About Authors:
Anil kumar
Jubilant chemsys Limited, Noida-201301
Babu Banarsi Das National Institute of Technology and Management
Akhilash Das Nagar, Lucknow
Abstract
Presence of moisture influences chemical stability, crystal structure, powder flow, compaction lubricity, dissolution rate, and polymer film permeability in solid dosage forms and lead to growth of microorganisms, change in thixotropy in semi-solid dosage forms. Moreover, unit operations obviously depending on the amount and state of water present are also influenced by it. Therefore, moisture influences the properties of individual active ingredients and excipients, and it is essential to characterize the effect of moisture on these individual components. This article lay emphasis on determination of moisture by various methods and illustrates the changes induced by moisture on several product and process attributes
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1187
INTRODUCTION:
The term moisture, usually defined as wetness conferred by an unidentified liquid1, is assumed here to be due to water. Thus the scope of this article is the characterization of and consequences due to relatively small amounts of water associated with solids of pharmaceutical interest. Chemical stability, crystal structure, powder flow, compaction lubricity, dissolution rate, and polymer film permeability are some properties of pharmaceutical interest that have been demonstrared to be influenced by the presence of moisture. Wet granulation, extrusion, spheronization, tray drying, freeze drying, spray drying, fluid bed drying, tableting, and aqueous film coating are some unit operations that obviously depend on the amount and state of water present. Moisture can and does influence the properties of individual active ingredients and excipients, and it is essential, as a first step, to characterize the effect of moisture on these individual components2.
HISTORICAL PERSPECTIVE:
In the area of moisture in pharmaceutical products, it is possible to identify three stages in the scientific and regulatory history. The first stage dealt more or less exclusively with the amount of water present in pharmaceuticals, most of which were products of natural origin, with regard to issues of potency and commerce. In the second stage, there was a realization that water could affect the chemical and physical properties of drugs and dosage forms. The fact that the water might exist in different states was exemplified by partitioning the water into “bound” or “free” moisture3. The third stage came with the realization that even small amounts of “bound” moisture could have a dramatic impact on properties and processes of pharmaceutical interest4
COMPENDIAL STANDARDS
The method for moisture determination in USP 24/NF 195 are the best, classical, addressing only the determination of moisture content6. The USP offers two methods for the determination of moisture content in solids: titrimetry (Karl Fisher titration) and gravimetric (e.g., thermal gravimetric analysis).
BACKGROUND
Conventions, Definitions, and Terminology
. Moisture content is expressed on a dry weight basis. The sorption isotherm7is the most widely used expression to quantify a substance’s affinity for water. It is, as the term implies, a relationship at a constant temperature.
n = f(x) ----------------- (1)
Where n, the number of moles of water sorbed, is a function of x, the partial pressure of water in the atmosphere at that temperature. The correspondence of n to moisture content and x to relative humidity, as defined in Table. 1. suggests that this functional relationship may be stated in numerous ways.
