg polymer/cm2were investigated regarding drug release in 0.1 mole. lit-1 HCl. Furthermore, the onset of floating, the floating duration and the floating strength of the device were determined. In addition, benchtop MRI studies of selected samples were performed. Coated tablets with 10 mg polymer/cm2SR/IR, 8.5:1.5 coat exhibited the shortest lag times prior to drug release and floating onset, the fastest increase in and highest maximum values of floating strength. The drug release was delayed efficiently within a time interval of 24 h by showing linear drug release characteristics.
Jang et al 92 has prepared a gastroretentive drug delivery system of DA-6034, a new synthetic flavonoidderivative, for the treatment of gastritis was developed by using effervescent floating matrix system (EFMS). The therapeutic limitations of DA-6034 caused by its low solubility in acidic conditions were overcome by using the EFMS, which was designed to cause tablets to float in gastric fluid and release the drug continuously. The release of DA-6034 from tablets in acidic media was significantly improved by using EFMS, which is attributed to the effect of the solubilizers and the alkalizing agent such as sodium bicarbonate used as gas generating agent. DA-6034 EFMS tablets showed enhanced gastroprotective effects in gastric ulcer-induced beagle dogs, indicating the therapeutic potential of EFMS tablets for the treatment of gastritis.
Sungthongjeen et al93 have prepared a floating multi-layer coated tablets based on gas formation. The system consists of a drug-containing core tablet coated with a protective layer (hydroxypropyl methylcellulose), a gas forming layer (sodium bicarbonate) and a gas-entrapped membrane, respectively. Eudragit® RL 30D was chosen as a gas-entrapped membrane due to its high flexibility and high water permeability. The obtained tablets enabled to float due to the CO2-gas formation and the gas entrapment by polymeric membrane. The effect of formulation variables on floating properties and drug release was investigated. The floating tablets using direct-compressed cores had shorter time to float and faster drug release than those using wet-granulated cores. The increased amount of a gas forming agent did not affect time to float but increased the drug release fromthe floating tablets while increasing coating level of gas-entrapped membrane increased time to float (more than 8 hours) and slightly retarded but sustained drug release.
Rajnikanth and Mishra 94 have developed a floating in situ gelling system of clarithromycin (FIGC) using gellan as gelling polymer and calcium carbonate as floating agent for potentially treating gastric ulcers, associated with Helicobacter pylori. Gellan based FIGC was prepared by dissolving varying concentrations of gellan in deionized water to which varying concentrations of drug and sucralfate were dispersed well. The addition of sucralfate to the formulation significantly suppressed the degradation of clarithromycin at low pH. FIGC showed a significant anti-H. pylori effect than that of clarithromycin suspension. The in situ gel formulation with sucralfate cleared H. pylori more effectively than that of formulation without sucralfate. In addition, the required amount of clarithromycin for eradication of H. pylori was found to be less from FIGC than from the corresponding clarithromycin suspension. It was concluded that prolonged gastrointestinal residence time and enhanced clarithromycin stability resulting from the floating in situ gel of clarithromycin might contribute better for complete clearance of H. pylori.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CONCLUSION
Development of an efficient gastro retentive dosage form for stomach specific drug delivery is a real challenge. So, in order to produce the desired gastro retention various approaches have been employed, out of which, floating drug delivery system has emerged as most promising technique. Floating drug delivery system is one of the approaches in which undergo sol to gel transition in acidic stomach conditions and provide stomach specific release of drug for longer duration while being buoyant on the gastric fluid surface. Such systems provide the advantage of better absorption of drugs which are absorbed from the upper part of stomach. As the system remains in the stomach for longer duration local action of drug due to prolonged contact time to gastric mucosa is increased. This leads to less frequent dosing and improved efficiency of the treatment. By understanding the floating and gel forming behavior of polymers we can look forward to improve the gastric retention and hence bioavailability improvement of various pharmacologically active agents. Similarly, good stability and better drug release than other conventional dosage forms make such system more reliable.
