{ DOWNLOAD AS PDF }
ABOUT AUTHORS:
Deepika Gautam1*, Deepti Gautam2
1Department of Chemistry, Lucknow University,
Lucknow, Uttar Pradesh, India
2Department of Nursing, Era’s Lucknow Medical college and Hospital,
Lucknow, Uttar Pradesh, India
*yashdeepika1@gmail.com
ABSTRACT
Different type of natural and synthetic agents for the treatment of Type 2 diabetes mellitus improve the metabolic profile but do not reestablished normality. They also reduce chronic diabetic complications, but they do not remove completely them. Thus, for the treatment of type2 diabetes mellitus new agents with novel actions are required to complement and extend the capabilities of existing treatments. Insulin resistance and beta-cell failure, which are main cause in the pathogenesis of Type 2 diabetes, in this review we discussed about some natural and synthetic molecule and their targets and some old oral ant diabetic drug and their mode of action.
INTRODUCTION
Diabetes mellitus (DM) is a metabolic disorder characterized by increase glucose level in blood and changes in lipid and protein metabolism[1]. Because the body cannot release or use insulin normally. insulin is a hormones which is released by pancreas. which maintaining sugar level in blood[2]. Diabetes mellitus are of two type one is insulin dependent[3] and another is non-insulin dependent. insulin dependent diabetes called type 1(IDDM), also known as "juvenile diabetes" because it occurs in the young children and adolescents. it is an autoimmune disorder where antibodies are produced against the β cells which release insulin, are destroyed[3].
Non-insulin dependent called type 2 diabetes mellitus(NIDDM) or "adult-onset diabetes “because occurs in adult. Serious prolong complications include some serious disease like heart disease, stroke, kidney failure, foot ulcers and damage to the eyes[1] DM can be found worldwide and the population is increasing. According to WHO projections, about 300 million or more people will be affected by diabetes by the year 2025.[4] The estimated number of diabetic patients in 2030 will be more than double that in 2005.[4] according to IDF in 2009 India has the largest number of people — 50.8 million suffering from diabetes in the world, followed by China (43.2 million) and the United States (26.8 million). India continues to be the "diabetes capital" of the world, and by 2030, nearly 9 per cent of the country's population is likely to be affected from the disease, warns the fourth edition of the World Diabetes Atlas launched by the IDF at the 20th World Diabetes Congress in Montreal, Canada. Worldwide, as of 2013, an estimated 382 million people have diabetes, with type 2 diabetes making about 90% of the cases.[5,6] This is equal about 8.3% of the adults population,[6]equal rates in both women and men.[7] Worldwide in 2012 and 2013 diabetes resulted in 1.5 to 5.1 million deaths per year, making it the 8th leading cause of death.[8,9] Diabetes overall at least doubles the risk of death. The number of people with diabetes is estimated up to rise to 592million by 2035.[10] The economic costs of diabetes globally was estimated in 2013 at USD 548 billion[9] and in the United States in 2012 USD 245 billion.[11]
Some Oral anti-diabetic drugs-
Type of anti-diabetic drugs-
· Sulfonylureas/insulin tropics
· Biguanides
· a-Glycosidase inhibitors
· Thiazolidinedione
· DPP-4 inhibitors (Glistens)
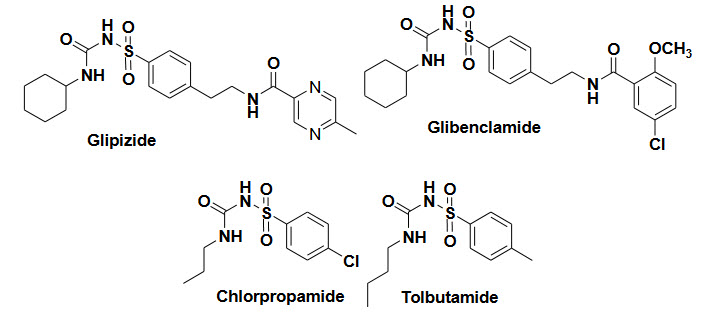
Sulfonylureas/insulin tropics-mechanism and targets-
Sulfonylureas reduce the blood glucose level by stimulating the release of insulin from the pancreatic β-cell and sensitivity of peripheral tissue to insulin, number of insulin receptor and suppressing gluconeogenesis in the liver
It bind to receptors on the pancreatic β-cell, and block the k+ on these receptor.it reduce potassium conductance and increased insulin secretion.
