 About Authors:
About Authors:
1*Ajeet, 2Shelly Singh
1Department of Medicinal Chemistry, S. D. College of Pharmacy and Vocational Studies,
Bhopa Road, Muzaffarnagar, U.P., India, PIN-251001
2Department of Pharmaceutics, Mahatma Gandhi College of Pharmaceutical Sciences,
Jaipur, Rajasthan, India.
* ajeet_pharma111@rediffmail.com
Abstract
Iontophoresis, a 250 years old technology for delivering the drug is simply based on the use of electrical potential. Being so old, it is still having too popped up just because of its unlimited aspects to work with. In the present review, I tried to compile the vast aspects of a drug delivery system termed as iontophoresis. Iontophoresis is one of the most interesting and challenging endeavors facing the pharmaceutical scientist. The systemic drug delivery systems often require large dose and are associated with gastrointestinal side effects, while topical application of solutions, suspensions, and ointments show variability in absorption patterns. Iontophoresis technique is capable of expanding the range of compounds that can be delivered through ocular, transdermal, ural, transungual, buccal or by nasal route.
[adsense:336x280:8701650588]
Reference ID: PHARMATUTOR-ART-1318
IMPORTANT IN THIS ARTICLE:
* Iontophoresis- specificity towards drugs
Introduction
In 1747 Pivati described the method of iontophoresis. It is the introduction of soluble salts into the tissue of the body by means of direct electrical current for gaining the therapeutic effect (Singh and Maibach, 1994).
Transdermal administration of drugs has assumed an important place in modern drug therapy. This mode of administration is suitable for non-ionized drugs requiring a relatively small dosage. The drug traverses the skin governed primarily by the laws of passive diffusion of the non-ionized drug through the rate-limiting membrane, the stratum corneum. Ionized drugs, however, do not easily penetrate this barrier and are not generally suitable for routine transdermal dosage forms unless an external source of energy is provided to drive the drug across the skin. In iontophoresis (IP), this external source of energy is in the form of an applied direct electrical current. Electrical energy will assist the movement of ions across the stratum corneum according to the basic electrical principles of “like charges repel each other and opposite charges attract”. (Loyd V. Allen)
Theoretical factors affecting the Iontophoresis described by Dr. Loyd V. Allen
Ion Penetration
When salts or drugs are dissolved in aqueous solutions, ionized or electrically charged particles are formed. This process of ion formation is called dissociation or ionization. Ionized drugs do not normally penetrate into surface tissues sufficiently using passive transdermal delivery to achieve a therapeutic level. The problem of membrane penetration of ionic drugs can be overcome by providing an energy source which increases the rate of penetration. Electrical energy, in the form of a small direct current, will assist the movement of ions. According to electrical principles, as previously mentioned, like charges repel each other and opposite charges attract. Thus, positive drug ions are repelled from a positively charged electrode and negative drug ions are repelled from a negatively charged electrode.
Physics and Mathematical Relationships
There are a few relationships that are important in IP.
Ohm’s Law states:
V = IR
where V is electromotive force in volts, I is current in amps and R is resistance in ohms. The importance of this relationship is that at constant voltage, any change in resistance results in a change in current level. Very often, the resistance decreases during a procedure; as a result the current, in milliamps, will increase, unless the current device is programmed to deliver a constant current, then the voltage will vary to compensate for changes in resistance.
Coulombs Law states:
Q = IT
where Q is the quantity of electricity, I is current in amps and T is time in minutes. Thus, “mA•min” is used to describe the “current dosage” used during IP.
Faraday’s Law states:
D = (IT) (IZI F)
where D is the amount of drug delivered (in gm-equivalents), I is current in amps, T is time, IZI is valance and F is Faraday’s Constant. From this relationship, the more electricity delivered, the more drug delivered. Faraday’s Law has been used by some to provide information concerning the rate of deposition of the drug at the skin surface. However, due to the complexity of the factors involved during the process of IP, theoretical predictions based on it are difficult.
[adsense:468x15:2204050025]
Involved variables discussed by Dr. Loyd V. Allen
As can be seen or derived from the above relationships, there are a number of variables that may affect the process of IP; in vitro, or in vivo or both. Each individual variable may impact either the in vivo or in vitro slightly different. The primary differences in the in vitro and in vivo situation concerns the donor cell or electrode system and a receptor cell (in vitro) or patient (in vivo). In the in vitro system, the donor cells are typically glass, plastic or the electrode system; the receptor cells typically are glass or plastic. The membrane is generally an animal skin, although in some studies different artificial membranes have been used. As the current is applied, the drug is generally sampled from the receptor cell and the concentration followed over time. The in vivo situation involves the use of the drug in an electrode system (as the donor “cell”) and the patient (as the receptor “cell”). The concentration of the drug in the patient can be determined from the blood level or from skin biopsies. The actual depth of skin penetration can be determined by analyzing different layers of the skin biopsy. Variables affecting the IP process include: the drug concentration, drug salt form, pH of the drug micro environment, the current intensity and duration, competing ions in the electrode solution/matrix, stability of the drug during the IP process, the type of matrix containing the drug and current density. Additionally, patient anatomical factors and the presence and extent of inflammation can influence the depth of drug penetration.