ACKNOWLEDGEMENT
The authors are thankful to librarians of various Institutions & libraries like NISCAIR New Delhi, and to the R. V. Northland Institute of Pharmacy, G.B. Nagar, Gautam Buddha Nagar for providing literature survey facility to carry out the work.
REFERENCES
1. Kagan L., Hoffman A., “Systems for region selective drug delivery in gastrointestinaltract, biopharmaceutical considerations”, Expert Opin. Drug Delivery, 2008; 5:681-692.
2. Bardonnet P., Faivre V., Pugh W., Piffaretti J., Falson F., Gastroretentive dosage forms, overview and special case of Helicobacter pylori, J. Control. Release, 2006; 111:1-18.
3. Agyilirah G. A, Green M., Ducret R., “Evaluation of the gastric retention properties of a cross linked polymer coated tablet versus those of a non?disintegrating tablets”, International Journal Pharm, 1991; 75:241?247.
4. Deshpande A. A., Shah N. H., Rhodes C.T., Malick W., “Development of a novel controlled release system for gastric retention” Pharm. Res, 1997; 14:815?819.
5. Hirtz J. “The GIT absorption of drugs in man: a review of current concepts and methods of investigation”, Br J Clin Pharmacol, 1985; 19:77-83.
6. Deshpande A. A., Shah N. H., Rhodes C. T, Malick W, “Development of a novel controlled release system for gastric retention”, Pharm. Res, 1997; 14:815-819.
7. Kawashinia Y, Niwa T, Takcuchi H, Hino T, Itoh Y, “Hallow microspheres for use as a floating controlled drug delivery system in the stomach”, J. Pharm. Sci, 1992; 81(2):135-140.
8. Ichikawa M., Watemblc S., Miyake V. A., “Multiple unit oral floating dosage systems I: Preparation and in-vivo evaluation of' floating and sustained release characteristics”, J. Pharm. Sci, l99l; 80:l062-1066.
9. Washington N., “Investigation into the barrier action of an alginate gastric reflux suppressant, Liquid Gaviscon”, Drug Investig., 1987; 2:23-30.
10. Fabrcgas J. L, Cucala C. G., Pous J., Sites R. A., “In vitro testing of an antacid formulation with prolonged gastric residence time”, Drug Dev. Ind. Pharm., 1994; 20:1199-1212.
11. Ponchel G., Irache J. M., “Specific and nonspecific bioadhesive particulate system for Oral delivery to the gastrointestinal tract”, Adv. Drug. Del. Rev., 1998; 34:191-219.
12. Redniek A. B., Tucker S. J., “Sustained release bolus for animal husbandry”, US Patent 3,507,952, 1970.
13. Bechgaard H., Ladefoged K., “Distribution of pellets in the gastrointestinal tract: The influence on transit time exerted by density or diameter of pellets”, J. Pharm. Pharmacol., 1978; 30:690-692.
14. Hwang S. J., Park H., “Gastric retentive drug delivery systems”, Cri. Rev. Ther. Drug Carr. Syst., 1998; 15:234-284.
15. Ito R., Mchida Y., Sannan T., Nagai T., “Magnetic granules: a novel system for specific drug delivery to oesophageal mucosa in oral administration”, Int. J. Pharm., 1990; 61:109-117.
16. Fujimori J., Machida Y., Nagai T., Preparation of magnetically-responsive tablet and confirmation of its gastric residence in beagle dogs”, STP Pharm. Sci., l994; 4:425-430.
17. Foda N. H., and Ali S. M., “Gastro retentive drug delivery systems as a potential tool for enhancing the efficacy of antibiotics: A Review”, International Journal of Pharma and Biosciences, 2011; 2(2):94P-104P.
18. Nasa P., Mahant S., and Sharma D., “Floating systems: a novel approach towards gastro retentive drug delivery systems”, International Journal of Pharmacy and Pharmaceutical Sciences, 2010; 2(3):2-7.