|
Term |
Symbol(s) |
Definitions |
|
Sorption |
|
The spontaneous acquisition of a component (waterin this case) from the atmosphere by a system. |
|
Adsorption |
|
Sorption confined to the surface of the solid. The amount of water adsorbed is directly proportional to the surface area available. |
|
Absorption |
|
Sorption characterized by penetration of the sorbed component into the bulk structure of the solid. The amount of water absorbed does not depend on surface area. |
|
Desorption |
|
The spontaneous loss of sorbed component (water). |
|
|
|
The substance or sorbent responsible for Sorption. |
|
Adsorbent |
|
Ostensibly the substance responsible for adsorption. The term “absorbent” is not in common usage. Adsorbent is used without discriminating between adsorption or absorption. |
|
Adsorbate |
MC, W |
The substance being adsorbed (water in this case). |
|
|
|
|
|
Term |
Symbol |
Definition |
|
Moisture content |
WD |
The total amount of water present with the adsorbent. |
|
Dry weight basis |
%MCD |
Anexpression of moisture content related to the weight of dry solid. WD = wt. water/wt.of dry solid Amount of moisture present with 100g of dry solid % MCD = 100WD |
|
Wet weight basis |
Ww %MCW |
An expression of moisture content referring to the to the total weight of the sample. Amount of moisture present in 100g of sample. %MCW = 100WW |
|
Equibilirium Moisture Content |
EMC, %EMC, WD.eq WW.eq |
The moisture content (sometimes expressed on a content percentage basis) at equilibrium under specified conditions of temperature, pressure, and vapour composition |
|
Partial pressure of water in the atmosphere. |
P/PO x |
The vapour pressure of water in the atmosphere (P) expressed as a fraction of the saturation vapour pressure of pure liquid pressure of pure liquid water (PO) at the same temperature. |
|
Relative humidity |
%RH, 100P/P0 100x |
The vapour pressure of water in the atmosphere (P) usually expressed as a percentage of the saturation vapour pressure of pure liquid water (P0) at the same temperature. |
|
Bound mixture |
Water associated with a solid exhibiting a vapor pressure less than P. |
|
|
Hygroscopicity |
A term synonymous with sorption, implying an acquired amount or state of water sufficient to affect the physical or chemical properties of the substance. |
|
|
Water of hydration |
Water present in regular positions within a crystal Lattice. |
|
|
Efflorescence |
Spontaneous loss of water of hydration. |
|
|
Deliquescence |
Sorption sufficient to produce dissolution of the substance. |
|
|
Free moisture, Unbound Moisture |
Water present in a solid exhibiting a vapor pressure greater than P. This water is some times referred to as “freezable” water. |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
ANALYTICAL METHODS: -
There is no single analytical method that suffices in the characterization of moisture associated with solids. The approach is a judicious combination of the following techniques.
Why Measuring Moisture?
Most natural products contain moisture. The water content per se is seldom of interest. Rather, it shows whether a product intended for trade and production has standard properties such as:
– Storability
– Agglomeration in the case of powders
– Microbiological stability
– Flow properties, viscosity
– Dry substance content
– Concentration or purity
– Commercial grade (compliance with quality aggrements)
– Nutritional value of the product
– Legal conformity (statutory regulations
governing food)
In a moisture determination by the physical separation of water from the solid, it is important to recognize that free and bound moisture must be dissociated from the solid by an applied stress, using
Solid x H2O -------> Solid.y H2O + (x-y) H2O
Where x is the moles of water initially associated with the solids, Y the moles of water still associated with the solid, and (x-y) the amount of water released as a consequence of the stress. The stresses used differ according to the conditions (e.g., high temperature, low vapor pressure, anhydrous solvent systems). Hence, the moisture contents determined by different methods may very well be different.
Thermal Methods
Thermal gravimetric analysis (TGA) is undoubtedly the most widely used method of moisture content determination. The sensitivity and sophistication of TGA instruments ranges from the classical moisture balance (LOD) to specially designed microbalances enclosed in chambers that may be evacuated. TGA determines mass changes as a function of temperatures
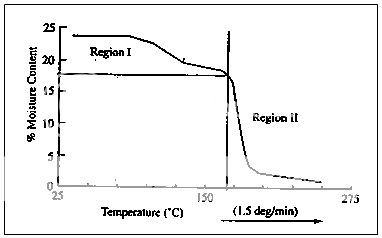
Fig. 1 Thermal gravimetric analysis of dicalcium phosphate dihydrate.
The result of a TGA of dicalcium phosphate dihydrate is shown in Fig. 1. The profile represents the weight loss with increasing temperature (1.50C/min) in an environment containing a desiccant. The total weight loss is the difference between the initial weight and the final constant weight of the dry solid. There are two regions in the profile, the first beginning at about 900C and the second at about 1700C.