Drug Concentration:
Increased uptake by the skin during and after IP with an increase in drug concentration has been reported. This is generally true until a plateau level is reached at which no further increase in flux is observed.
Drug Salt Form:
It has been reported that different salt forms have different specific conductivities and that conductivity experiments in vitro will provide information concerning the general suitability of a drug for IP. The salt form of drugs must be considered along with the pH of the solution for determining the amount of drug in the ionized state.
pH of the Drug Micro environment:
Laboratory findings vary on the effect of pH and drug behavior. According to the Henderson- Hasselbalch equation, pH is the determining factor governing the amount of drug present in the ionized state. For optimum IP, it is desired to have a relatively large proportion of the drug in the ionized state. However, this must be counterbalanced with delivery of a drug at a pH that is tolerable and safe for the patient.
Current Intensity and Duration:
From Faraday’s Law we know that in an electrolytic solution the transported quantity of electricity depends on the strength of the current and the duration of its passage. Thus, this law would suggest that the same number of ions should be transported at different strengths of current if the time for current flow is inversely related to their strengths. However, generally speaking, we also know that in some cases, higher current may deliver more drug than lower current, possibly due to induced changes in skin permeability by the higher current, resulting in a greater flow of drugs. The rate at which the ions are introduced into the body with various current strengths can play an important role. When the current is stronger, more ions penetrate at one time. The strength of the current used also depends on the sensitivity and tolerance of patient.
Competing Ions in the Electrodes:
Electrical current is carried by positive and negative ions in solution. There is no major distinction between ions of the same charge even though they are composed of different chemical elements. Therefore, solutions for IP should be as pure as practical and generally contain as few extraneous substances as possible. Drug solutions should be prepared with purified water (deionized, distilled, reverse osmosis). It is has been shown that the presence of excipients in dosage forms, i.e. preservatives in injections as well as compounds used as external buffers, will alter the amount of drug delivered. In vitro, the total current will be carried by drug ions along with the same charges as drug ions in the donor cell plus the counter ions present in the receptor cell. Therefore, the competing ions in the donor cell and the counterions in the receptor cell will be affecting the actual current carried by the drug moiety. During IP, there is a shift in pH due to hydrolysis of water which may result in a loss of efficiency of drug transfer due to presumably competing ions. Buffers may be built into the electrode to minimize this effect, but the buffer materials should be bound, or immobile, and not released for IP transport, as they would then compete with the active drug.
Stability of the Drug During the IP Process:
The drug undergoing IP must be stable in the solution environment up to the time of IP and also during the iontophoretic process. Oxidation or reduction of a drug not only decreases the total drug available but the degradation compounds, if they possess the same charge as the drug ion, will compete with the drug ion and reduce the overall transmembrane rate of the drug.
Type of Matrix Containing the Drug, Gel vs. Solution:
The migration of the drug under the influence of the electrical current will be different as the matrices are different. This can be related to differences in viscosities, material electrical charge and porosities.
Current density:
Current density is the quantity of current delivered per unit surface area. The following criteria should be considered in selecting proper current densities for IP: (1) the current should be sufficiently high to provide a desired drug delivery rate; (2) it should not produce harmful effects to the skin; (3) there should be a quantitative relationship between the flux and the applied current; and (4) there should be electrochemical stability of the drug.
Patient Anatomical Factors:
Patient anatomical factors that influence the depth of penetration that are variable from patient to patient include skin thickness at the site of the application, presence of subcutaneous adipose tissue and the size of other structures, including skeletal muscle. Additionally, the presence and severity of inflammation can influence drug penetration due to the increased temperature (which may increase penetration rate) and the elevated level of blood and fluids present that may serve to transport the drug throughout the body.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Iontophoresis- specificity towards drugs
Lidocaine
Lidocaine has been extensively studied for treatment and prophylaxis of local pain. Using iontophoresis as the method of delivery reduces systemic exposure to this antiarrythmic agent, thus lowering the possibility for cardiac side effects. One common problem is a short duration of action, which is explained by the vasodilation that lidocaine causes, increasing its own clearance. In order to alleviate this problem, combination with epinephrine is indicated to cause local vasoconstriction and thus hold the lidocaine in the tissues in close proximity to the electrode. The efficacy of lidocaine in providing dermal anesthesia has been proven by several randomized controlled clinical trials.(Greenbaum, 2001; Wanke, 1991; Wallace et. al., 2001; Ashburn et. al. 1997; Galinkin et. al., 2002; Miller et. al., 2001; Squire et. al., 2000; Kim et. al., 1999; Sadler et. al., 1999; Meyer et. al., 1990; Russo et. al. 1980). Studies comparing the efficacy of eutectic mixture of local anesthetics (EMLA) to iontophoresis with lidocaine for pre venipuncture analgesia have found mixed results. These findings support the use of iontophoresis as a viable alternative to EMLA (Greenbaum, 2001; Wanke, 1991; Wallace et. al., 2001; Ashburn et. al., 1997). Several studies have evaluated the cost effectiveness of delivering medications (mostly lidocaine) via iontophoresis. One study comparing the use of iontophoresed lidocaine versus general or spinal anesthesia found that the cost of iontophoresis was half that of other anesthesia for performing bladder surgeries (Jewett et. al., 1999). Other studies have compared the costs of using EMLA versus iontophoresis and all have found iontophoresis to be a more cost effective method of drug delivery.