19. Patil, J. M., Hirlekar R. S., Gide P.S., and Kadam V. J., “Trends in floating drug delivery system”, Journal of Scientific and Industrial Research, 2006; 65:11-21.
20. Hoffman A., and Kagan L., “Selection of drug candidates for gastroretentive dosage forms: pharmacokinetics following continuous intragastric mode of administration in a rat model”, European Journal of Pharmaceutics and Biopharmaceutics, 2008; 69:238-246.
21. Wilding I. R., Coupe A. J., and Davis S. S., “The role of ã-scintigraphy in oral drug delivery”, Advance Drug Delivery Review, 2001; 46:103-124.
22. Weitschies W., Kosch O., Kosch H. M. O., and Trahms L., “Magnetic marker monitoring: an application of bio-magnetic measurement instrumentation and principles for the determination of the gastrointestinal behavior of magnetically marked solid dosage forms”, Advance Drug Delivery Review, 2005; 57:1210-1222.
23. Arunachalam A., Karthikeyan M., Konam K., Prasad H. P., Sethuraman S., Ashutoshkumar S., and Manidipa S., “Floating drug delivery systems: A Review”, International Journal of Pharmaceutical Science and Research, 2011; 2(1):76-83.
24. Vantrappen G.R., Peeters T. L., Janssens J., “The secretory component of interdigestive migratory motor complex in man”, Scand J Gastroenterol., 1979; 14:663-667.
25. Wilson C. G., Washington N., “The stomach its role in oral drug delivery system in bio-logical barriers to drug Absorption”, Chichester, UK, Eills Horwood, 1989; 47-70.
26. Talukder R., and Fassihi R., “Gastroretentive Delivery Systems: A Mini Review”, Drug Dev Ind Pharm., 2004; 30:1019–1028.
27. Garg S., Sharma S., “Gastroretentive Drug Delivery System”, Business Briefing: Pharmatech., 2003; 160?166.
28. Babu V. B. M., Khar R. K., “In vitro and In vivo studies of sustained release floating dosage forms containing salbutamol sulphate”, Pharmazie, 1990; 45:268-270.
29. Kikani H. N, “A Thesis on, Floating Drug Delivery System”, The North Gujarat University, Patan, 2000-2001; 11-12.
30. Desphande A. A., Rhodes C. T., Shah N. H., Malick A.W., “Controlled release systems for prolonged gastric residence”, Drug Dev. Ind. Pharm., 1996; 22:531-539.
31. Penners G., Lustig K., Jorg P. V. G., “Expandable pharmaceutical forms”, U.S. patent 5651985 july 1997.
32. Lenaerts V. M., Gurny R., “In Bioadhesive Drug Delivery Systems”, CRC Press, Boca Raton, FL, 1990.
33. Jaimini M., Rana A. C., and Tanwar Y. S., “Formulation and evaluation of famotidine floating tablets”, Curr. Drug Deliv., 2007; 4:51-55.
34. Sharma N., Agarwal D., Gupta M. K., and Khinchi M. P., “A comprehensive review on floating drug delivery system”. International Journal of Research In Pharmaceutical And Biomedical Sciences, 2011; 2(2):428-441.
35. Bardonnet P. L., Faivre V., Pugh W. J., Piffaretti J. C., and Falson F., “Gastro retentive dosage forms: overview and special case of Helicobacter pylori”, Journal of Controlled Release, 2006; 111:1-18.
36. Riner J. L., Byford R. L., Stratton L. G., and Hair J.A., “Influence of density and location on degradation of sustained-release boluses given to cattle”. American Journal of Veterinary Research, 1982; 43(11):2028-2030.
37. Ganapati R., Bhimagoni K. S., and Anegundha S., “Floating drug delivery of a locally acting H2-antagonist: an approach using an in situ gelling liquid formulation”, Acta Pharmceutica, 2009; 59:345-354.