Temperature ranges, quantitative mass changes and their dynamics can be determined from room temperature to 1600 °C. The profile represents the weight loss with increasing temperature (1.50 C/min) an environment containing a desiccant. The total weight loss is the difference between the initial weight and the final constant weight of the dry solid.Thermogravimetric methods are suitable for practically all thermally stable substances with a moisture content >0.1%.
Differential scanning calorimetry (DSC) is a thermal method that measures the energy change accompanying a nonadiabatic process. A small sample of the moist solid contained in a metal sample container is exposed to a controlled increase in temperature. Water dissociated from the sample is swept out of the heating chamber by a nitrogen stream and the dynamic energy consumption (mcal/sec) required to keep the sample at the same temperature as an empty sample container recorded. When the temperature is increased at a constant rate, the area under the DSC curve reflects the energy, in the form of heat, associated with the phase change.
The principal advantage of the thermal methods is convenience; however, these analyses are not specific for water, and exposure to high temperature may be an unrealistic stress or alter the sample. An acceptable determination of moisture content using a thermal method provides a result with minimal residual moisture and only minor alteration of the solid. Thermal methods can be combined with a specific titration of water by bubbling the evolved gas from TGA or DSC through the titration medium.
Karl Fisher Titration
For over 60 years, the specific titration of water has used a reagent developed by Karl Fisher, which consists of iodine, sulphur dioxide, and pyridine in methanol. In its simplest form, the Karl Fisher titration is a one-point determination of moisture content8. The principal advantage is specificity for water. It is also a non-thermal method, which is very sensitive and can be easily automated. The main disadvantage is that the solid must dissolve in the titration medium to be sure that the total amount of moisture is released. If the analysis is carefully designed in such a way that moisture is extracted from the solid to the same degree each time, accurate and reproducible results can be obtained for solids that do not dissolve.
Principle: The Karl Fischer method is used for many substances as a reference method. It is a chemical analysis procedure, which is based on the oxidation of sulfur dioxide by iodine in a methanolic hydroxide solution. In principle, the following chemical reaction takes place:
H2O + I2 + SO2 + CH3OH + 3RN -----> [RNH] SO4CH3 + 2[RNH] I
The titration can be performed volumetrically or coulometrically. In the volumetric method a Karl Fischer solution containing iodine is added until the first trace of excess iodine is present. The amount of iodine converted is determined from the burette volume of the iodine-containing Karl Fischer solution. In the coulometric procedure, the iodine participating in the reaction is generated directly in the titration cell by electrochemical oxidation of iodide until again a trace of unreacted iodine is detected. Faraday's law can be used to calculate the amount of iodine generated from the quantity of electricity required.
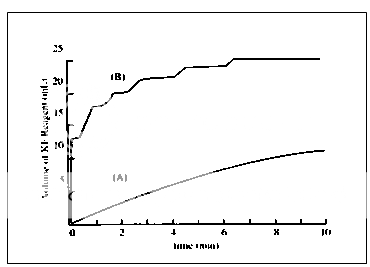
Fig. 2 Normalized KF titration for dicalcium PO4
Application: The Karl Fischer titration is a moisture determination method specific for water and is suitable for samples with a high moisture content (titrimetry) and also for those with water contents in the ppm range (coulometry). It was originally developed for nonaqueous liquids, but is also suitable for solids if these are soluble or if the water they contain can be removed by heating in a stream of gas or by extraction.
Advantages: Accurate reference method, coulometry also suitable for trace analysis and water detection.
Spectroscopic Methods
The most useful spectral methods for the Chacterization of water in solids are Fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR), and powder X-ray diffraction (XRD).
Although moisture contents often present values that are close to being stoichiometric, X-ray confirmation of the differing crystal structure should be a requisite for designation as a hydrate9
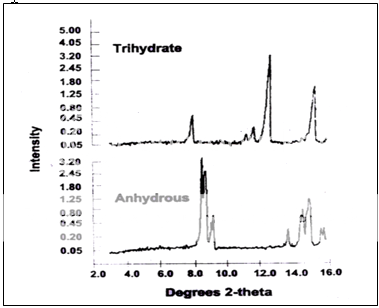
Fig. 3 Partial X-ray diffraction patterns of ampicillin samples.
