{ DOWNLOAD AS PDF }
ABOUT AUHTORS
Nisha Sharma, Mahroz, Deepak Chowrasia*
University Institute of Pharmacy,
Chhatrapati Shahu Ji Maharaj University, Kanpur, U.P., India
chowrasia.deepak@gmail.com
ABSTRACT
Chemically chalcones are 1,3–diphenyl-2-propene-1-one, containing dual aromatic rings which are linked to each other via carbon bridge system enveloping keto-ethylenic core structure. Owing to the presence of conjugated double bond and electron dense aromatic ring system, the molecule posses less redox potential; thus greater probability for characteristic electron transfer reactions. Naturally, conjugated systems of these types are abundantly present in edible plants and are considered to be precursors of bioactive flavonoids and bioflavonoids. Chalcones and their derivatives find numerous industrial applications such as artificial sweeteners, scintillator, polymerization catalyst, fluorescent whitening agent, organic brightening agent, stabilizer against heat, visible light, and ultraviolet radiation. As a chemo-identifying agent, chalcones have been found useful in elucidating structure of natural products like hemlock, tannin, cyanomaclurin, ploretin, eriodictyol and homoeriodictyol, and naringenin. Pharmacologically, the same molecule acts as a versatile and universally accepted moiety for design and development of numerous bioactive synthetic analogues in search of ideal medicine to conquer human pathological conditions. The present paper thus designs to explore and study various prospective of chalcones and their derivatives in terms of recent developments and pharmacological importance.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2458
|
PharmaTutor (ISSN: 2347 - 7881) Volume 5, Issue 1 Received On: 22/08/2016; Accepted On: 16/09/2016; Published On: 01/01/2017 How to cite this article: Sharma N, Mahroz, Chowrasia D; Structure based Antibacterial Activity Of 1,3-Diaryl-2-Propen-1-Ones And Their Recent Pharmacological Interests; PharmaTutor; 2017; 5(1); 42-47 |
INTRODUCTION
Chalcones are excellent scaffolds not only for synthetic manipulations but also act as a unique template responsible for numerous pharmacological activities1. Biphenylic bridged core structure of chalcone provides firm backbone for generation of potential pharmacophore when derivatized2-3 yields multiple potent and highly selective medicinal active compounds with differential biological activities4-14. Chalcones are the close conger of naturally occurring bioactive flavonoid, isoflavonoids & their analogues but differ from them in having dual (instead of triple) ring system. Xanthohumol, cardamonin, and flavokawains A, B, C are only few bioactive naturally occurring chalcones derivatives that posses potent anticancer, vasorelaxant and anti-inflammatory properties15-17. However, occurs naturally isolation of chalcones employs tedious multi-step time-consuming complicated methodologies which rarely compatible & comparable with end product yield.
Chemistry of Chalcones
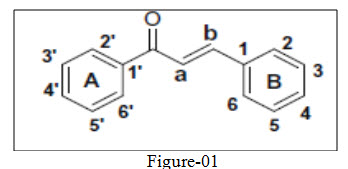
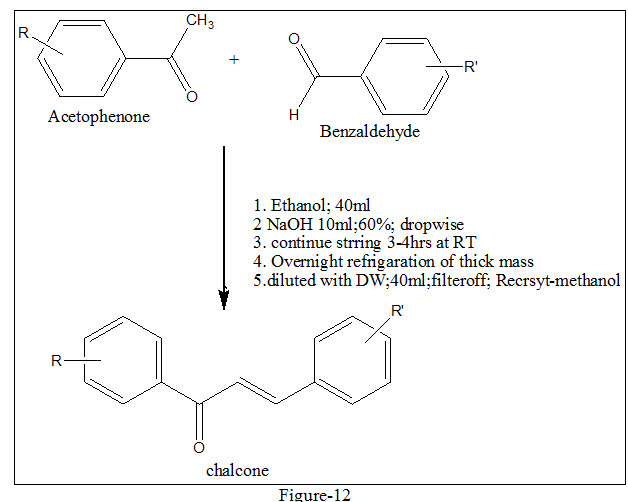
Chalcone (1,3-diaryl-2-propen-1-ones) also termed as benzalacetophenone or benzylidene acetophenone was initially named by Kostanecki and Tambor18. These compounds contain two aromatic ring systems A and B linked by highly reactive keto-ethylenic group –CO-CH=CH- (See figure 01) endowed with potent pharmacological activity such as antioxidant and antimicrobial, but also contribute towards efficient chromogenic property of this molecule. The conjugated double bonded carbon bridge and completely delocalized pi-electrons of dual aromatic ring system ultimately not only fulfilled its criteria for relative low redox potential but bequeath the molecule with higher possibility of easier electron transfer aromatic substitution reactions. This thus makes molecule prone for laboratory derivatization and subsequent molding into differential scaffold by interchanging the position of various substituent on aromatic rings intra-molecularly. Besides having induced chemical differentiability they act as key intermediate for synthesis of heterocyclic ring system19-22 with well defined diversified biological activities. Numerous methods are available for the synthesis of chalcones, the most convenient and popular method is the Claisen-Schmidt reaction (figure-12), relay on base assisted condensation of equimolar quantities of arylmethlketone with aryl aldehyde in the presence of absolute alcohol. The limiting factor for reaction is concentration of alkali, which usually ranges from 10-60 %. Rather than alkali other condensing agents such as amino acid, aqueous solution of borax, perchloric acid, Pepperdine, boron trifloride, alkali metal alkoxide, and magnesium tert-butoxide.
Recent developments
Chalcones are precursor for biosynthesis flavonoids. Literatures are surplus with compounds reported to having backbone analogues to that of chalcone and their multidisciplinary pharmacological action23,4-14 including antiviral, antibacterial, antifeedant, antimycobacterial, antiinvasive, antiplatelet, cytotoxic, anti-HIV, antihyperlipidemic, hypoglycemic agent, antiulcerative, antiprotozoal, antioxidant, antimalarial, analgesic, anti-inflammatory, cytoprotective, antitumor, antileishmanial, antihistamine etc. Although other related compounds also posses these activity but not to an extent comparable with this (chalcone). Thus chalcones attract considerable attention globally from scientific community to explore this molecule further in order to improve activity of existing molecule, but also search for novel derivatized chalcone based lead moiety which may in future leads to a potential therapeutic agent. Here is the brief discussion on some of the well defined chalcone based pharmacophore responsible for antimicrobial activity.
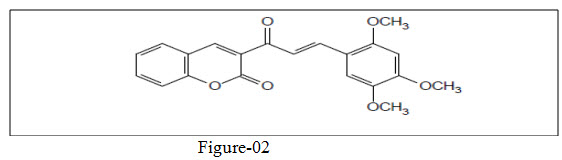
Prasad et. al.25 synthesize benopyran-2-one substituted chalcone (figure 02) based antimicrobial agent against E.coli and B. subtilis and reveals activity of these compound in a manner proportional to electron releasing group on ring.
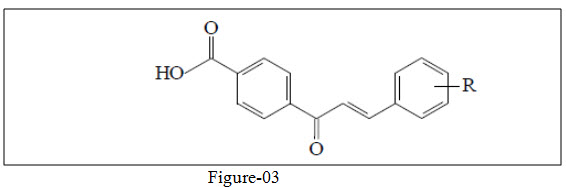
Nielsen et al.26 studied bioisosteric effect & hydrophilicity of various substitution on aromatic ring of chalcone and their derivatives and find out that substitution of halogen atom on ring increase not only the solubility
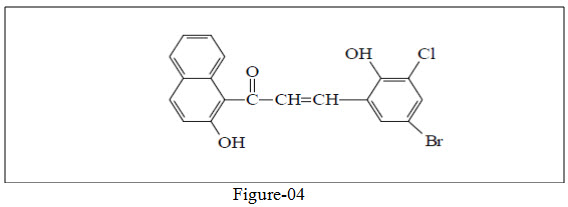
Prasad et. al.27 synthesize naphthalene based chalcone derivative and studied its antimicrobial and antifungal property.
Rao et al.28 syntheise halogen based chalcone derivative and elucidate its antimicrobial action predominantly against E. Coli.
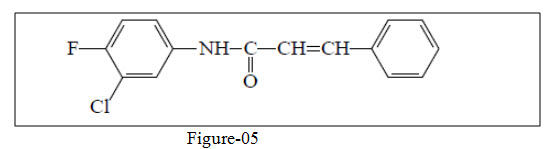
Nowakowska et al.29 synthesized a series of substituted chalcones and tested for their antibacterial and antifungal activities.
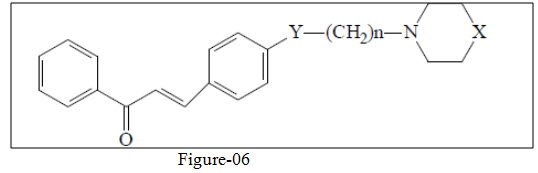
Okunade et al.30 synthesized dihydrochalcones having antibacterial activity.
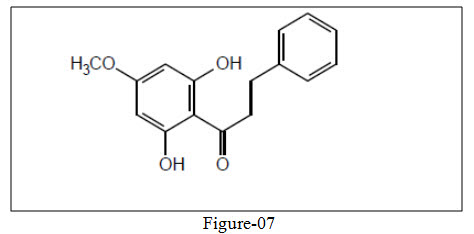
Boeck et al.31 synthesized novel xanthoxylin-derived chalcones showing antifungal activity.
