{ DOWNLOAD AS PDF }
ABOUT AUHTORS
Deepak Chowrasia1*, Nisha Sharma1, Mohammad Arshad2
1 University Institute of Pharmacy,
CSJM University, Kanpur, U.P., India
2Molecular Endocrinology Lab, Department of Zoology,
Lucknow University, Lucknow, U.P., India
* chowrasia.deepak@gmail.com
ABSTRACT
Preliminary screening of different crude extract of. M. azedarach was evaluated against human cancer cell lines viz. MCF-7 (breast cancer), SaOS-2 (osteosarcoma), and A431 (epidermoid carcinoma) to search for better herbal based anticancer agent. Solvents used were water, methanol, ethanol, n-butanol, & n-hexane. It has been found that among solvents, methanolic extract of M. azedarach shows comparatively superior activity suggesting presence of phytoconstituents comprising polar functionalities.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2465
|
PharmaTutor (ISSN: 2347 - 7881) Volume 5, Issue 2 Received On: 04/10/2016; Accepted On: 26/10/2016; Published On: 01/02/2017 How to cite this article: Chowrasia D, Sharma N, Arshad M; In-vitro antiproliferative activity of M. Azedarach; PharmaTutor; 2017; 5(2); 46-49 |
INTRODUCTION
Cancer is a complex genomic disease responsible for sever ill-heath globally. The disease accounted 8.2 million deaths in 2012 & continues to swing worldwide with an estimate of 14 million new cases [1]. Expansive treatment, unaffordable medicines, drastic adverse drug reactions, limited oncological clinician, poverty, ignorance, lack of knowledge, restricted resources are some of the major roadblocks in fueling better cancer treatment thus advancing the disease towards end-up progression [2-3]. In spite of significance elucidation in carcinogenic molecular mechanism & intensive clinical trials the disease remains principal community problem globally with only three well defined & universally framed interventions (depending upon stages) viz. chemotherapy, radiotherapy, & surgery each with their own pros & cons. Chemotherapy thou a versatile global affirmative with remarkable pharmacological appurtenance however suffers limitations of non-selective targeting, sever toxicity, and tumor acquired resistance thus need of novel anticancer agent with better therapeutic profile, selectivity, specificity, and “reach by all” is need of present era [4]. Plants itself endowed with self cure regimen of phytochemicals providing immunity against pathological conditions, could equally beneficial in treating human ailments. Researcher worldwide dynamically targeted attention towards development of phytomedicine based anticancer agents, thus screened & studied numerous plants hence successfully pinpoint thousands or more to possess significant anticancer properties [5-10] among Meliaceae family hold distinct position [11-22]. M. Azedarach is an evergreen small to medium size deciduous tree in Mahogany family Meliaceae, distributed globally however specifically native to central & western china, Malaysia, Burma, & northern regions of India [23]. The plant houses essential phytochemicals responsible for multiple pharmacological activities, preclinical in-vitro profiling further extent its usage in treating eczema, headaches, chickenpox infection, burn, piles, gingivitis, paroxysmal fever, rheumatisim, pimples, scrofula etc. In view of above segmentation and our own intention towards development of novel herbal based anticancer agents, in-vitro screened M. Azedarach extract to three different cancer cell lines to elucidate its potential towards next generation anticancer agent.
MATERIAL & METHODS
Collection of plant material
Leaves from mature plant of M. Azedarach were used for this study was obtained from local milkman of District Kanpur Uttar Pradesh (India) and were characterized as per the available literature present at Institute of Pharmacy, CSJM University, Kanpur (India). The leaves were further washed thoroughly from clean distilled water, dry in shade under strict temperature control (40◦C-45◦C) to render any decomposition of active constituents. Once dried fully, they were milled into fine powder which is almost shady green in color.
Preparation of the extract
The powdered leafs were cold macerated in methanol for 2-weeks at room temperature with intermittent agitation. The alcoholic extract obtained was dried under reduced pressure & subjected to polarity induced solvent-solvent fractionation. The solvents were further dried under reduced pressure and crude extract so obtained were finally refrigerated for further usage.
Cell lines & culture
The human cancerous cell lines viz. MCF-7 (breast cancer), SaOS-2 (osteosarcoma), and A431 (epidermoid carcinoma) were obtained from NCCS, Pune, India. The cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Himedia) with 4.0 mM L-glutamine, 1.5 g/L NaHCO3, 1.0 mM sodium pyruvate, 1.0 % penicillin and streptomycin solution and supplemented to contain 10 % (v/v) fetal calf serum (Himedia). Cells were grown at 37ºC, 5% CO2 in a humidified air.