- The X-ray method is not just useful from the qualitative perspective. In samples that contain both anhydrous and hydrate forms, diagnostic regions of the pattern can be identified, and the relative areas of peaks in these regions may be used to establish the relative amounts of each phase9.
- Infrared analysis of water associated with a solid centers on an assessment of the degree to which the environment influences the stretching frequency associated with the –OH group. The –OH stretching mode for free water in the gaseous state has a characteristic energy of 3655 cm-1. The frequency of this stretching is lowered when water is condensed and/or bound. Ice has a characteristic –OH stretching frequency of 3400 cm-1. By comparison of the FTIR spectrafor the anhydrous form with those of the sample with water, the –OH bands for water can be identified. The distinctive, sharp peak for crystalline water is shown multiple bands may be seen in the spectrum.
- Solid state NMR is a method that promises to increase the understanding of the state of water in solids and its specific influence on the chemicals of interest.
Influence of Moisture on Product and/or Process Attributes
No attempt will be made to provide a comprehensive summary of the numerous studies that have been conducted and which show how water influences the processing and stability of pharmaceutical solids and dosage forms11,14,15
In an earlier section, the potential for a water-soluble substance to deliquesce was discussed. The emphasis here is on less obvious effects of moisture on solid dosage forms, and three associated areas that link to information presented earlier in this article are discussed: 1) moisture induced changes in the state of solid, 2) the effect of moisture on the performance of excipients in the manufacture of compressed tablets, and 3) the chemical stability of bioactive agents alone and in combination with excipients.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Moisture-Induced Changes
The moisture can have a dramatic effect on the physical character of a substance has been demonstrated recently by Carstensen and Van Scoik25. Amorphous sucrose spheres were prepared by Lyophilization characteristically produces a highly porous, amorphous solid cake. Its moisture content increases considerably in the first few days of the study. However, the moisture absorbed by the porous amorphous sucrose phase eventually caused a collapse of the structure and a commensurate reduction in moisture content because of the dramatic decrease in available “surface
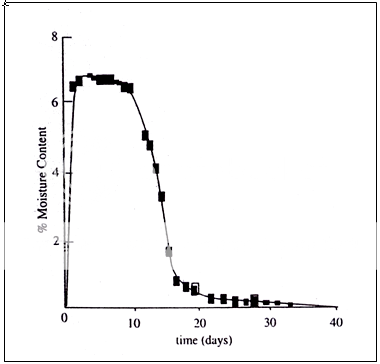
Fig. 4 Moisture uptake by amorphous sucrose at 230C at 33% RH.
convert to the crystalline form at the rate that was shown to convert to the crystalline form at a rate that was dependent on relative Not only did the physical structure change from a loose to a more dense amorphous structure, but also the amorphous sucrose was shown to humidity. This example is consistent with the increase emphasis being placed on changes in state of the solid as a result of sorption. One of the difficulties associated with a thermodynamic treatment of solids is the problem of dealing with changes in surface area as a continuous variable. An early thermodynamic treatment to address this problem was presented by Copeland and Young in 196127. These authors considered a change in the number of moles of adsorbent as the addition or removal of particles with the same specific surface area, and, therefore, presented a basis for treating thermodynamic properties of powder systems as continuous functions
Wu and Copeland28 used this approach in the characterization of barium sulphate. They found clear evidence to discount the “inert” adsorbent theory and stressed that even though thermodynamic variables of adsorbents are generally smaller than these for adsorbates, this can be misleading. Adsorbents properties are average properties of the respective component. When the adsorbed moisture is homogeneously distributed within the solid, this estimate is reasonable. However, if the process is adsorption and only the first few layers of the adsorbent are affected, the thermodynamic changes would be dramatically increased. Very few pharmaceutical studies involve the determination of thermodynamic properties of the adsorbent, which is promising area for further research.
The need to address changes in adsorbent is discussed by Zografi3. He observes that water absorbed in to the bulk structure of a solid can act as a plasticizer and depress the glass transition temperature. At temperatures above the glass transition point, the mobility of molecules or segments of molecules in the system increases3. The change from the “glassy state” to the “rubbery state” can account for a number of physical chemical processes of pharmaceutical interest, including the collapse and subsequent crystallization of lyophilized cakes, direct compaction properties, powder caking, permeability of coatings and packaging materials, and solid state chemical stability3. Recognition of this fact has been the single greatest recent advance in establishing a framework for understanding and predicting the impact of moisture.
Zografi and Kontny corrected the experimentally determined monolayer capacities of microcrystalline cellulose for degree of crystallinity and found reasonably consistent values. This result supported the conclusion that water in microcrystalline cellulose is confined to the noncrystalline regions.
Effect of Moisture on Excipients and Tablet Manufacture
The bulk properties of cellulose are generally influenced by adsorbed moisture. The effect of the change in bulk solid properties for
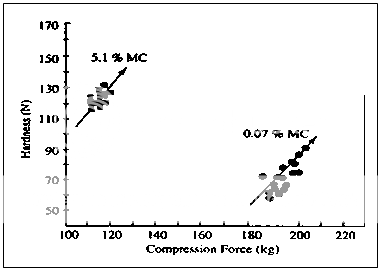
Fig. 5 Compression force vs. hardness plots for microcrystalline cellulose tablets with 0.07 and 5.1% MC.
microcrystalline cellulose has been demonstrated by a tableting operation in a very simplistic manner32,33. Dry microcrystalline cellulose (%MC = 0.07) was compared with material with a moisture content above the associated with completion of the monolayer (%MC = 5.1). A thermodynamic picture of the character of water in these samples can be based on the adsorbate thermodynamic properties: DH > 3.5 kcal/mole (14.65 kJ/mole), DG > 2.3 kcal/mole (9.6 kJ/mole), DS < 4.12 entropy units (e.u.) per mole with the dry solid, and DH = 1.5 kcal/mole (6.27 kJ/mole), DG = 0.53 kcal/mole (2.21 kJ/mole), DS = 3.35 e.u./mole for moisture at the 5.1 % level, or 1.5 times monolayer capacity. (The differential entropy goes through a maximum near the level where a monolayer is completed, and the large difference in free energy between the two states can be accounted for primarily in terms of the bonding of the water to the solid.)
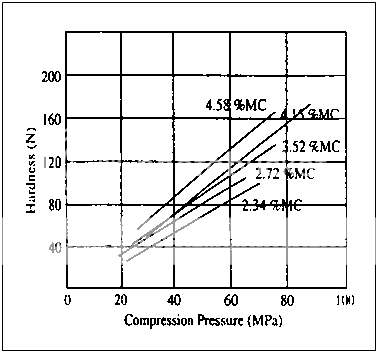
Fig. 6Hardness vs. compression pressure for compressible sugar with different moisture contents.
In each case, preconditioned material was placed in the hopper of an instrumented tablet machine and the performance of these two materials was compared at constant machine settings. The sample with 5.1% MC produced tablet that weighed slightly less (an effect on flow and bulk density), and as a result was exposed to lower compression force. However, the moist microcrystalline cellulose resulted in harder tablets, even though it had been exposed to lower compression force. The loci of points on the compression force versus hardness profile for the tablets indicate a different fundamental behavior for the two materials.
In a similar, yet more extensive study, the compaction of compressible sugar was examined for materials preconditioned at different relative humidities. The hardness versus compression profiles for these samples show a group of lines whose slope appear to be a function of moisture content. This relationship is also demonstrated in Fig.7, where slope is used as a compressibility index is a linear function of moisture content; samples with “desorbed” moisture did not differ from those with adsorbed moisture.