Dexamethasone
Dexamethasone is an extremely effective corticosteroid with strong anti-inflammatory effects and a long half-life. Using this medication via iontophoresis enables steroids to be delivered to joints and tissues, while bypassing the various unwanted side effects that accompany systemic treatment with corticosteroids. Its use for treating joint, fascia and tendon inflammation has been well studied (Gudeman et. al., 1997; Crawford et. al., 2002; Schiffman et. al., 1996; Li et. al., 1996). One double-blind, randomized controlled trial involving 39 patients suffering from plantar fasciitis found significant benefit from the addition of dexamethasone 0.4% iontophoresis to the standard treatment regimen during the 2-3 week treatment. However, the duration of increased effect was lost at the four-week follow-up. Therefore, although iontophoresis may not provide a long-term benefit, it does provide for accelerated improvement crucial to performance athletes and highly active patients (Gudeman et. al., 1997). Another interesting application involves combination therapy with dexamethasone and lidocaine combination solution applied at the positive electrode. When the electrical current is turned on, lidocaine is driven into the tissues, toward the negatively charged electrode. Although the dexamethasone in solution is not repelled by the positive electrode, the solution flow into the tissues, driven by the lidocaine is sufficient to draw a substantial amount of dexamethasone into the tissues as well.
NSAIDs
The efficacy of NSAIDs has been documented for a long period of time. However, many patients experience routine gastro-intestinal side effects after taking them systemically. Topical preparations are available in compounded form. Deeper penetration may be facilitated by preparation of an iontophoresis solution (Tashiro et. al., 2001). One randomized controlled trial involving 40 patients compared the pain relief produced by iontophoretically delivered sodium salicylate compared to diclofenac for lateral epicondylitis and found that pain in both resisting wrist extension and pressure were more significantly decreased in the diclofenac group (Demirtas et. al., 1998). Ketoprofen and Ketorolac solutions have also been successfully used with iontophoresis (Tashiro et. al., 2000a, 2000b).
Antibiotics
The use of antibiotics has been historically documented for various dermatologic infections, ear chondritis and burn infections. Reports of the use penicillin and gentamicin as well as the antiseptic effects of zinc and iodine are available. The available data on the use of this gentamicin via iontophoresis are conflicting. Historical data presents this use as a productive application of iontophoresis. The theory of attempting to deliver antibiotics to a tissue with limited vascularity appears strong. However, in a randomized trial comparing patients who received gentamicin with those who only received standard ear care, there were no differences other than the gentamicin group developing gentamicin resistant organisms (Desai et. al., 1991).
Hyaluronidase
The enzymatic action of hyaluronidase has been used for a variety of indications. Its ionic nature andability to promote fluid resorption makes it an ideal molecule for iontophoresis. Historically this drug was available as the product, Wydase? . However, this product has been discontinued by the manufacturer and thus is only available through compounding pharmacies. The mechanism should decrease the damage involved with hematomas and edema. It is important to remember that hyaluronidase requires a special buffer solution and also has limited shelf life. Also, this product requires refrigeration when it is in solution.