38. Patel J. K., Chavda J.R., and Modasiya M.K., “Floating in situ gel based on alginate as carrier for stomach-specific drug delivery of famotidine”, International Journal of Pharmaceutical Sciences and Nanotechnology, 2010; 3(3):1092-1104.
39. Kro Gel I., and Bodmeier R., “Development of a multifunctional matrix drug delivery system surrounded by an impermeable cylinder”, Journal of Controlled Release, 1999; 61:43-50.
40. Streubel A., Siepmann J., and Bodmeier R., “Floating matrix tablets based on low density foam powder: effects of formulation and processing parameters on drug release”, European Journal of Pharmaceutical Sciences, 2003; 18:37-45.
41. Reddy L. H., and Murthy R. S., “Floating dosage systems in drug delivery”, Critical reviews in therapeutic drug carrier systems, 2002; 19(6):553-585.
42. Shaha S.H., Patel J.K., Pundarikakshudu K., and Patel N.V., “An overview of a gastro-retentive floating drug delivery system”, Asian Journal of Pharmaceutical Sciences, 2009; 4(1):65-80.
43. Shah S. H., Patel J.K., and Patel N.V., Stomach specific floating drug delivery system: A Review”, International Journal of Pharmtech Research, 2009; 1(3):623-633.
44. Streubel A., Siepmann J., and Bodmeier R., “Drug delivery to the upper small intestine window using gastroretentive technologies”, Current opinion in pharmacology, 2006; 6:501-508.
45. Reddy L. H., Murthy R. H, “Floating dosage systems in drug delivery”, Crit. Rev. Ther. Drug Carr. Syst., 2002; 19(6):553-585.
46. Garg S., and Sharma S., “Gastroretentive Drug Delivery System”, Business Briefing: Pharmatech., 2003; 160-166.
47. Rubinstein A., Friend D. R, “Specific delivery to the gastrointestinal tract, in: Domb A.J (Ed.), Polymeric Site-Specific Pharmacotherapy”, Wiley, Chichester, 1994; 282-283.
48. Ozdemir N., Ordu S., Ozkan Y., “Studies of floating dosage forms of furosemide: In vitro and In vivo evaluation of bilayer tablet formulation”, Drug Dev. Ind. Pharm., 2000; 26:857-866.
49. Penners G., Lustig K., Jorg P. V. G., “Expandable pharmaceutical forms”, US patent 5, 651, 985, 1997.
50. Talwar N., Sen H., Staniforth J. N., “Orally administered controlled drug delivery system providing temporal and spatial control”, US patent 6261601, 2001.
51. Sheth P. R., and Tossounian J. L., U.S. Patent no. 4140755, 1979.
52. Roy H. M, U.S. Patent no. 4055178, 1977.
53. Whitehead L., Fell J., Sharma H. L., “Floating dosage forms: an In vivo study demonstrating prolonged gastric retention”, J. cont. Rel., 1998; 55:3-12.
54. Sato Y., Kawashima Y., “Physicochemical properties to determine the buoyancy of hollow microspheres (microballoons) prepared by the emulsion solvent diffusion method”, Eur. J. Pharm. Sci., 2003; 55:297-304.
55. Joseph N. H., Laxmi S., Jayakrishnan A. A., “Floating type oral dosage form for piroxicam based on hollow microspheres: In vitro and In vivo evaluation in rabbits”, J. Cont. Rel., 2002; 79:71-79.
56. Yang L., Fassihi R., “Zero order release kinetics from self correcting floatable configuration drug delivery system”, J. Pharm. Sci., 1996; 85:170-173.
57. Yang L., Esharghi J., Fassihi R., “A new intra gastric delivery system for the treatment of helicobacter pylori associated gastric ulcers: In vitro evaluation”, J. Cont. Rel., 1999; 57:215-222.
