About Authors:
Hemant Rathod*, M.P.Khinchi, Dilip Agrawal, Natasha Sharma
*Department of Pharmaceutics
Kota College of Pharmacy,
Kota, Rajasthan, India
*hemant2787@gmail.com
Abstract
Fast dissolving, fast melting, chewable and orally dissolving or disintegrating tablets are solid dosage forms that disintegrate rapidly and dissolve in the mouth without water. These products have staying power in the marketplace because they appeal to consumers and support increased compliance among users as well as provide effective life-cycle management. The principle challenge with orally disintegrating tablets (ODTs) is to develop tablet formulations that deliver rapid disintegration, pleasant mouth feel and high breaking force for tablet robustness. Superdisintegrants affect a range of formulation parameters; including the rate of disintegration, tablet breaking force, and mouth feel i.e. Polyplasdone XL-10 superdisintegrant provides optimal performance in ODT formulations.
REFERENCE ID: PHARMATUTOR-ART-1828
Introduction
Tablet disintegration has received considerable attention as an essential step in obtaining fast drug release. The emphasis on the availability of drug highlights the importance of the relatively rapid disintegration of a tablet as a criterion for ensuring uninhibited drug dissolution behavior.[1]Disintegrants are substances or mixture of substances added to the drug formulations, which facilitate dispersion or breakup of tablets and contents of capsules into smaller particles for quick dissolution. Superdisintegrants, are those substances, which facilitate the faster disintegration with smaller quantity in contrast to disintegrants. The disintegration of dosage forms are depends upon various physical factors of disintegrants/superdisintegrants which are as follow:
1. Percentage of disintegrants present in the formulation.
2. Proportion of disintegrants used.
3. Compatibility with other excipients.
4. Presence of surfactants.
5. Hardness of the tablets.
6. Nature of Drug substances.
7. Mixing and types of addition. [2, 3]
Researchers these days are looking for a new, safe and effective disintegrating agents which can disintegrate tablets rapidly even at a tablet crushing strength of greater than 3.5 Kg. On analyzing the behavior of disintegration time in the oral cavity as well as wetting time by surface free energy we came to know, that for a faster wetting a molecule should have high polar component of surface free energy and the agents which meet these special requirements are called as superdisintegrants. [4] The ease of availability of these agents and the simplicity in the direct compression process suggest that their use would be a more profitable alternative in the preparation of ODT than the sophisticated and patented techniques. [5]
Superdisintegrants are another version of super-absorbing materials with tailor-made swelling properties. These materials are not planned to absorb significant amounts of water or aqueous fluids, but planned to swell very fast. Superdisintegrants are used as a structural weakener for the disintegrable solid dosage forms. They are physically dispersed within the matrix of the dosage form and will expand when the dosage form is exposed to the wet environment. [6]These newer substances are more effective at lower concentrations with greater disintegrating efficiency and mechanical strength.[7]Superdisintegrants are generally used at a low level in the solid dosage form, typically 1 - 10 % by weight relative to the total weight of the dosage unit. [8]Their particles are generally small and porous, which allow for rapid tablet disintegration in the mouth without an objectionable mouth-feel from either large particles or gelling. The particles are also compressible which improves tablet hardness and its friability.[6] Effective superdisintegrants provide improved compressibility, compatibility and have no negative impact on the mechanical strength of formulations containing high-dose drugs.[9]
Mechanism of Action of Superdisintegrants
There are five major mechanisms for tablet disintegration as follows:-
1 Swelling
2 Porosity and Capillary Action (Wicking)
3 Deformation
4 Enzymatic reaction
5 Due to disintegrating particle/particle repulsive forces
1 Swelling
Swelling is believed to be a mechanism in which certain disintegrating agents (such as starch) impart the disintegrating effect. By swelling in contact with water, theadhesiveness of other ingredients in a tablet is overcome causing the tablet to fall apart. [10]
E.g. Sodium starch glycolate, PlatagoOvata. [11, 12, 13] (Fig.1)
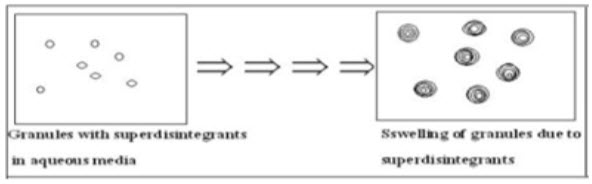
Figure 1 Disintegration by Swelling
2 Porosity and Capillary Action (Wicking)
Effective disintegrants that do not swell are believed to impart their disintegrating action through porosity and capillary action. Tablet porosity provides pathways for the penetration of fluid into tablets. The disintegrant particles (with low cohesiveness & compressibility) themselves act to enhance porosity and provide these pathways into the tablet. Liquid is drawn up or “wicked” into these pathways through capillary action and rupture the interparticulate bonds causing the tablet to break apart.E.g.Crospovidone, Croscarmellose Sodium. [14] (Fig.2)
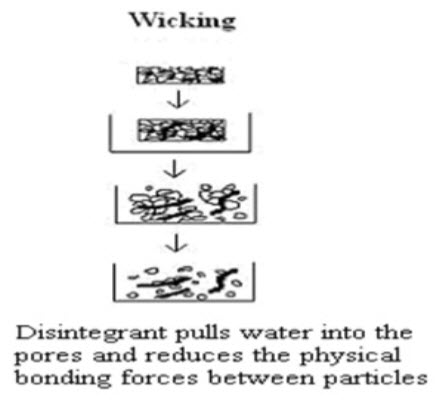
Figure 2 Disintegration by Wicking
3 Deformation
Starch grains are generally thought to be “elastic” in nature meaning that grains that are deformed under pressure will return to their original shape when that pressure is removed. But, with the compression forces involved in tableting, these grains are believed to be deformed more permanently and are said to be “energy rich” with this energy being released upon exposure to water. In other words, the ability for starch to swell is higher in “energy rich” starch grains than it is for starch grains that have not been deformed under pressure. [15] (Fig.3)

Figure 3 Disintegration by Deformation
4 By Enzymatic Reaction
Enzymes present in the body also act as disintegrants. These enzymes dearth the binding action of binder and helps in disintegration. Due to swelling, pressure is exerted in the outer direction that causes the tablet to burst or the accelerated absorption of water leads to an enormous increase in the volume of granules to promote disintegration. [16] (Fig. 4)
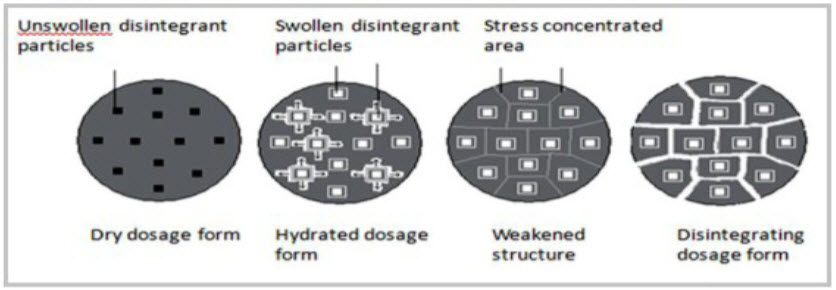
Figure 4 Disintegration by Enzymatic Reaction
5 Due to disintegrating particle/particle repulsive forces
Another mechanism of disintegration attempts to explain the swelling of tablet made with “nonswellable” disintegrants. Guyot-Hermann has proposed a particle repulsion theory based on the observation that nonswelling particle also cause disintegration of tablets. The electric repulsive forces between particles are the mechanism of disintegration and water is required for it. Researchers found that repulsion is secondary to wicking. It is believed that no single mechanism is responsible for the action of most disintegrants. But rather, it is more likely the result of inter-relationships between these major mechanisms. [17](Fig.5)
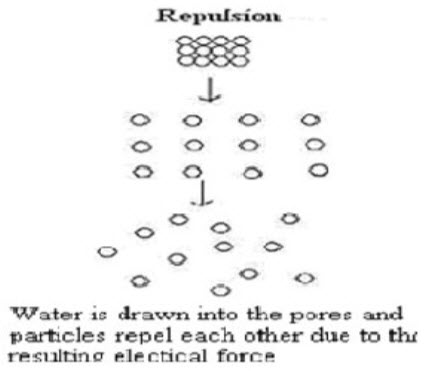
Figure 5 Disintegration by Repulsion
Types of Superdisintegrants
The Superdisintegrants can be classified into two categories on the basis of their availability:
1 Natural Superdisintegrants
2 Synthetic Superdisintegrants.
1 Natural Superdisintegrants
These superdisinegrating agents are natural in origin and are preferred over synthetic substances because they are comparatively cheaper, abundantly available, non-irritating and nontoxic in nature. The natural materials like gums and mucilages have been extensively used in the field of drug delivery for their easy availability, cost effectiveness, Eco friendliness, emollient and non-irritant nature, non-toxicity, capable of multitude of chemical modifications, potentially degradable and compatible due to natural origin. There are several gums and mucilages are available which have superdisintegrating activity. [18]
Isapghula Husk Mucilage (Plantago ovata)
Isapghula Husk consists of dried seeds of the plant known as plantago ovata. The plant contains mucilage in the epidermis of the seeds. Mucilage of plantago ovata has various characteristics like binding, disintegrating and sustaining properties. Mucilage can be used as superdisintegrant to formulate fast dissolving tablets because it has very high percentage of swelling index (around 89±2.2%v/v) as compared to the other superdisintegrating agents. The rapid disintegration of the FDTs is due to the swelling of superdisintegrants to create enough hydrodynamic pressure for quick and complete disintegration of the tablet. The rate at which swelling develops and significant force of swelling also determine its disintegrating efficiency. [19, 20]
Chitosan
Chitosan is a natural polymer obtained by deacetylation of chitin which is the second most abundant polysaccharides in nature after cellulose. Superdisintegrant property of chitosan has been utilized to develop a fast mouth dissolving tablet by utilizing a novel method of treatment. Similar to the other superdisintegrants chitosan too generously engulf water when in contact with aqueous media and burst due to the pressure exerted by their capillary action thereby impart instantaneous disintegration of the dosage form and resulting in formation of a uniform dispersion in the surrounding media which behave like a true suspension formed inside the body leading to rapid and complete absorption of drug. [21]
Guar Gums
Guar gum is naturally occurring guar seed extract, containing about 80% of galactomannan (guaran), 10% moisture, 5-7% protein and trace amounts of heavy metals and ash. It is free flowing, completely soluble, neutral polymer and is approved for use in food. It is not sensitive to pH, moisture contents or solubility of the tablet matrix. It is not always pure white and sometimes varies in color from off-white to tan tends to discolour with time in alkaline tablets. As a disintegrant, guar gum has been found to be superior to some common disintegrants such as corn starch, celluloses, alginates and magnesium aluminium silicate. Particle size can affect disintegration, with finer particle sizes having greater disintegrating capabilities. It is available in the market under the trade name jaguar. [22, 23]
Agar
Agar is the dried gelatinous substance obtained from Gelidium amansii (Gelidanceae) and several other species of red algae like, Gracilaria (Gracilariaceae) and Pterocadia (Gelidaceae). Agar is yellowish gray or white to nearly colorless, odorless with mucilaginous taste and is accessible in the form of strips, sheet flakes or coarse powder. Agar consists of two polysaccharides as agarose and agaropectin. Agarose is responsible for gel strength and Agaropectin is responsible for the viscosity of agar solutions. It is a potential candidate to act as a disintegrant due to its high gel strength. Gums are used in concentration from 1 to 10%. However, these are not as good disintegrating agents as others because capacity development is relatively low. [24]
2 Synthetic Superdisintegrants
A group of superdisintegrants including Croscarmellose sodium (Ac-Di-Sol), sodium starch glycolate (Primogel, Explotab) and crospovidone (Polyplasdone XL) alleviate most of these problems. Use of the superdisintegrants in fast dispersible tablet is possible as tablet shows optimum physical properties. [25]
Advantages of Synthetic Superdisintegrants
1 Effective in lower concentrations than starch.
2 Less effect on compressibility and flow ability.
3 More effective intragranularly. [26]
Limitations of Synthetic Superdisintegrants
1 More hygroscopic (may be a problem with moisture sensitive drugs)
2 Some are anionic and may cause some slight in-vitro binding with cationic drugs (not a problem in-vivo). [27]
3 An acidic medium significantly reduces the liquid uptake rate and capacity of sodium starch glycolate and croscarmellose sodium, but not crospovidone. [28, 29]
4. The degree of swelling of Primogel (sodium starch glycolate) and Polyplasdone XL101 (crospovidone) is minimized following wet granulation formulation. Finally, the medium ionic strength was found to have an adverse effect on the swelling capacity of croscarmellose. [30, 31]
Crospovidone
Unlike other superdisintegrants, which rely principally on swelling for disintegration, crospovidone use a combination of swelling and wicking. Due to its high crosslink density, crospovidone swells rapidly in water without gelling. Crospovidone particles are found to be granular and highly porous which facilitates wicking of liquid into the tablet and particles to generate rapid disintegration. [32]Larger particles provide a faster disintegration than smaller particles. [33] Crospovidone disintegrants are highly compressible materials as a result of their unique particle morphology. [32]Crospovidone can also be used as solubility enhancer. It is available in two particle sizes in the form of Polyplasdone XL and Polyplasdone XL-10.
Croscarmellose Sodium
It is an internally cross linked polymer of carboxymethyl cellulose sodium. It has high swelling capacity with minimal gellingresulting in rapid disintegration. [32] Due to fibrous structure, croscarmellose particles also show wicking action. [35] In tablet formulations, croscarmellose sodium may be used in both direct compression and wet-granulation processes. When used in wet-granulation, the croscarmellose sodium should be added in both the wet and dry stages of the process (intra- and extra-granularly) so that the wicking and swelling ability of the disintegrant is best utilized. [34, 35]
Sodium Starch Glycolate
Sodium Starch Glycolate is the sodium salt of a carboxymethyl ether of starch. These are modified starches made by crosslinking of potato starch as it gives the product with the best disintegrating properties. [37] The degree of cross-linking and substitution are important factors in determining the effectiveness of these materials as superdisintegrants.[36]The effect of the crosslinking is to reduce both the water soluble fraction of the polymer and the viscosity of dispersion in water. The natural predried starches swell in water to the extent of 10-20 percent and the modified starches increase in volume by 200-300 percent in water. The mechanism by which this action takes place involves rapid absorption of water leading to an enormous increase in volume of granules that result in rapid and uniform disintegration. These are available as explotab and primogel which are low substituted carboxy methyl starches. [22]The effect of introduction of the large hydrophilic carboxymethyl groups is to disrupt the hydrogen bonding within the polymer structure. This allows water to penetrate the molecule and the polymer becomes cold water soluble. [36]
Method of Incorporation of superdisintegrants
The incorporation of superdisintegrants in the dosage forms are mainly of three types:
1 Intragranular or during granulation
In this process the superdisintegrants are blend with other powders and granulation is carried out. Thus the superdisintegrants are incorporated within the granules.
2 Extragranular or prior to compression
In this process, the superdisintegrants are mixed with prepared granules before compression.
3 Incorporation of superdisintegrants at intra and extra granulation steps
In this process part of superdisintegrants are added to intragranular and a part to extragranules. This method usually produces better results and more complete disintegration than type 1 and type 2. [38]
Conclusion
Now a days Fast Dissolving Tablets have a broader market in Pharmaceutical Industry. FDT’S are mostly used for its fast dissolution and thus by fast absorption and at last immediate action which can be only achieved by using superdisintegrants. The present article revealed about superdisintegrants and their important in pharmaceutical Industry.
REFERENCE
1.Bhowmik, Chiranjib.B, Krishnakanth, Pankaj, R. M. Chandira. Fast Dissolving Tablet: An Overview. Journal of Chemical and Pharmaceutical Research. 1(1) (2009) 163-177.
2.Schimidt P C, and Brogramann B., Pharmaceutical Technology. 1988 (34), 22.
3.Cohen Y, and Lach J L, Journal of Pharmaceutical Sciences. 1963(52), 122
4.Goel H, Vora N, Rana V: A novel approach to optimize and formulate fast disintegrating tablets for nausea and vomiting. AAPS PharmSciTech 2008; 9(3): 774-781.
5.Mohanachandran PS, Sindhumol PG and Kiran TS: Superdisintegrants: an overview. Journal of Pharmaceutical Sciences Review and Research 2011; 6(1): 105-109.
6.Omidian H and Park K: Swelling agents and devices in oral drug delivery. Journal of Drug Delivery Science and Technology 2008; 18 (2): 83-93.
7.Bhardwaj S, Jain V, Sharma S, Jat RC and Jain S: Orally disintegrating tablets: a review. Drug Invention Today 2010; 2(1): 81-88.
8.Belet MH and Derle DV: Analysis of patents pertaining to superdisintegrants used in tablet manufacturing. Journal of intellectual Property Rights 2008; 13: 601-604.
9.Konapure AS, Chaudhari PS, Oswal RJ, Kshirsagar SS, Antre RV and Chorage TV: Mouth dissolving tablets-an innovative technology. International Journal of Applied Biology and Pharmaceutical Technology 2011; 2(1): 496-503.
10.D. Bikashapathi, K. Saikrishna, U. A. Kumar & G. Sabitha, Fast Dissolving Table: An Update. International Research Journal of Pharmacy. 2(3) (2011) 45-53.
11. formulationvinensia.com
12.N. G. R. Rao, T. Ketan, S. Bala. Formulation and evaluation of fast dissolving Tablets of Metoprolol Tartrate using Natural superdisintegrant, International Journal of Pharmaceutical and Clinical Research. 2 (2010) 40-45.
13.D.chougule, Ghodke Dhananjay, R.R. Shah, Rahul Ghaste. Fast Dissolving Tablets: An Overview. (2010).
14.G. G. Gajare, S. R. Bakliwal, B. R. Rane, N. A. Gujrathi, S. P. Pawar, Mouth Dissolving Tablet: A Review. International Journal of Pharmaceutical Research and Development (IJPRD). 6 (2011) 280-296.
15.G. P. Kumar, R. Nirmala, Fundamental Aspects of Superdisintegrants: A Concise Review. Journal of Global Pharma Technology. 4 (2012) 1-12.
16.R. Pahwa, N. Gupta. Superdisintegrants inthe Development of Orally Disintegrating Tablets: A Review. International Journal of Pharmaceutical Science and Research. Vol. 2 (2011) 2767-2780.
17.V. D. kumar, I. Sharma, V. Sharma. A comprehensive review on fast dissolving tablet technology. Journal of Applied Pharmaceutical Science. 01 (05); (2011) 50-58.
18.S. Shirsand, S. Suresh, M. Para, P. Swamy, D. N. Kumar. Plantagoovata mucilage in the design of fast disintegrating tablets. Indian Journal ofPharmaceutical Science. 71 (2009) 41-45.
19.Shirsand SB, Sarasija S, Para MS, Swamy PV and Kumar DN: Plantago ovata mucilage in the design of fast disintegrating tablets. Indian Journal of Pharmaceutical Sciences 2009; IP: 210. 212. 120. 94.
20.Ghenge G, Pande SD, Ahmad A, Jejurkar L and Birari T: Development and characterisation of fast disintegrating tablet of Amlodipine besylate using mucilage of plantago ovata as a natural superdisintegrant. International Journal of PharmTech Research 2011; 3(2): 938-945.
21.Nagar M and Yadav AV: Cinnarizine orodispersible tablets: a Chitosan based fast mouth dissolving technology. International Journal of PharmTech Research 2009; 1(4): 1079-1091.
22.Uddhav S Bagul. (2006). Current status of tablet disintegrants: a review. Retrieved March 5, 2011 from Pharmainfo.net. pharmainfo.net/reviews/current-status-tablet-disintegrantsa-review.
23.Shah B: Textbook of Pharmacognosy and Phytochemistry. Elsevier Health Sciences Publishers, First Edition 2009; 164-165.
24.Setia A, Goyal N and Kansal S: Formulation and evaluation of Ciprofloxacin hydrochloride dispersible tablets using natural substances as disintegrates. Pelagia Research Library Der Pharmacia Sinica 2011; 2(1): 36-39.
25.S. Bhise, G.Chaulang, P. Patel, B. Patel, A. Bhosale, S. Hardikar. Superdisintegrants as solubilizing agent. Research J. Pharm. and Tech. 2(2) (2009) 387-391.
26.R. Bala, S. Khanna& P. Pawar. Polymers In Fast Disintegrating Tablet A Review. Asian Journal of Pharmaceutical and Clinical Research, 5 (2012) 8-14.
27.John C Carter. (2002-06). The role of disintegrants in solid oral dosage form manufacturing. Carter Pharmaceutical Consulting, Inc. Retrieved March 25, 2011 from carterpharmaceutical consulting.com/articles/The-role of disintegrants. html.
28.Chen CR, Lin YH, Cho SL, Yen SY and Wu HL: Investigation of the dissolution difference between acidic and neutral media of Acetaminophen tablets containing a super disintegrant and a soluble excipient. Chem Pharm Bull 1997; 45: 509–512.
29. Zhao N and Augsburger LL: The influence of swelling capacity of super disintegrants in different pH media on the dissolution of Hydrochlorothiazide from directly compressed tablets. AAPS Pharm SciTech 2005; 6: 120–126.
30.Bussemer T, Peppas NA and Bodmeier R: Evaluation of the swelling, hydration and rupturing properties of the swelling layer of a rupturable pulsatile drug delivery system. European Journal of Pharmaceutics and Biopharmaceutics 2003; 56: 261–270.
31.Zhao N and Augsburger LL: The influence of granulation on super disintegrant performance. Pharm Dev Technol 2006; 11: 47–53.
32.Raymond CR: Handbook of Pharmaceutical Excipients. Alpha Publishers, Fifth Edition 2006.
33. Polyplasdone superdisintegrants product overview. ISP Pharmaceuticals. April 11, 2011. < anshulindia. com/ pdfs/polyplasdone%20Lit.pdf>.
34.Goel H, Rai P, Rana V and Tiwary AK: Orally disintegrating systems: innovations in formulation and technology. Recent Patents on Drug Delivery & Formulation 2008; 2: 258-274.
35. Camarco W, Ray D and Druffner A: Selecting superdisintegrants for orally disintegrating tablet formulations. Pharmaceutical Technology Supplement 2006.
36.Superdisintegrants: an introduction to chemistry and performance. April 12, 2011. <dmvfonterraexcipients.com/products/~/media/DFEA18D0FB9945A984F16949D2B56B95.ashx>.
37.Newman AW, Mueller RL, Vitez IM and Kiesnowski CC: Starch and starch derivatives. Encycolpedia of Pharmaceutical Technology, Informa Healthcare USA 2007.
38.H. Shihora, S. Panda. Superdisintegrants, Utility in Dosage Forms: A Quick Review. Journal of Pharmaceutical Science andBio scientificResearch (JPSBR) 1 (2011) 148-153.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