Acetic Acid
The acetate ion in this chemical is believed to reduce calcium deposits. The published studies and case reports on this application are unfortunately conflicting with regard to both acetate penetration and clinical efficacy. One study found reductions in both the size and density of calcium deposits as confirmed by x-ray. Another case report found complete absorption of deposits at 3 months (Psaki et. al., 1955). And a study involving 35 patients with heel pain found “significant” decreases in pain with 27 months of follow-up (Japour et. al., 1999). However, a small (n=21) randomized controlled trial found no significant differences in calcium density or range of motion (Bose et. al., 2001). Therefore, the success of this treatment may be questionable but is not out of the realm of possibility and should be kept in the armamentarium.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Iontophoresis- a lots of aspects
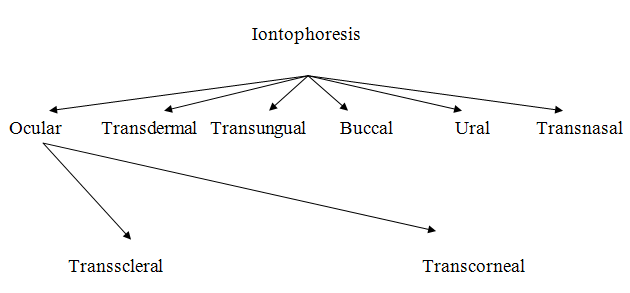
Ocular Iontophoresis
Delivery of drug to the inner eye still presents a critical problem in ocular therapeutics. Topical administration cannot effectively reach the ocular fluids, where as systemic drug delivery has restricted access due to blood-aqueous and blood-retinal barriers. The retrobulbar and subconjuctival injections do not produce adequate drug levels, while direct intracameral or intravitreal delivery leads to intraocular complications (Monti et. al., 2003). Ocular iontophoresis could be a solution for such problems. Ocular iontophoresis was first investigated in 1908 by the German investigator, Wirtz, who passed an electric current through electrolyte-saturated cotton sponges placed over the globe for the treatment of corneal ulcers, keratitis and episcleritis (Wirtz, 1908). Basically, in ocular iontophoresis, a donor electrode is placed in the eye while another electrode is placed on the body surface to complete the electrical circuit. The drug to be delivered into the eye is loaded in the donor electrode. An electric field is applied across the eye to enhance the delivery of the drug into the eye (Sarah et. al., 2008). It has been suggested that this technique is relatively easy, convenient for use, safe, and provides fast and higher drug concentration in the specific ocular site, thereby providing solution to the low bioavailability of drugs. It is also well tolerated at low electric current density (Parkinson et. al., 2003; Hughes et. al., 1984; Kalia et. al., 2004). However, it causes minimum discomfort to patients and is not entirely harmless for ocular tissues (Rootman et. al., 1988; Grossman et. al., 1989). Iontophoresis has been evaluated for various drugs into the eye (Halhal et. al., 2004; Myles et. al., 2005). This technique can reproducibly deliver therapeutic concentrations of various ophthalmic drugs, such as corticoids, antibiotics, peptides, and proteins, to both segments of the eye. The drugs can be delivered either by transscleral or transcorneal iontophoresis. Transscleral iontophoresis presents more advantages when compared to transcorneal delivery, owing to scleral larger surface area, enhanced delivery of the drugs to the posterior segment and least possibilities of systemic absorption.
Transscleral Iontophoresis
In phakic animals, the lens-iris diaphragm limits penetration of a topically applied drug to the posterior tissues of the eye, such as the vitreous and retina. Transscleral iontophoresis overcomes this barrier and delivers drugs directly into the vitreous and retina through the choroids (Eljarrat et. al., 2006). Transscleral iontophoresis of steroids (dexamethasone and methyl prednisolone) can be the alternative treatment for many ocular inflammations. Detailed pharmacokinetic studies have been performed on transscleral iontophoresis for various drugs (Grossman et. al., 1990). Every drug resulted with different patterns of distribution in the vitreous. Controlled distribution concentration for 1 to 6 hours into the vitreous chamber has been reported for carboplatin after transceral iontophoresis. A previous study also revealed that the transscleral iontophoretic treatment can be used to obtain high concentrations of drugs to the posterior segments of the eye using a short transscleral iontophoresis with low current, without removing the conjunctiva (Esther et. al., 2004). Several investigators have conducted clinical studies using transscleral iontophoresis of the anti-inflammatory corticosteroid, methyl-prednisolone hemisuccinate (SoluMedrol). The procedure was safe, well tolerated and easily applied for the treatment of severe ocular inflammation thereby reducing the systemic side effects of corticotherapy (Chauvaud et. al., 2000).
Transcorneal Iontophoresis
The transcorneal iontophoresis has demonstrated good results for various investigations. Its application in the treatment of corneal ulcers offers a potentially effective method of management. Gentamicin, tobramycin and ciprofloxacin iontophoresis have resulted in significantly fewer bacterial colonies in the cornea compared with frequent eye-drops instillation (Frucht-Pery et. al., 2006). Using this method, it has been revealed that after a short iontophoretic treatment of 1 mA for only 1 min with transcorneal iontophoresis of dexamethasone, a 30 fold higher concentrations in the cornea (1363.7F 436.3 Ag/g) is achieved when compared with the common treatment of frequent drop instillation every 5 min for 1 hour (Esther et. al., 2005).
Berdugo et. al. have used transcorneal iontophoresis (300 µA for 5 min) to enhance in vivo delivery of an AS-ODN against VEGFR-2 using rat cornea. The AS-ODNs penetrated into all corneal layers (Berdugo et. al., 2003). In earlier studies, the β-blocking agents, timolol maleate and betaxolol hydrochloride, showed significantly increase in permeation through transcorneal iontophoresis. However, iontophoresis does not always show good result for penetration, as iontophoresis of vancomycin, a complex glycopolypeptide antibiotic, has resulted in poor corneal penetration compared with the other antibiotics, due to its high molecular weight (1448 Dalton) that highly influence the effectiveness of the iontophoretic drug delivery (Choi et. al., 1988).