58. Streubel A., Siepmann J., Bodmeier R., “Floating matrix tablets based on low density foam powder: effect of formulation and processing parameters on drug release”, Eur. J. Pharm. Sci., 2003; 18:37-45.
59. Wu W., Zhou Q., Zhang H. B., Ma G. D., Fu C. D., “Studies on Nimodipine sustained release tablet capable of floating on gastric fluids with prolonged gastric resident time”, Yao Xue Xue Bao., 1997; 32:786-790.
60. Nur A. O, Zhang J. S., “Captopril floating and/or bioadhesive tablets: design and release kinetics”, Drug Dev. Ind. Pharm., 2000; 26:965-969.
61. Iannuccelli V., Coppi G., Sansone R., Ferolla G., “Air compartment multiple-unit system for prolonged gastric residence, Part II, In vivo evaluation”, Int. J. Pharm., 1998; 174:55-62.
62. Tardi P., Troy H., European patent no. EP 1432402. 2002.
63. Ikura, Hiroshi, Suzuki, Yoshiki., United States Patent 4777033. 1988.
64. Umezawa, Hamao., United States Patent 4101650. 1978.
65. Iannuccelli V., Coppi G., Bernabei M. T., and Cameroni R., “Air compartment multiple-unit system for prolonged gastric residence”, Part I. Formulation study, Int.J.Pharm., 1998; 174:47-54.
66. Stops F., Fell J. T., Collett J. H., Martini L. G., “Floating dosage forms to prolong gastro-retention-the characterization of calcium alginate beads”, Int. J. Pharm., 2008; 350:301-311.
67. Ichikawa M., Watanabe S., Miyake Y., “A new multiple unit oral floating dosage system. I: Prepration and In vitro evaluation of floating and sustained-release kinetics”, J. Pharm.Sci., 1991; 80:1062-1066.
68. Thanoo B. C., Sunny M. C., and Jayakrishnan A., “Oral sustained-release drug delivery systems using polycarbonate microspheres capable of floating on the gastric fluid”, J. Pharm. Pharmacol., 1993; 45:21-24.
69. Sato Y., Kawashima Y., Takeuchi H., and Yamamoto H., “In vivo evaluation of riboflavin-containing microballoons for floating controlled drug delivery system in healthy human volunteers”, J. Cont. Rel., 2003; 93:39- 47.
70. Washington N., “Investigation into the barrier action of an alginate gastric reflux suppressant, Liquid Gaviscon”, Drug Investig., 1987; 2:23-30.
71. Foldager J., Toftkjor H., “Antacid composition”, US Patent 5068109, 1991.
72. Fabrcgas J. L, Cucala C. G., Pous J., “Sites R.A. In vitro testing of an antacid formulation with prolonged gastric residence time”, Drug Dev. Ind. Pharm., 1994; 20:1199-1212.
73. El-Kamel A. H., Sokar M. S., Algamal S. S., Naggar V. F., “Preparation and evaluation of ketoprofen floating oral drug delivery system”, Int. J. Pharm., 2001; 220:13-21.
74. Kawashima Y., Niwa T., Takeuchi H., Hino T., Ito Y., “Preparation of multiple unit hollow microspheres (microballoons) with acrylic resins containing tranilast and their drug release characteristics (In vivo)”, J. Cont. Rel., 1991; 16:279-290.
75. Jayanthi G., Jayaswal S. B., Srivastava A. K., “Formulation and evaluation of terfenadine microballoons for oral controlled release”, Pharmazie, 1995; 50:769-770.
76. Gu T. H., “Pharmacokinetics and pharmacodynamics of diltiazem floating tablets”, Chung Kao Yao Li Hsuesh Pao., 1992; 13:527-531.
77. Ichikawa M., Watanabe S., Miyake Y., “A new multiple-unit oral floating dosage system. II: In vivo evaluation of floating and sustained-release characteristics with para amino benzoic acid and isosorbide dinitrate as model drugs”, J. Pharm. Sci., 1991; 80:1153-1156.