In-vitro Antiproliferative Assay
The antiproliferative activities of extracts were evaluated by MTT reduction assay as per the standard protocol[1], based on the enzymatic reduction phenomenon of MTT dye. Briefly, the cells were seeded in 100 μL complete medium in each well of 96-well culture plate for 24 hrs at 37oC in humidified, 5% CO2 atmosphere. Stocks of leaf extract was prepared in DMSO and diluted in culture media. Further stock solutions were serially diluted in the medium to the desired concentrations and added to the wells in triplicate in such a way that the final concentration of DMSO would not exceed 0.01%. DMSO (0.01%) was used as a vehicle control. After 21 hrs of treatment, 10 μL of MTT (5 mg/mL of media without phenol red and serum) solution was added in each well and the plates were further incubated for 3 hrs at 37oC until formazan blue crystal developed. Then the supernatant was discarded from each well and 100μL of DMSO was added to solublize formazan crystals for 10-min at 37oC. The absorbance was recorded at 540 nm by a microplate reader (BIORAD-680). The percentage viability was calculated by using the formula;
% Cell viability = [(OD of control - OD of treated) ̸ (OD of control)] X 100
The plot of % cell viability versus sample concentration was applied to calculate the lethal concentration to 50% of the cells (IC50).
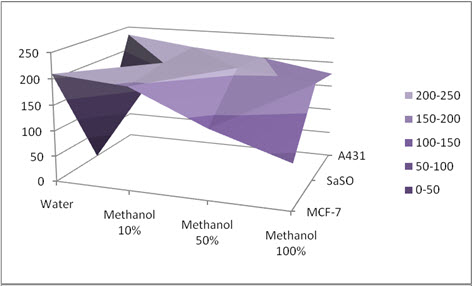
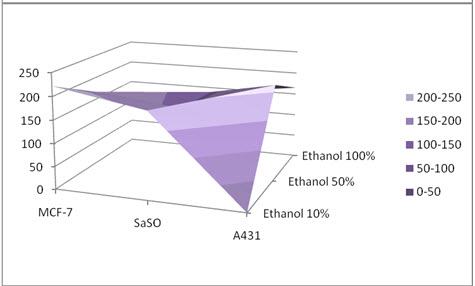

Figure 01: Antiproliferative activity of crude extract
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CONCLUSION
The crude extracts were screened in-vitro for antiproliferative activity against MCF-7 (breast cancer), SaOS-2 (osteosarcoma), and A431 (epidermoid carcinoma) cell lines adopting MTT assay. As per American National Cancer Institute (NCI) the criteria for antiproliferative activity of crude extract is IC50 ≤ 30 µg/ml [24], plant extract derived from all three solvents comparatively thus shows mild antiproliferative activity (Figure-01). It was concluded that extract derived from methanol fraction shows superior activity comparatively, possibly indicating presence of polar constituents responsible for antiproliferative activity and may be used as in-future anticancer agent
REFERENCES
1. Chowraia, Deepak et. al. Synthesis, characterization and anti cancer activity of some fluorinated 3,6-diaryl-[1,2,4]triazolo[3,4-b][1,3,4] thiadiazoles; Arabian Journal of Chemistry (2013).
2. Chowrasia, Deepak et. al., Chalcone as a principle pharmacphore for design & development of novel anticancer agents; Pharmacophore, 2016 (5). Accepted
3. WHO fact sheet N 297; Feb.-2015.
4. Skipper HE, Schabel FM, Wilcox Ws.( 1964), Experimental evaluation of potential anti-cancer agents. XIII On the criteria and kinetics associated with ‘curability’ of experimental leukemia. Cancer chemotherapy reports, 1-111.
5. Biemar, F., Foti, M. (2013); Global progress against cancer-challenges and opportunities; Cancer Bio. Med., 10(4); 183-186.
6. Kviecinski MR, Felipe KB, Schoenfelder T, de Lemos Wiese LP. Rossi MH, Gonçalez E, Felicio JD, Filho DW, Pedrosa RC. Study of the antitumor potential of Bidens pilosa (Asteraceae) used in Brazilian folk medicine. J Ethnopharmacol. 2008; 117: 69–75.
7. Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies.Blood. 2005; 106: 408-418.
8. Yan-Wei H, Chun-Yu L, Chong-Min D, Jian , Wen-Qian, W, Zhen-Lun G. Induction of apoptosis in human hepatocarcinoma SMMC-7721 cells in vitro by flavonoids from Astragalus complanatus. J Ethnopharmacol. 2009; 123: 293–301.
9. Kintzios E. Terrestrial plant-derived anticancer agents and plant species used in anticancer research. Crit Rev Plant Sci. 2006; 25: 79–113.
10. Park HJ, Kim MJ, Ha E, Chung JH. Apoptotic effect of hesperidin through caspase 3 activation in human colon cancer cells, SNU-C4. Phytomedicine. 2008; 15: 147–151.
11. Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and anincreasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45-66.
12. Akhtar, Y.; Yeoung, Y.R.; Isman, M.B. Comparative bioactivity of selected extracts from Meliaceae and some commercial botanical insecticides against two noctuid caterpillars, Trichoplusia ni and Pseudaletia unipuncta. Phytochem. Rev. 2008, 7, 77-88.
13. Carpinella, C.; Ferrayoli, C.; Valladares, G.; Defago, M.; Palacios, S. Potent limonoid insect antifeedant from Melia Azedarach. Biosci. Biotechnol. Biochem. 2002, 66, 1731-1736.
14. Coria, C.; Almiron, W.; Valladares, G.; Carpinella, C.; Ludueña, F.; Defago, M.; Palacios. S. Larvicide and oviposition deterrent effects of fruit and leaf extracts from Melia azedarach L. on Aedes aegypti (L.) (Diptera: Culicidae). Bioresource Technol. 2008, 99, 3066-3070.
15. Kamaraj, C.; Rahuman, A.A.; Bagavan, A.; Mohamed, M.J.; Elango, G.; Rajakumar, G.; Zahir, A.A.; Santhoshkumar, T.; Marimuthu, S. Unit Ovicidal and larvicidal activity of crude extracts of Melia azedarach against Haemonchus contortus (Strongylida). Parasitol. Res. 2010, 106, 1071-1077.
16. Carpinella, M.C.; Giorda, L.M.; Ferrayoli, C.G.; Palacios, S.M. Antifungal effectt of different organic extracts from Melia azedarach L. on phytopathogenic fungi and their isolated active components. J. Agric. Food Chem. 2003, 51, 2506-2511.
17. Carpinella, M.C.; Ferrayoli, C.G.; Palacios, S.M. Antifungal synergistic effect of scopoletin, a hydroxycoumarin isolated from Melia azedarach L. fruits. J. Agric. Food Chem. 2005, 53, 2922-2927.
18. Ntalli, N.G.; Menkisoglu-Spiroudi, U.; Giannakou, I. Nematicidal activity of powder and extracts of Melia azedarach fruits against Meloidogyne incognita. Ann. Appl. Biol. 2010, 156, 309-317.
19. Vishnukanta, A.C.R. Melia azedarach: A phytopharmacological review. Pharmacogn. Rev. 2008, 2, 173-179.
20.Bueno, C.A.; Alché, L.A.; Barquero, A.A. 1-Cinnamoyl-3,11-dihydroxymeliacarpinin delays glycoprotein transport restraining virus multiplication without cytotoxicity. Biochem. Biophys. Res. Commun. 2010, 393, 32-37.
21. Wu, S.B.; Ji, Y.P.; Zhu, J.J.; Zhao, Y.; Xia, G.; Hu, Y.H.; Hu, J.F. Steroids from the leaves of Chinese Melia azedarach and their cytotoxic effects on human cancer cell lines. Steroids 2009, 74, 761-765.
22. Mazumder, M.E.H.; Rahman, S. Pharmacological evaluation of Bangladeshi medicinal plants for antioxidant activity. Pharm. Biol. 2008, 46, 704-709.
23. Ramya S, Jepachanderamohan PJ, Alaguchamy N, Kalayanasundaram M and Jayakumararaj R. In Vitro Antibacterial Prospective of Crude Leaf Extracts of Melia azedarach Linn. against Selected Bacterial Strains. Ethnobot. Leaf. 2009; 13: 254-258.
24. Itharat A, Houghton PJ, Eno-Amooquaye E, Burke PJ, Sampson JH, Raman A. In vitro cytotoxic activity of Thai medicinal plants used traditionally to treat cancer. J Ethnopharmacol. 2004; 90: 33–38.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