Voigt et. al. used the combined transcorneoscleral iontophoresis to enhance intraocular penetration of rat antinitric oxide synthase II oligonucleotides (anti-NOSII AS-ODNs) in the rat model of endotoxin-induced uveitis (EIU). The anti-NOSII AS-ODNs were detected intact in all the corneal layers, iris/ciliary body, peripheral retinal layers as well as conjunctiva and sclera 1 hour post-iontophoresis (Voigt et. al. 2002).
Preliminary toxicity to the eye after iontophoresis indicated a reversible inflammation with a current intensity of 5.1 mA/cm2 for 2 min. Further, iontophoresis effect on the eye surface was evaluated by histopathology of the corneal tissue 5 min and 8 h after iontophoretic current of 0.5 and 1 mA for 1 and 2 min (2.5 and 5.1 mA/cm2). Minor reversible epithelial defects and stromal edema were found 5 min after the iontophoretic treatment, which disappeared or diminished 8 hours afterwards.
Transdermal Iontophoresis
Transdermal iontophoresis is the application of an electrical potential that maintains a constant electric current across the skin and enhances the delivery of ionized as well as unionized moieties (Yiping et. al., 2005). It offers various advantages such as easier termination of therapy, better control of drug delivery, improving delivery of polar drugs as well as high molecular weight substances, benefits of bypassing hepatic metabolism and reducing considerably the inter and intra-individual variability (Williams et. al., 1992) and ability to be used for systemic delivery or local delivery of drugs.
Transungual Iontophoresis
Nowadays, persons suffering from nail diseases are growing. Fungal infections in the nail can lead to severe health problems if left untreated in immuno-suppressed individuals (Repka et. al., 2002). Many nail diseases are notoriously difficult to cure owing to the nail barrier and the deep-seated target site underneath the nail plate. Long treatments are usually needed and relapses are common. Oral drug delivery is somewhat successful in treating the nail disorders, but side effects may be severe due to considerable high doses required. Topical monotherapy is considered less efficient in treating nail disorders, such as onychomycosis, due to poor trans-nail bioavailability of drugs (Murdan, 2002). There are two main factors that could limit the accumulation and activity of drugs in the nail on topical application.
Buccal Iontophoresis
Buccal administration of drugs is advantageous for those drugs that encounter degradation in the gastrointestinal tract or severe hepatic first-pass metabolism and require the administration of large doses to reach effective therapeutic levels in the target site (Hao et. al., 2003). Side effects are minimized. Among the epithelial tissues, the buccal mucosa offers good performance for local/systemic pharmacological actions because of its permeability. Since a major limitation in the development of a buccal drug delivery device could be the low permeability of the buccal mucosa, because of relatively small surface area available for absorption and poor retention of the drug and/or drug formulation at the site of absorption. The drug passively crosses the membrane whereas the application of electric fields promotes drug diffusion. The application of a current density of 1 mA/cm2 or more determines a good improvement (Giannola et. al., 2007)
Transnasal Iontophoresis
The delivery of the drugs to the brain is a challenge, as systemically administered drugs fails to pass through the blood brain barrier to enter the brain. A great deal of efforts has been invested in developing the ways to open or defeat the blood brain barrier in order to deliver drugs from blood to the brain. Direct nose to brain delivery of small molecules, peptides and proteins are well known phenomenon. Transnasal. iontophoresis is another technique in the field of drug delivery to the brain.
Lerner et. al. (2004), studied the transnasal iontophoresis of octreotide in rabbits. He placed electrodes containing a drug reservoir into the deep nasal cavity with a return electrode placed at the back of head. The current strength, 3 mA, was applied for 60 min. The experiments resulted to elevated levels of octreotide in brain, with varying results due to electrode and tissue damage during insertion of electrode.