78. Rouge N., Cole E. T., Doelker E., Buri P., “Buoyancy and drug release patterns of floating mini tablets containing piretanide and atenolol as model drugs”, Pharm. Dev. Technol., 1998; 3:73-84.
79. Cheuh H. R., Zia H., Rhodes C. T., “Optimization of Sotalol floating and bioadhesive extended release tablet formulation”, Drug Dev. Ind. Pharm., 1995; 21:1725-1747.
80. Gustafson J. H., Weissman L., Weinfeld R. T., “Clinical bioavailability evaluation of a controlled release formulation of diazepam”, J. Pharmacokinet. Biopharm., 1981; 9:679-691.
81. Simoni P., Cerre C., Cipolla A., “Bioavailabilty study of a new sinking, enteric coated ursodeoxycholic acid formulation”, Pharmacol. Res., 1995; 31:115-119.
82. drugdeliverytech.com/ME2/dirmod.asp?sid=4306B1E9C3CC4E07A4D64E23FBDB232Candnm=Back+Issuesandtype=Publishingandmod=Publications%3A%3AArticleandmid=8F3A7027421841978F18BE895F87F791andtier=4andid=CC1CDA11871540EFAFAB50839AFFD292.
83. Karande A. D., Yeole P. G., “Comparative Assessment of Different Dissolution Apparatus for Floating Drug Delivery Systems”, Dissolutiontech, 2006; 13(1):20-23.
84. Gohel M. C., Mehta P. R., Dissolutiontech., 2004; 11(4):22-25.
85. Agnihotri S. A., Jawalkar S. S., and Aminabhavi T. M., “Controlled release of cephalexin through gellan gum beads: Effect of formulation parameters on entrapment efficiency, size, and drug release”, Eur. J. Pharm. Biopharm., 2006; 63:249–261.
86. Fell J., Digenis C. G., “Imaging and behaviour of solid oral dosage forms in vivo”, Int. J. Pharm. 1984; 22(1):1-15.
87. Harries D., Sharma H. L., “GI transit of potential bioadhesive formulations in man: A scintigraphic study”, J. Cont. Rel., 1990; 12(1):45-53.
88. Timmermans J., Gansbeke V. B., Moes A. J., “Assessing by gamma scintigraphy the in vivo buoyancy of dosage forms having known size and floating force profiles as a function of time”, Vol I. Proceedings of the 5 International Conference on Pharmacy Technology. Paris, France: APGI, 1989; 42-51.
89. Sawicki W., “Pharmacokinetics of verapamil and norverapamil from controlled release floating pellets in humans”, Eur. J. Pharm. Biopharm., 2002; 53:29-35.
90. Ninan M., Lu X., Wanga Q., Zhanga X., “Development and evaluation of new sustained-release floating microspheres”, Int. J. Pharm., 2008; 358:82-90.
91. Strübing S., Abbouda T., Renata C., Metza H., “New insights on poly (vinyl acetate)-based coated floating tablets: Characterisation of hydration and CO2 generation by benchtop MRI and its relation to drug release and floating strength”, European J. Pharm. And Biopharm., 2008; 69:708-717.
92. Janga S., Lee J., Park S., “Gastroretentive drug delivery system of DA-6034, a new flavonoid derivative, for the treatment of gastritis”, Int. J. Pharm., 2008; 356:88-94.
93. Sungthongjeena S., Sriamornsak P., and Puttipipatkhachorn S., “Design and evaluation of floating multi-layer coated tablets based on gas formation”, Euro. J. Pharm. and Biopharm. 2008; 69:255-263.
94. Rajinikanth P., and Mishra B., “Floating in situ gelling system for stomach site-specific delivery of clarithromycin to eradicate H.pylori”, J. Cont. Rel., 2008; 25:33-41.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE