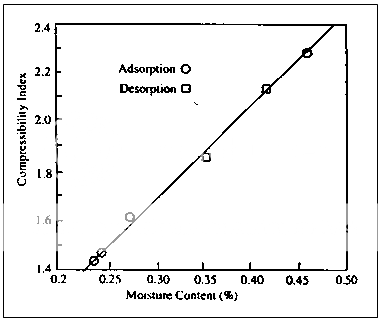
Fig. 7Compressibility of compressible sugar as a function of moisture content.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Chemical Stability of Bioactive Agents
Despite recent advances in understanding the influence of moisture on the physical state of the solid, it is perhaps the effect of sorbed moisture on the chemical stability of moisture sensitive drugs that is most important, particularly because many new bioactive agents are expensive moisture-sensitive proteins.
The literature related to the study of the influence of moisture on the properties and stability of proteins is extensive and growing rapidly. The examination of water associated with proteins and polymers is relevant to the pharmaceutical scientist dealing with the formulation and processing of small drug molecules as well because many excipients used belong in this large-molecule category. Hageman18 has presented a review of protein stability from the pharmaceutical perspective. He ascribes the effect of water content and activity on the solid-state stability of proteins to
· Changes in dynamic activity.
· Changes in conformational stability.
· Participation of water as a reactant or inhibitor, and
· Participation of water as a medium for mobilization of reactants.
Using an analysis of the hydration data of lysozyme and other proteins, Hageman describes critical ranges of hydtration based on certain properties of bound water. Below monolayer capacity, these physical properties of water do not change significantly, and this water has very little mobility. Between 6% and 25% water, the properties of bound water change dramatically, above 25% water, the properties of bound water are similar to bulk water.
In Fig. 8, Hageman classifies the role of moisture in bimolecular reactions into three cases. The increases in reaction rate are attributed to a change in state of the water associated with the solid as reflected by a lower effective viscosity. In case I, there is a continual increase in reaction rate with increasing water content above the monolayer. When all the reactant has been solubilized and further water dilutes the medium. Case II results. If the dilution is extensive, or if water is a product inhibitor of decomposition, a rate reduction can be observed (Case III). Case III behaviour is an example of the effect of moisture on the progress of the Malliard reaction for the glucose-containing formulations of α-N-acetyl-L-lysine, poly-L-lysine, insulin, casein, and plasma proteins19. The fact that there can be a maximum degradation rate at a humidity other than 100% RH is observed in other situations as well.
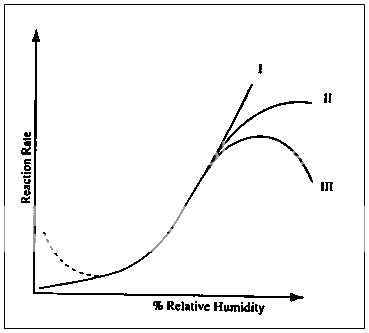
Fig. 8 Effect of sorbed water on the reaction rates of bimolecular reactions in the solid state.
The presence of excipients in a formulation can influence product stability. The conceptually appealing strategy of including a “moisture scavenger” in a formulation is based on this. In glucose-containing systems, it was demonstrated that liquid and solid humectants can influence the mobility of water in the system. The location of the maximum rate of reaction was found to vary from 40% to 80% RH, depending on the additives. The addition of liquids such as glycerol or propylene glycol lowered the mobilization point and facilitated the reaction at lower humidities. The addition of the solid humectant sorbitol reduced the reaction rate dramatically by decreasing free water for mobilization of reactants.
Carstensen7 has presented a summary of the effects of moisture on the stability of smaller molecules by addressing three cases: decomposition with non-depleting moisture, simultaneous decomposition in the solid and dissolved states, and decomposition with limited amounts of water. In the context of this discussion, emphasis here is placed on hydrolytic degradation processes.
Leeson and Mattocks29 by initially modeled the first phase with abundant moisture assuming that a layer of moisture sufficiently mobile to permit dissolution of the drug substance surrounded the solid particle. If the layer remains saturated with drug, this approach yields an apparent zero order degradation process given
-dM/dt = Ks[H2O] …………(2)
Where –dM/dt is the rate of drug loss, k is a rate constant appropriate for the order of the reaction, S is the saturation concentration of the drug in the layer, and (H2 O) is the concentration of water. Although this sample approach often suffices in form to describe data observed in a stability study, it should not be accepted to account for the effects of moisture present in the different states described previously.
The degradation of aspirin powder after 120 days at 100% RH and 250 C, for example, was found to be more than ten times greater than what would be expected based on suspension data22. In addition, the actual rate of aspirin degradation has been found to increase with time34,35. Carstensen presented a theory to describe this non-linear behaviour based on the fact that the formation of salicylic acid actually exposes moisture adsorption sites of higher energy on aspirin35, thereby leading to the acquisition of more water. This explanation accounts for an increase in rate with time, but does not explain why the rate is high for the powder then a suspension where the supply of water is in huge excess. This increase rate could be due to specific acid catalysis in a film of relatively small volume where the ph is dramatically affected by the hydrolysis itself35.
Finally, the complicated situation faced when a moisture sensitive drug is combined with an hydrophilic excipient is shown in Fig. 9, In this study, aspirin was combined with 4% of the disintegrant and stored for 120 days at 25oC at the respective humidity. The moisture contents stabilized within the first few days, and the degradation results are presented as moles of the degradation product salicylic acid per mole of water. Point A is the result for aspirin powder alone at 100% RH.
Fig. 9Effect of humidity on the salicylic acid content in aspirin-disintegrant mixtures at 120 days and 250C.
These result demonstrate many of the points presented here. At 100%RH, the reaction rate is lower than that expected for croscarmellose and sodium starch glycolate, because the disintegrants swelled and in doing so diminished the available moisture. Normalizing based on the total moisture present overstates the amount of water available to react and underestimates the actual reaction rate. At the other extreme (20%RH), the analysis is also biased by overstating the amount of water available, but for a different reason. The water associated with the system here is tightly bound to the disintegrants and not available. At intermediate humidities, depending on the excipient, the observed rate of degradation of the solid is higher than expected. This behaviour may be a consequence of the activated sorption process and a specific acid catalysis described by Carstensen35. Nevertheless, results of this sort are disconcerting for the product development pharmacist interested in making a stable tablet that disintegrates quickly.
SUMMARY
Real progress has been made in the last 10 years, providing pharmaceutical scientists with a solid basis for understanding the interaction of water with solids of pharmaceutical interest. Much of this progress has been the consequence of a paradigm shift: the model of the solid as an inert substrate is almost never valid. Further characterization of the state of water in solid-water systems may ultimately provide a basis for the design of stable formulations and permit the establishment of performance-based specifications for pharmaceutical excipients.
REFERENCES
1. Webster’s Ninth New Collegiate Dictionary, CT, 1991; 28, 432.
2. Mitrevej, A.; Hollenbeck, R.G. Int. J. Pharm. 1983, 14, 243.
3. Zografi, G. Drug Dev. Ind. Pharm. 1988, 14, 1905.
4. Gibbs, J.W. The Collected Works of J. Willard Gibbs; Yale University Press: New Haven, 1948; 1.
5. The United States Pharmacopoeia 24th Rev., The National Formulary, 19th Ed. United States Pharmacopeial Convention, Inc. Rockville MD, 2000.
6. Hollenbeck, R.G. Determination of water: Compendial Viewpoint Proceedings of the Seventh Wisconsin Update Conference, Carstensen, J.T. Ed.; Extension Servicesin Pharmacy, University of Wisconsin, Madision, 1988; 77,91.
7. Carstensen, J.T. Drug Dev. Ind. Pharm. 1988, 14, 1927.
8. Mitrevej, A.; Hollenbeck, R.G. Pharmaceut. Technol. 1982, 6 (10), 48.
9. Connors, K.A. Drug Dev. Ind. Pharm. 1988, 14, 1891.
10. Brittan, H.G.; Bugay, D.E.; Bogdanowich, S.J.; DeVincentis, J. Drug Dev. Ind. Pharm. 1988, 14, 2029.
11. Adamson, A.A. Physical Chemistry of Surfaces, 4th Ed.; John Wiley & Sons: NY, 1982.
12. Harkins, W.D.; Jura, G.J. Am. Chem. Soc. 1944, 66, 919.
13. Franks, F. Water: A Comprehensive Treatment; Plenum Press: New York, 1972; 1 and 5, 1975.
14. Carstensen, J.T. Ppharmaceutics of Solids and Solid Dosage Forms; Wiley: New York NY, 1977.
15. Conners, K.A.; Amidon, G.L.; Stella, V.J. Chemical Stability of Pharmaceuticals; Wiley-Interscience: New York, 1986.
16. York, P. Int. J. Pharm. 1983, 14, 1.
17. Umprayn, K.; Mendes, R.W. Drug Dev. Ind. Pharm. 1989, 13 (4 -5), 653.
18. Hageman, M.J. Drug Dev. Ind. Pharm. 1988, 14, 2047.
19. Plotkowiak, Z. Pharmazie 1989, 44 (2), 837.
20. Fassihi, A.R.; Persicaner, P.H.R. Int. Pharm. 1987, 37 (1 – 2), 167.
21. Hageman, M.J.; Water Sorption and Solid State Stability of Proteins. Stability of Protein Pharmaceuticals; Ahern, T.J., Manning, M.C., Eds.; Plenum Press: New York, 1992.
22. Shukla, J.; Price, J.C. Pharm. Res. 1991, 8 (3), 336.
23. Ahlneck, C.; Alderborn, G. Int. J. Pharm. 1989, 56 (2), 143.
24. Teng, C.D.; Alkan, M.H.; Groves, M.J. Drug Dev. Ind. Pharm. 1986, 12 (11 – 13), 2325.
25. Cartensen, J.T.; Van Scoik, K. Pharm. Res. 1990, 7, 1278.
26. Groves, M.J.; Teng, C.D.; The Effect of Compaction and Moisture on Some Physical and Biological Properties of Proteins. Stability of Protein Pharmaceuticals; Ahern, T.J., Manning, M.C., Eds.; Plenum Press: New York, 1992.
27. Copeland, L.E.; Young, T.F. Adv. Chem. Ser. 1961, 33, 348.
28. Wu, Y.C.; Copeland, L.E. Adv. Chem. Ser. 1961, 33, 357.
29. Leeson, L.J.; Mattocks, A.M.J. Am. Pharm. Assoc. Sci. Ed. 1958, 47 (5), 329.
30. Zografi, G.; Kontny, M.J.; Yang, A.Y.S.; Brenner, G.S. Int. J. Pharm. 1984, 18 (1 – 2), 99.
31. Ben-Rayana, E.; Seddas, A.; Ruelle, P.; Ho, N.T.; Kesselring, U.w. mol. Cryst. Liq. Cryst. 1990, 187, 617.
32. Blair, T.C.; Buchton, G.; Breezer, A.E.; Bloomfield, S.K. Int. J. Pharm. 1990, 63 (3), 257.
33. Sadeghnejad, G.R.; York, P.; Stanley-Wood, N.G.; Water Vapour Interaction with Pharmaceutical Cellulose Powders. Pharmaceutical Technology: Drug Stability; Rubenstein, M.H., Ed.; Ellis Horwood Limited: West Sunsex, UK, 1989.
34. Mitrevej, A. A Comprehensive Study of Water Vapor Sorption by Aspirin-Disintegrant Blends and its Relationship to Aspirin Stability; Ph.D. Thesis, 1982.
35. Carstensen, J.T.; Attarchi, F.J. Pharm. Sci. 1988, 74 (4), 314.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