Ural Iontophoresis
Iontophoresis improves the tissue penetration of locally applied drugs. Thus, high local drug tissue levels can be achieved without general side effects. Karen (1999) studied iontophoresis using a specialized urethral catheter delivery system, equipped with an iontophoresis electrode and showed that it could safely deliver lidocaine to the prostate without a significant increase in serum levels. Possible applications in urology in addition to local anesthesia such as delivery of antibiotics for prostatitis, chemotherapy for prostate and bladder cancer, gene therapy, and prostate enzymatic ablation for benign prostate hyperplasia were also suggested. This exciting technology could certainly play a significant role in future.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Extra ordinary considerations in concern to Iontophoresis
In vitro and in vivo transdermal studies of atenolol using iontophoresis (Ozge Inal et. al., 2008)
He prepared Matrix formulations of Eudragit E 100: NE 40D polymers (100:0, 70:30, 60:40, 50:50% w/w) with 20% w/w of triacetine and 5% w/w of atenolol by film casting method with different solvents (methanol, 2-propanol and acetone). In vitro release of atenolol from the films were studied by vertical Franz diffusion cells in HEPES buffer (pH 7.4) for 78 h. Direct currents of 0.1 and 0.5 mA/cm 2 were applied for 6 h to the formulations with Ag/AgCl electrodes. Also, transdermal application for the Eudragit E 100: NE 40 D (70:30% w/w) formulation was compared by iontophoresis or oleic acid (2.5% w/v) with control group on Wistar rats. As a result, the in vitro release rate of atenolol from films were increased with iontophoresis by increasing the current density (from 0.240 to 0.424 mg/cm 2 for 70:3% w/w formulation) and also increased with the amount of Eudragit NE 40D (from 0.646 to 1.30 mg/cm 2 at the end of 78 h). It is obtained from the in vivo studies that oleic acid provided a higher plasma and skin concentration (0.825 mg/mL and 12.5 mg/cm 2 , respectively) than iontophoresis treatment (0.399 mg/mL and 1.81 mg/cm 2 , respectively) due to the different mechanisms. However, the results showed that iontophoresis is a good alternative for enhancing the transdermal delivery of atenolol.
Reverse iontophoresis: a new approach to measure blood glucose level (Congo Tak-Shing Ching et. al., 2007)
This study focused on the investigation of the possibility of monitoring blood glucose levels in humans by non-invasively extracting glucose and lactate from blood through the skin using reverse iontophoresis. In vitro reverse iontophoresis studies have indicated that the optimum switching mode for reverse iontophoresis of lactate and glucose are continuous direct current and direct current with electrode polarity reversal every 15 minutes, respectively. The application of a current combined with electrode polarity reversal every 15 minutes has been suggested for use in humans. The reverse iontophoresis technique was applied to 10 healthy volunteers. Glucose and lactate were successfully extracted through the subjects’ skin into the methylcellulose gel of the electrodes. A fair-good correlation (r 2 = 0.62) between the subject’s blood glucose level and the ratio of glucose to lactate levels in the collection gels was observed after two outliers were removed from the regression equation. The result suggests that it may be possible to non-invasively monitor the blood glucose levels using this new approach free of the need for calibration with a blood sample.
References
- Ashburn, M.A., et. al., 1997. Iontophoretic administration of 2% lidocaine HCl and 1:100,000 epinephrine in humans. Clin. J. Pain. 13, 22-26.
- Berdugo, M., Valamanesh, F., Andrieu, C., Klein, C., Benezra, D., Courtois, Y., Behar-Cohen, F., 2003. Delivery of antisense oligonucleotide to the cornea by iontophoresis. Antisense Nucleic Acid Drug Dev. 13(2),107–114.
- Bose, S., et. al. 2001. Electrically-assisted transdermal delivery of buprenorphine. J. Controlled Release. 73, 197-203.
- Chauvaud, D., Behar-Cohen, F.F., Parel, J.M., Renard, G., 2000, Transscleral Iontophoresis of corticosteroids:Phase II clinical trial. Invest. Ophthalmol. Vis. Sci. 41(4), S79.
- Choi, T.B., Lee, D.A., 1998. Transscleral and transcorneal iontophoresis of vancomycin in rabbit eyes. J. Ocul. Pharmacol. 4(2), 153–164.
- Crawford, F., Atkins, D., Edwards, J., 2002. Interventions for treating plantar heel pain (Cochrane Review). The Cochrane Library. 4. Oxford:Update Software.
- Demirtas, R.N., Oner, C., 1998. The treatment of lateral epicondylitis by iontophoresis of sodium salicylate and sodium diclofenac. Clin. Rehab. 12, 23-29.
- Desai, M.H., et. al. 1991. The role of gentamicin iontophoresis in the treatment of burned ears. J. Burn. Care. & Rehabilitation. 12, 521-524.
- Eduard, Lerner, N., Elske, Van, Zanten, H., Gregory, Stewart, R., 2004. Enhanced delivery of octreotide to the brain via transnasal iontophoretic administration. J. Drug Target. 12(5), 273-280.
- Eljarrat-Binstock, E., Domb, A.J., 2006. Iontophoresis: A non-invasive ocular drug delivery. J. Control Release. 110(3), 479–489.
- Esther, eljarrat-binstock, Frederik, Raisku, Joseph, Frucht-Pery, Abrahamj, Domb., 2004. Hydrogel probe for iontophoresis drug delivery to the eye. J. Biomater. Sci. Polymer. Edn. 15(4), 397–413.
- Esther, Eljarrat-Binstock, Frederik, Raiskup, Joseph, Frucht-Pery, Abraham, Domb, J., 2005. Transcorneal and transscleral iontophoresis of dexamethasone phosphate using drug loaded hydrogel. J. Control Release. 106(3), 386–390.
- Frucht-Pery, J., Raiskup, F., Mechoulam, H., Shapiro, M., Eljarrat-Binstock, E., Domb A., 2006. Iontophoretic treatment of experimental pseudomonas keratitis in rabbit eye using gentamicin loaded hydrogel. Cornea. 25(10), 1182-1186.
- Galinkin, J.L., Rose, J.B., Harris, K., Watcha, M.F., 2002. Lidocaine iontophoresis versus eutectic mixture of local anesthetics (EMLA) for IV placement in children. Anesthesia & Analgesia. 94, 1484-1488.
- Giannola, L.I., De, Caro, V., Giandalia, G., Siragusa, M.G., Campisi, G., Florena, A.M., Ciach, T., 2007. Diffusion of naltrexone across reconstituted human oral epithelium and histomorphological features. Eur. J. Pharm. Biopharm. 65(2),238–246.
- Greenbaum, S.S., 2001. Iontophoresis as a tool for anesthesia in dermatologic surgery: an overview. Dermatologic Surgery. 27, 1027-1030.
- Grossman, R., Chu, D.F., Lee, D.A., 1990. Regional ocular gentamicin levels after transcorneal and transscleral iontophoresis. Invest. Ophthalmol. Vis. Sci. 31(5), 909–916.
- Grossman, R., Lee, D.A., 1989. Transscleral and transcorneal iontophoresis of ketoconazole in the rabbit eye. Ophthalmol. 96(5), 724-729.
- Gudeman, S.D., Eisele, S.A., Heidt, R.S., Colosimo, A.J., Stroupe, A.L., 1997. Treatment of plantar fasciitis by iontophoresis of 0.4% dexamethasone. A randomized, double-blind, placebo-controlled study. Am. J. Sports. Med. 25, 312-316.
- Halhal, M., Renard, G., Courtois, Y., BenEzra, D., Behar, Cohen, F., 2004. Iontophoresis: from the lab to the bed side. Exp. Eye Res. 78, 751–757.
- Hao, J., Heng, P.W.S., 2003. Buccal delivery systems. Drug Dev. Ind. Pharm. 29(8), 821–832.
- Hughes, L., Maurice, D.M., 1984. A fresh look at iontophoresis. Arch. Ophthalmol. 102, 1825–1849.
- Japour, C.J., Vohra, R., Vohra, P.K., Garfunkel, L., Chin, N., 1999. Management of heel pain syndrome with acetic acid iontophoresis. J. Am. Podiatric Med. Assoc. 89, 251-257.
- Jewett, M.A.S., et. al. 1999. Electromotive drug administration of lidocaine as an alternative anesthesia for transurethral surgery. J. Urology. 161, 482-485.
- Kalia, Y.N., Naik, A., Garrison, J., Guy, R.H., 2004. Iontophoretic drug delivery. Adv. Drug Deliv. Rev. 56(5), 619– 658.
- Karen, Hanson, A., 1999. Identifying Iontophoresis. (New urologic drug delivery device). Urol. Nurs. 19, 46.
- Kim, M.K., Kini, N.M., Troshynski, T.J., Hennes, H.M., 1999. A randomized clinical trial of dermal anesthesia by iontophoresis for peripheral intravenous catheter placement in children. Ann. Emergency Med. 33, 395-399.
- Li, L.C., Scudds, R.A., Heck, C.S., Harth, M., 1996. The efficacy of dexamethasone iontophoresis for the treatment of rheumatoid arthritic knees: a pilot study. Arthritis Care & Research. 9, 126-132.
- Loyd V. Allen, http://www.empi.com/uploadedFiles/Healthcare_Professionals/technote4.pdf , Factors affecting the iontophoresis process. Accessed 18.05.2012.
- Meyer, D.R., Linberg, J.V., 1990. Vasquez RJ. Iontophoresis for eyelid anesthesia. Ophthalmic Surgery. 21, 845-848.
- Miller, K.A., Balakrishnan, G., Eichbauer, G., Betley, K., 2001. 1% lidocaine injection, EMLA cream, or “numby stuff” for topical analgesia associated with peripheral intravenous cannulation. Am. Acad. Nurse. Anesth. J. 69, 185-187.
- Monti, D., Saccomani, L., Chetoni, P., Burgalassi, S., Saettone, M.F., 2003. Effect of iontophoresis on transcorneal permeation ‘in vitro’ of two b-blocking agents, and on corneal hydration. Int. J. Pharm. 250(2), 423-429.
- Murdan, S., 2002. Drug delivery to the nail following topical application. Int. J. Pharm. 236(1-2), 1-26.
- Myles, M.E., Neumann, D.M., Hill. J.M., 2005. Recent progress in ocular drug delivery for posterior segment disease:emphasis on transscleral iontophoresis. Adv. Drug Deliv. Rev. 57(14), 2063–2079.