Side effects-Hypoglycemia,weight gain
Example and their structure-
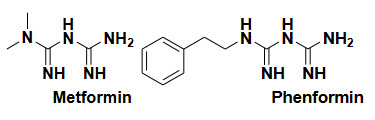
Biguanides-mechanism and action is not clear,suppressing hepatic gluconeogenesis
Side effect-Gastrointestinal disturbances, lactic acidosis
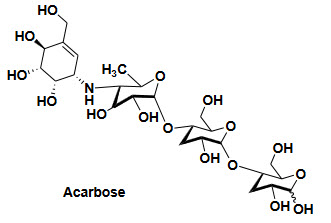
α-Glucosidase inhibitors-it reduce the glucose absorption from upper intestines and do not causes hypoglycemia
Eide effect-Gastrointestinal disturbance
Example and their structure-
Thiazolidinediones- they are agonist at the PPARϒ receptor. It increase insulin mediated glucose transport in to muscle and fat tissue and reduce hepatic gluconeogenesis and lower incidence of hypoglycemia
Side effect-cause severe hepatotoxicity and weight and anemia
Example and their structure-
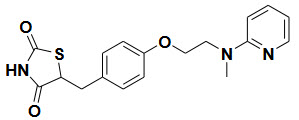
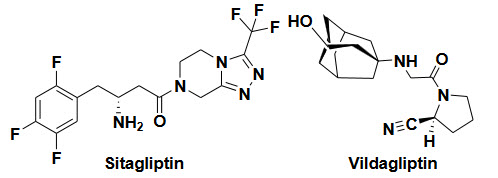
DPP-4 inhibitors (Gliptins)- Reduce glucagon and blood glucose levels by inhibiting DPP-4
Side effect- Nasopharyngitis, Headache, Nausea, Hypersensitivity, Skin reactions
Example and their structure-
Some anti-diabetic agent -
|
S.N. |
Name and source |
Activity & Mechanism |
|
1 |
Genistein12 branches of Tetracera scandens (Dilleniaceae |
Glucose-uptake activity in basal and insulin-stimulated L6 myotubes (0–25 mM), acting by AMPK activation and GLUT4 and GLUT1 (IC50 range = 20–37 µM) |
|
2 |
3’,5’-diprenylgenistein branches of Tetracera scandens (Dilleniaceae) |
Glucose-uptake activity in basal and insulin-stimulated L6 myotubes (0–25 mM), acting by AMPK activation and GLUT4 and GLUT1 (IC50 range = 20–37 µM) |
|
3 |
Alpinumisoflavone branches of Tetracera scandens (Dilleniaceae) |
Glucose-uptake activity in basal and insulin-stimulated L6 myotubes (0–25 mM), acting by AMPK activation and GLUT4 and GLUT1 (IC50 range = 20–37 µM) |
|
4 |
Derrone branches of Tetracera scandens (Dilleniaceae |
Glucose-uptake activity in basal and insulin-stimulated L6 myotubes (0–25 mM), acting by AMPK activation and GLUT4 and (IC50 range = 20–37 µM) |
|
5 |
6,8-diprenylgenistein branches of Tetracera scandens (Dilleniaceae |
Glucose-uptake activity in basal and insulin stimulated L6 myotubes (0–25 mM), acting by AMPK activation and GLUT4 and GLUT1 over-expressio (IC50 range = 20–37 µM) |
|
6 |
Vanilic acid & sulphate Derivatives13 Green algae Cladophora socialis |
Inhibition of protein tyrosine phosphatase 1B (PTP1B),an important enzyme in regulating the insulin receptor, with IC50 values of 3.7µM |
|
7 |
Cinamaldehyde Cinanamonum zeylanicum Biume |
Inhibition of protein tyrosine phosphatase 1B (PTP1B),an important enzyme in regulating the insulin receptor, with IC50 values of 1.7 µM
|
|
8 |
Cinchonain Ib Eriobotrya japonica14 LINDL (Rosaceae) leaves |
Enhanced insulin secretion from INS-1 cells (rat insulinoma cell), as well as reduced plasma insulin level in rats after 108 mg /kg oral administration
|
|
9 |
Steppogenin-4’-O-β-D-glucoside15
root bark of Morus alba L. (Moraceae) |
Showed a hypoglycemic effect at 50 mg kg_1 (p.o.) in alloxan-induced diabetic mice
|
|
10 |
Two bis(catechol glycoside) esters16
leaves of Dodecadenia grandiflora (Lauraceaes |
Compounds (100 mg kg_1 bw, p.o.) showed significantantihyperglycemic activity in STZ-induced diabetic rats |
|
11 |
kraussianone-1 & kraussianone-217 Roots of Eriosema kraussianum N. E. Br. (Fabaceae) |
Compounds 1and 12 (20–80 mg/ kg p. o.) resulted in dose-dependent hypoglycaemia in rats, with glibenclamide (10 mg/ kg bw, p. o.) as the positive control |
|
12 |
Davidigenin18 Artemisia dracunculus L. (Asteraceae), |
This extract inhibited aldose reductase (ALR2) activity by 58% to 77% at 3.75 mg/ mL |
|
13 |
6,demethoxycapillarisn Artemisia dracunculus L. (Asteraceae), |
Inhibited phosphoenol pyruvate carboxykinase (PEPCK) mRNA levels related to the gluconeogenesis pathway, with IC50 values of 43µM,it also activated the PI3K pathway, similarly to insulin, |
|
14 |
4-hydroxyderricin19 ethanol extract of Angelica keiskei Koidzumi (Apiaceae/Umbelliferae) |
Prevented progression of diabetes in genetically impaired KK-Ay mice, which develop diabetes and show hyperglycemia with aging because of insulin resistance (positive control: 0.05% diet of pioglitazone).30showed acute blood glucose lowering effects (50 mg kg_1 bw) in ALX-induced diabetic rats and promoted glucose-induced insulin secretion after oral treatment in hyperglycemic rats. |
|
15 |
Xanthoangelol19 ethanol extract of Angelica keiskei Koidzumi (Apiaceae/Umbelliferae) |
. Prevented progression of diabetes in genetically impaired KK-Ay mice, which develop diabetes and show hyperglycemia with aging because of insulin resistance (positive control: 0.05% diet of pioglitazone).30showed acute blood glucose lowering effects (50 mg kg_1 bw) in ALX-induced diabetic rats and promoted glucose-induced insulin secretion after oral treatment in hyperglycemic rats. |
|
16 |
Apigenin-5-O-[α-L-rhamnopyranosyl-(1-4)-6-O-β-Dacetylglucopyranoside]20 leaves of Cephalotaxus sinensis |
Anti-hyperglycemic
|
|
17 |
Apigenin- 5-O-[a-L-rhamnopyranosyl-(1-4)-6-O-β-D-glucopyranoside20 leaves of Cephalotaxus sinensis |
Anti-hyperglycemic |
|
18 |
Apigenin21 leaves of Cephalotaxus sinensis |
Increased level of glucose transporterGLUT-4 was also seen from mice adipocytes treated with 14 (0.1mg, 2 mg/ml. |
|
19 |
Apigenin-O-{20-O-α-L-rhamnopyranosyl)-β-L-fucopyranoside21 Averrhoa carambola L. |
Acute blood glucose lowering effects (50 mg/ kg bw) in ALX-induced diabetic rats and promoted glucose-induced insulin secretion after oral treatment in hyperglycemic rats |
|
20 |
Apigenin-6-C-β-L-fucopyranoside 21 Averrhoa carambola L |
(50 mg/kgbw, p.o.) Lowered blood glucose in hyperglycemic rats, promoted glucose-induced insulin secretion, and stimulated glycogen synthesis |
|
21 |
Pongamol22 fruit of Pongamia pinnata (L.) Pierre (Fabaceae) |
Exhibited anti-hyperglycemic activity. In streptozotocin (STZ)-induced diabetic rats, the blood glucose lowering effects of pongamol were 22% , |
|
22 |
Karanjin22 fruit of Pongamia pinnata (L.) Pierre (Fabaceae) |
exhibited anti hyperglycemic activity. In streptozotocin (STZ)-induced diabetic rats, the blood glucose lowering effects of karanjin 20% |
|
23 |
Kaempferol23 Euonymus alatus (Celastraceae) |
compound 1 (5–50 mM) significantly improved insulin-stimulated glucose uptake in mature 3T3-L1 adipocytes, and they also served as weak partial agonists in a PPAR-g reporter gene assay without inducing differentiation of 3T3-L1 preadipocytes, an effect shown by traditional PPAR-g agonists |
|
24 |
Quercetin23 Euonymus alatus (Celastraceae |
(5–50 mM) Significantly improved insulin-stimulated glucose uptake in mature 3T3-L1 adipocytes, and they also served as weak partial agonists in a PPAR-g reporter gene assay without inducing differentiation of 3T3-L1 preadipocytes, an effect shown by traditional PPAR-g agonists |
|
25 |
Aspalathin24 (FabaceaeAspalathus linearis),
|
Increase glucose uptake by L6 myotubes at 1–100 mM concentrations in a dose-dependent manner, and to increase insulin secretion from cultured RIN-5F cells at 100 mM |
|
26 |
Coagulin C25 aqueous extract of Withania coagulans Dunal (Solanaceae) |
Inhibited post-diet glucose rise |
|
27 |
Karaviloside26XI bitter melon (Momordica charantia) |
Stimulated glucose transporter 4 (GLUT4) translocation to the cell membrane, which was associated with increased activity of AMPK |
|
28 |
Stigmasterol27 bark of Butea monosperma (Lam.) Kuntze (Fabaceae)
|
Reduced serum triiodothyronine(T3),thyroxin (T4) and glucose concentrations were found as well as decreased activity of hepatic G-6-Pase and increased insulinlevels, indicating that it exhibits both thyroid-inhibiting and hypoglycemic properties |
|
29 |
Costunolide28 Roots of Costus Speciosus
|
Decreased glycosylated hemoglobin (HbA1c), serum total cholesterol, LDL cholesterol,and triglyceride levels were seen as well as increased plasma insulin, tissue glycogen, HDL cholesterol, and serum protein |
|
30 |
Spicatanol29 Hedychium spicatum Ham. Ex Smith (Zingiberaceae) |
Intestinal a-glucosidase inhibitory activities IC50 of 34.1 µM. |
|
31 |
Palbinone30 Paeonia suffruticosa Andrew (Paeoniaceae)
|
Exhibited the most potent activity by increasing the levels of phospho-AMPK, phospho-ACC, and phospho-GSK-3b in a dose-dependent manner, and triggering glucose uptake and glycogen synthesis in insulin-resistant human HepG2 cells |
|
32 |
Swietenine31 seeds of Swietenia macrophylla King. (Meliaceae) |
Exhibited significant hypoglycemic activity comparable to that of human insulin in an in vitro glucose utilization assay |
|
33 |
Scopoletin (7-hydroxy-6-methoxycoumarin)32 leaves of Aegle marmelos Linn. Corr (Rutaceae). |
Inlevo-thyroxine-treated animals, decreased levels of serum thyroid hormones, glucose, and hepatic G-6-Pase were seen in the scopoletin-administrated group |
|
34 |
Moracin M33 root bark of Morus alba L. (Moraceae), |
Moracin M(100 mg /kg, p.o.) decreased the fasting blood glucose level |
|
35 |
Mullberroside A33 root bark of Morus alba L. (Moraceae), |
Hypoglycemic effects in alloxan-induced diabetic mice |
|
36 |
Epicatechin34 (Green tea) |
Preventing T1D BY modulating immune function and thereby preserving islet mass |
|
37 |
1,5 anhydro-D-glucitol35 (nearly all food ) |
Potent and selective renal sodium-glucose co transporter 2 (SGLT2) inhibitor with anti-hyperglycemic activity |
|
38 |
Caftaric acid 36 (Corni Fructus) |
Hypoglycemic and Beta-Cell Protective |
|
39 |
Dapagliflozin 37 |
Selective Renal Sodium-Dependent Glucose Cotransporter 2 (SGLT2) Inhibitor for the Treatment of Type 2 Diabetes |
|
40 |
GPR-14238 |
EC50-1.5µM F=3.9% in cynomolgus monkey |
|
41 |
4-(5-(4-(piperidin-1-yl)piperidin-1-yl)-1,3,4-thiadiazol-2-yl)-2-(pyridin-2-yl)morpholine39 |
Histamine H3 receptor antagonist |
|
42 |
(2R)-2-(3-{3 [(4Methoxyphenyl)carbonyl]-2-methyl-6(trifluoromethoxy)-1H-indol-1-yl}phenoxy)butanoic Acid40 |
Peroxisome Proliferator-Activated Receptor γ Modulator for the Treatment of Type 2 Diabetes Mellitus with a Reduced Potential to Increase Plasma and Extracellular Fluid Volume |
|
43 |
Canagliflozin41 |
Highly potent and selective SGLT2 inhibitor and showed pronounced anti-hyperglycemic effects in high-fat diet fed KK (HF-KK) mice |
|
44 |
Tofogliflozin42 |
Highly Selective Sodium Glucose Cotransporter 2 (SGLT2) Inhibitor for the Treatment of Type 2 Diabetes IC50(hSGLT2):2.9 nM IC50(hSGLT1): 8,444 nM Selectivity : (hSGLT1)/ (hSGLT2)=2,912 fold F(%)(mice)=75% (monkey) 85% |
|
45 |
(2S,3R,4R,5S,6R)-2-(4-(4-ethoxybenzyl)-2-methoxy-3-methylphenyl)-tetrahydro-6-(hydroxymethyl)-2H-thiopyran-3,4,5-triol43 |
Potent, Selective Sodium-Dependent Glucose Cotransporter 2 (SGLT2) Inhibitor for Type2 Diabetes Treatment IC50:2.26Nm (hSGLT2) IC50:3990Nm(hSGLT1) |
CONCLUSION
In this review we discussed about some recent discoveries of pure anti-diabetes compound and their structure and biological activities.in this review we discussed some natural and synthetic compound which differ from anti-diabetes drug in structure and mechanism of action. Which may be helpful in designing and synthesis of new drugs.
REFERENCES
1. "About diabetes". World Health Organization. Retrieved 4 April 2014
2. Greenspan's basic & clinical endocrinology (9th ed.). New York: McGraw-Hill Medical. pp. Chapter ISBN 0-07-162243-8.
3. "Diabetes Fact sheet N°312". WHO. October 2013. Retrieved 25 March 2014.
4. M. Jung, M. Park, H. C. Lee, Y. H. Kang, E. S. Kang and S. K. Kim,Antidiabetic agents from medicinal plants, Curr. Med. Chem., 2006,s13, 1203–18.
5. Williams textbook of endocrinology (12th ed.). Philadelphia: Elsevier/Saunders. pp. 1371–1435. ISBN 978-1-4377-0324-5
6. Shi, Yuankai; Hu, Frank B. "The global implications of diabetes and cancer".The Lancet 383 (9933): 1947–1948
7. Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, et al. (Dec 15, 2012). "Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010.". Lancet 380 (9859): 2163–96.
8. "The top 10 causes of death Fact sheet N°310". World Health Organization. Oct 2013
9. IDF DIABETES ATLAS. International Diabetes Federation. 2013. p. 7.ISBN 2930229853.
10. nternational Diabetes Federation: Diabetes Atlas". Retrieved 4 April 2014
11. American Diabetes, Association (Apr 2013). "Economic costs of diabetes in the U.S. in 2012.". Diabetes care 36 (4): 1033–46
12. M. S. Lee, C. H. Kim, D. M. Hoang, B. Y. Kim, C. B. Sohn,M. R. Kim and J. S. Ahn, Genistein-derivatives from Tetracerascandens stimulate glucose-uptake in L6 myotubes, Biol. Pharm.Bull., 2009, 32, 5048.
13. Y. Feng, A. R. Carroll, R. Addepalli, G. A. Fechner, V. M. Avery and R. J. Quinn, Vanillic acid derivatives from the green algae Cladophora socialis as potent protein tyrosine phosphatase 1B inhibitors, J. Nat. Prod., 2007, 70, 1790–2.
14. F. Qa’dan, E. J. Verspohl, A. Nahrstedt, F. Petereit andK. Z. Matalka, Cinchonain Ib isolated from Eriobotrya japonicainduces insulin secretion in vitro and in vivo, J. Ethnopharmacol.,2009, 124, 2247.
15. M. Zhang, M. Chen, H. Q. Zhang, S. Sun, B. Xia and F. H. Wu, Invivo hypoglycemic effects of phenolics from the root bark of Morusalba, Fitoterapia, 2009, 80, 475–7.
16. M. Kumar, P. Rawat, N. Rahuja, A. K. Srivastava and R. Maurya,Antihyperglycemic activity of phenylpropanoyl esters of catechol glycoside and its dimers from Dodecadenia grandiflora, Phytochemistry, 2009, 70, 1448–55.
17. J. A. Ojewole, S. E. Drewes and F. Khan, Vasodilatory and hypoglycaemic effects of two pyrano isoflavone extractives fromEriosema kraussianum N.E. Br. Fabaceae] rootstock in experimentalrat models, Phytochemistry, 2006, 67, 610–7.
18. D. Govorko, S. Logendra, Y. Wang, D. Esposito, S. Komarnytsky,D. Ribnicky, A. Poulev, Z. Wang, W. T. Cefalu and I. Raskin,Polyphenolic compounds from Artemisia dracunculus L. inhibit PEPCK gene expression and gluconeogenesis in an H4IIE hepatoma cell line, Am. J. Physiol.: Endocrinol. Metab., 2007, 293,E1503–10.
19. T. Enoki, H. Ohnogi, K. Nagamine, Y. Kudo, K. Sugiyama,M. Tanabe, E. Kobayashi, H. Sagawa and I. Kato, Antidiabeticactivities of chalcones isolated from a Japanese Herb, J. Agric.Food Chem., 2007, 55, 6013–7.
20. W. Li, R. J. Dai, Y. H. Yu, L. Li, C. M. Wu, W. W. Luan,W. W. Meng, X. S. Zhang and Y. L. Deng, Antihyperglycemic effect of Cephalotaxus sinensis leaves and GLUT-4 translocationfacilitating activity of its flavonoid constituents, Biol. Pharm. Bull.,2007, 30, 1123–9.
21. L. H. Cazarolli, P. Folador, H. H. Moresco, I. M. Brighente,M. G. Pizzolatti and F. R. Silva, Stimulatory effect of apigenin-6-C-beta-l-fucopyranoside on insulin secretion and glycogensynthesis, Eur. J. Med. Chem., 2009, 44, 4668–73.
22. A. K. Tamrakar, P. P. Yadav, P. Tiwari, R. Maurya andA. K. Srivastava, Identification of pongamol and karanjin as lead compounds with antihyperglycemic activity from Pongamiapinnata fruits, J. Ethnopharmacol., 2008, 118, 435–9.
23. X. K. Fang, J. Gao and D. N. Zhu, Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity, Life Sci., 2008, 82, 615–22.
24. A.Kawano, H. Nakamura, S. Hata, M. Minakawa, Y. Miura andK. Yagasaki, Hypoglycemic effect of aspalathin, a rooibos tea component from Aspalathus linearis, in type 2 diabetic model db/db mice, Phytomedicine, 2009, 16, 437–43.
25. R. Maurya, Akanksha, Jayendra, A. B. Singh and A. K. Srivastava,Coagulanolide, a withanolide from Withania coagulans fruits and antihyperglycemic activity, Bioorg. Med. Chem. Lett., 2008, 18,65347.
26. M. J. Tan, J. M. Ye, N. Turner, C. Hohnen-Behrens, C. Q. Ke,C. P. Tang, T. Chen, H. C. Weiss, E. R. Gesing, A. Rowland, D. E. James and Y. Ye, Antidiabetic activities of triterpenoids isolated from bitter melon associated with activation of the AMPK pathway, Chem. Biol., 2008, 15, 263–73.
27. S.Panda, M. Jafri, A. Kar and B. K. Meheta, Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma, Fitoterapia, 2009, 80, 123–6.28. J. Eliza, P. Daisy, S. Ignacimuthu and V. Duraipandiyan, Normoglycemic and hypolipidemic effect of costunolide isolated from Costus speciosus (Koen ex. Retz.)Sm. in streptozotocin-induced diabetic rats, Chem.-Biol. Interact., 2009, 179, 329–34.
29. P. Prabhakar Reddy, A. K. Tiwari, R. Ranga Rao, K. Madhusudhana, V. Rama Subba Rao, A. Z. Ali, K. SureshBabu and J. Madhusudana Rao, New Labdane diterpenes as intestinal alpha-glucosidase inhibitor from anti hyper glycemic extract of Hedychium spicatum (Ham. Ex Smith) rhizomes, Bioorg.Med. Chem. Lett., 2009, 19, 2562–5.
30. T. Ha do, D. T. Tuan, N. B. Thu, N. X. Nhiem, T. M. Ngoc, N. Yim and K. Bae, Palbinone and triterpenes from Moutan Cortex (Paeonia suffruticosa, Paeoniaceae) stimulate glucose uptake and glycogen synthesis via activation of AMPK in insulin-resistant human HepG2 Cells, Bioorg. Med. Chem. Lett.,\ 2009, 19, 5556–9.
31. A. Maiti, S. Dewanjee and R. Sahu, Isolation of hypoglycemicphytoconstituent from Swietenia macrophylla seeds, Phytother.Res., 2009, 23, 1731–3.
32. S. Panda and A. Kar, Evaluation of the antithyroid, antioxidative and antihyperglycemic activity of scopoletin from Aegle marmelosleaves in hyperthyroid rats, Phytother. Res., 2006, 20, 1103–5.
33. M. Zhang, M. Chen, H. Q. Zhang, S. Sun, B. Xia and F. H. Wu, In vivo hypoglycemic effects of phenolics from the root bark of Morusalba, Fitoterapia, 2009, 80, 475–7.34. Zhuo Fu, Julia Yuskavage, and Dongmin LiuDietary Flavonol Epicatechin Prevents the Onset of Type 1 Diabetes in Nonobese Diabetic Mice,J. Agric. Food Chem., 2013, 61 (18), pp 4303–4309
35. Atsushi Kato, Takahito Kunimatsu, Yukiko Yamashita, Isao Adachi, Kei Takeshita, and Fumihiro Ishikawa,Protective Effects of Dietary 1,5-Anhydro-D-glucitol as a Blood Glucose Regulator in Diabetes and Metabolic Syndrome,J. Agric. Food Chem., 2013, 61 (3), pp 611–617
36. Mei-Hsiang Lin, Hui-Kang Liu, Wei-Jan Huang, Chu-Chun Huang, Tzu-Hua Wu, and Fen-Lin Hsu,Evaluation of the Potential Hypoglycemic and Beta-Cell Protective Constituents Isolated from Corni Fructus To Tackle Insulin-Dependent Diabetes Mellitus,J. Agric. Food Chem., 2011, 59 (14), pp 7743–7751
37. Wei Meng, Bruce A. Ellsworth, Alexandra A. Nirschl, Peggy J. McCann, Manorama Patel, Ravindar N. Girotra, Gang Wu, Philip M. Sher, Eamonn P. Morrison, Scott A. Biller, Robert Zahler, Prashant P. Deshpande, Annie Pullockaran, Deborah L. Hagan, Nathan Morgan, Joseph R. Taylor, Mary T. Obermeier, William G. Humphreys, Ashish Khanna, Lorell Discenza, James G. Robertson, Aiying Wang, Songping Han, John R. Wetterau, Evan B. Janovitz, Oliver P. Flint, Jean M. Whaley and William N. Washburn,Discovery of Dapagliflozin: A Potent, Selective Renal Sodium-Dependent Glucose Cotransporter 2 (SGLT2) Inhibitor for the Treatment of Type 2 Diabetes,J. Med. Chem., 2008, 51 (5), pp 1145–1149
38. Narihiro Toda, Xiaolin Hao, Yasuyuki Ogawa, Kozo Oda, Ming Yu, Zice Fu, Yi Chen, Yongjae Kim, Mike Lizarzaburu, Sarah Lively, Shauna Lawlis, Michiko Murakoshi, Futoshi Nara, Nobuaki Watanabe, Jeff D. Reagan, Hui Tian, Angela Fu, Alykhan Motani, Qingxiang Liu, Yi-Jyun Lin, Run Zhuang, Yumei Xiong, Peter Fan, Julio Medina, Leping Li, Masanori Izumi, Ryo Okuyama, and Satoshi Shibuya,Potent and Orally Bioavailable GPR142 Agonists as Novel Insulin Secretagogues for the Treatment of Type 2 Diabetes,ACS Med. Chem. Lett., 2013, 4 (8), pp 790–794
39. Ashwin U. Rao, Ning Shao, Robert G. Aslanian, Tin-Yau Chan, Sylvia J. Degrado, Li Wang, Brian McKittrick, Mary Senior, Robert E. West, Jr., Shirley M. Williams, Ren-Long Wu, Joyce Hwa, Bhuneshwari Patel, Shuqin Zheng, Christopher Sondey, and Anandan Palani,Discovery of a Potent Thiadiazole Class of Histamine H3Receptor Antagonist for the Treatment of Diabetes, ACS Med. Chem. Lett., 2012, 3 (3), pp 198–202
40.John J. Acton, III, Taro E. Akiyama, Ching H. Chang, Lawrence Colwell, Sheryl Debenham, Thomas Doebber, Monica Einstein, Kun Liu, Margaret E. McCann, David E. Moller, Eric S. Muise, Yugen Tan, John R. Thompson, Kenny K. Wong, Margaret Wu, Libo Xu, Peter T. Meinke, Joel P. Berger and Harold B. Wood,Discovery of (2R)-2-(3-{3-[(4-Methoxyphenyl)carbonyl]-2-methyl-6-(trifluoromethoxy)-1H-indol-1-yl}phenoxy)butanoic Acid (MK-0533): A Novel Selective Peroxisome Proliferator-Activated Receptor γ Modulator for the Treatment of Type 2 Diabetes Mellitus with a Reduced Potential to Increase Plasma and Extracellular Fluid Volume,J. Med. Chem., 2009, 52 (13), pp 3846–3854
41. Sumihiro Nomura, Shigeki Sakamaki, Mitsuya Hongu, Eiji Kawanishi, Yuichi Koga, Toshiaki Sakamoto, Yasuo Yamamoto, Kiichiro Ueta, Hirotaka Kimata, Keiko Nakayama and Minoru Tsuda-Tsukimoto, Discovery of Canagliflozin, a Novel C-Glucoside with Thiophene Ring, as Sodium-Dependent Glucose Cotransporter 2 Inhibitor for the Treatment of Type 2 Diabetes Mellitus, J. Med. Chem., 2010, 53 (17), pp 6355–6360
42.Yoshihito Ohtake, Tsutomu Sato, Takamitsu Kobayashi, Masahiro Nishimoto, Naoki Taka, Koji Takano, Keisuke Yamamoto, Masayuki Ohmori, Marina Yamaguchi, Kyoko Takami, Sang-Yong Yeu, Koo-Hyeon Ahn, Hiroharu Matsuoka, Kazumi Morikawa, Masayuki Suzuki, Hitoshi Hagita, Kazuharu Ozawa, Koji Yamaguchi, Motohiro Kato, and Sachiya Ikeda,Discovery of Tofogliflozin, a Novel C-Arylglucoside with an O-Spiroketal Ring System, as a Highly Selective Sodium Glucose Cotransporter 2 (SGLT2) Inhibitor for the Treatment of Type 2 Diabetes,J. Med. Chem., 2012, 55 (17), pp 7828–7840
43.Hiroyuki Kakinuma, Takahiro Oi, Yuko Hashimoto-Tsuchiya, Masayuki Arai, Yasunori Kawakita, Yoshiki Fukasawa, Izumi Iida, Naoko Hagima, Hiroyuki Takeuchi, Yukihiro Chino, Jun Asami, Lisa Okumura-Kitajima, Fusayo Io, Daisuke Yamamoto, Noriyuki Miyata, Teisuke Takahashi, Saeko Uchida and Koji Yamamoto,1S)-1,5-Anhydro-1-[5-(4-ethoxybenzyl)-2-methoxy-4-methylphenyl]-1-thio-D-glucitol (TS-071) is a Potent, Selective Sodium-Dependent Glucose Cotransporter 2 (SGLT2) Inhibitor for Type 2 Diabetes Treatment, J. Med. Chem., 2010, 53 (8), pp 3247–3261.
REFERENCE ID: PHARMATUTOR-ART-2252
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 10 Received On: 19/07/2014; Accepted On: 24/07/2014; Published On: 01/10/2014How to cite this article: D Gautam, D Gautam; A Short Review on Anti-Diabetic Agent; PharmaTutor; 2014; 2(10); 89-105 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