- Ozge, Inal, Muge, Kilicarslan, Nuray, Ari, Tamer, Baykara, 2008. In vitro and in vivo transdermal studies of atenolol using iontophoresis. Acta Poloniae Pharmaceutica ñ Drug Research. 65(1), 29-36.
- Parkinson, T.M., Ferguson, E., Febbraro, S., Bakhtyari, A., King, M., Mundasad, M., 2003. Tolerance of ocular iontophoresis in healthy volunteers. J. Ocul. Pharmacol. Ther. 19(2), 145–151.
- Psaki, C., Carol, J., 1955. Acetic acid ionization: a study to determine the asorptive effects upon calcified iondinitis of the shoulder. Physical Therapy Rev. 35, 84.
- Repka, M.A., O’Hare, J., See, C.H., Gutta, K., Munjal, M., 2002. Nail morphology studies as assessments for onychomycosis treatment modalities. Int. J. Pharm., 245(1-2), 25–36.
- Rootman, D.S., Jantzen, J.A., Gonzalez, J.R., Fischer, M.J., Beuerman, R., Hill, J.M., 1998. Pharmacokinetics and safety of transcorneal iontophoresis of tobramycin in the rabbit. Invest. Ophthalmol. Vis. Sci. 29(9), 1397-1401.
- Russo, J., Lipman, A.G., Comstock, T.J., Page, B.C., Stephen, R.L., 1980. Lidocaine anesthesia: comparison of iontophoresis, injection, and swabbing. Am. J. Hospital Pharm. 37, 843-847.
- Sadler, P.J., Thompson, H.M., Maslowski, P., Liddle, A., Rowbotham, D.J., 1999. Iontophoretically applied lidocaine reduces pain on propofol injection. British. J. Anaesthesia. 82, 432-434.
- Sarah, Molokhia, A., Eun-kee, Jeong, William, Higuchi, I., Kevin, Li, S., 2008. Examination of barriers and barrier alteration in transscleral iontophoresis. J. Pharm. Sci. 97(2), 831-844.
- Schiffman, E.L., Braun, B.L., Lindgren, B.R., 1996. Temporomandibular joint iontophoresis: a double-blind randomized clinical trial. J. Orofacial Pain. 10, 157-165.
- Singh, P., Maibach, H.I., 1994. Iontophoresis in drug delivery: Basic principles and applications. Crit. Rev. Ther. Drug Car. Sys. 11, 161.
- Squire, S.J., Kirchhoff, K.T., Hissong, K., 2000. Comparing two methods of topical anesthesia used before intravenous cannulation in pediatric patients. J. Pediatric Health Care. 14, 68-72.
- Tashiro, Y., Kato, Y., Hayakawa, E., Ito, K., 2000. Iontophoretic transdermal delivery of ketoprofen: novel method for the evaluation of plasma drug concentration in cutaneous vein. Biol. Pharm. Bull. 23, 632-636.
- Tashiro, Y., Kato, Y., Hayakawa, E., Ito, K., 2000. Iontophoretic transdermal delivery of ketoprofen: effect of iontophoresis on drug transfer from skin to cutaneous blood. Biol. Pharm. Bull. 23, 1486-1490.
- Tashiro, Y., Shichibe, S., Kato, Y., Hayakawa, E., Itoh, K., 2001. Effect of lipophilicity on in vivo iontophoretic delivery. I. NSAIDs. Biol. Pharm. Bull. 24, 278-283.
- Voigt, M., De, Kozak, Y., Halhal, M., Courtois, Y., Behar, Cohen, F., 2002. Down-regulation of NOSII gene expression by iontophoresis of anti-sense oligonucleotide in endotoxin induced uveitis. Biochem. Biophys. Res. Commun. 295(2), 336–341.
- Wallace, M.S., et al., 2001. Topical delivery of lidocaine in healthy volunteers by electroporation,, electroincorporation, or iontophoresis: an evaluation of skin anesthesia. Reg. Aneth. Pain. Med. 26, 229-238.
- Wanke, L.A., Griffin, C.D., 1991. Iontophoresis therapy of dermatological conditions. Micromedex. Accessed 01.06.03.
- Williams, A.C., Barry, B.W., 1992. Skin absorption enhancers. Crit. Rev. Ther. Drug Carrier Syst. 9(3-4), 305–353.
- Wirtz, R., 1908. Die ionentherapie in der augenheilkunde. Klinische Monatsblatter fur Augenheilkunde, 46, 543–579.
- Yiping, Wang, Rashmi, Thakur, Qiuxi, Fan, Bozena, Michniak, 2005. Transdermal iontophoresis: Combination strategies to improve transdermal iontophoretic drug delivery. Eur. J. Pharm. Biopharm. 60(2), 179–191.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE