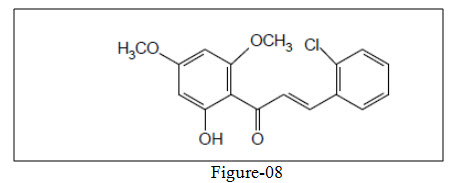
Sohly et al.32 isolated prenylated chalcones from the leaves of Malclura tinctoria possessing antifungal activity.
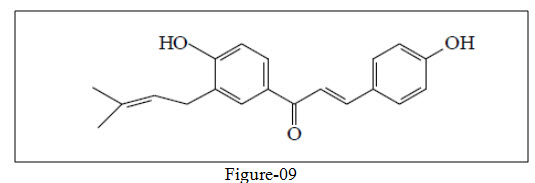
Stevaz et al.33 isolated a 2',4'-dihydroxy-3'-methoxychalcone from the methanolic extract of Zuccagnia punctata which exhibited antifungal activity.
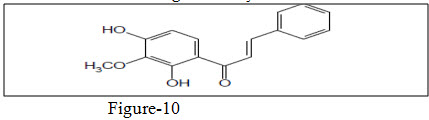
Tsuchiya et al.34 synthesized hydroxychalcones which exhibited antifungal activity.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
PHARMACOLOGICAL ACTIVITIES:
Antioxidant
Recent study indicates chalcones and their substituted derivatives can be used as effective antioxidants. Pathologically free radicals are responsible for various disease process such as cataracts, diabetes, generalized inflammation, liver disorder, and macular degeneration. These free radical reacting species initiates consequences of knock out process thus disturbed the normal biochemical processes in the body.
Anti-inflammatory
Importance of chalcones including their analogues especially hydroxyl derivatives are now proven to inhibit inflammatory process inside a cell by inhibiting NO synthesis and cyclooxygenase protein expression in lipopolysacchride cell. Beside this, some researches also indicate effect of chalcones in stabilizing macrophage which plays a prominent role in inflammatory process by mediators.
Cytotoxic activity
Mannich bases of phenolic azobenzenes demonstrated cytotoxic activity, and various Mannich bases analogs of chalcones exhibited potent cytotoxicity against murine P338 and L1210 leukemia cells as well as several human tumor cell lines. Mannich bases of heterocyclic chalcones are evaluated for cytotoxic activity against four human cancer cell lines PC-3, MCF-7, KB and KB-VIN. Mannich base of chalcones with morphine substitution at C3 or C5 pyridyl or phenyl at C2 substitution are found to possess good cytotoxic activity.
Hypoglycemic activity
Non-insulin dependent diabetes mellitus (NIDDM), a chronic metabolic disease characterized by insulin resistance and hyperglycemia. The disease is generally characterized by obesity, dyslipidemia, and hypertension leading to cardiovascular risks. Beta-3 adrenergic receptors are found on the cell surface of both white adipose and brown adipose tissue where their stimulation promotes lipolysis and thermogenesis respectively. Brown adipose tissue also plays an important role in the maintenance of glucose homeostasis, hence beta-3 adrenergic agonists are useful for treating diabetes as well as obesity. The aryloxypropanolamines were first described as beta-3 adrenergic receptor agonists.
Antihepatotoxic activity
Silymarin isolated from seeds of siybum marianum commonly known as MILK THISTLE has been used as a potent Antihepatotoxic agent against a variety of toxicants. It is a mixture of three isomers namely, silybum, silydianin, and silychristin. Silybin is the most active component containing 1,4-dioxane ring system however other lacks the same thus does not show prominent activity.
Antimicrobial activity
Chalcones containing electron releasing groups such as methoxy and hydroxyl showed better antibacterial activity than the derivative of same class that lacks such groups. Compounds having chalcone as a core pharmacophores with functional derivatives such as chloro, dichloro, fluro groups have exhibited greater antibacterial activity.
Antimalarial activity
Antimalarial property of some chalcones derivatives is derived from their ability to inhibit the parasitic enzyme cysteine protease. The enzyme is responsible for catabolizing globin into peptides with in the acidic food vacuole of the intra-erythrocytic malarial parasite. In the absence of cysteine protease action osmotic swelling occurs, food vacuolar functions are impaired, and parasite ultimately died off. It has been found that malaria blood stage cysteine protease is the key target enzyme of chalcones.
Antileishmanial activity
The conventional structure activity relationships show that antileishmanial activity is favored by chalcones that are more hydrophilic in nature, the hydroxychalcones are most active members in this class.
Tyrosinase inhibitor
Tyrosinase also known as polyphenol oxidase, is a copper-containing enzyme widely distributed in nature. It catalyzes two reactions; molecular oxygen in the melanin biosynthesis pathway, the hydroxylation of aminophenol’s to o-phenols, and the oxidation of the o-phenols to o-Quinone. This Quinone’s are highly reactive and tend to polymerize spontaneously to form brown pigments of high molecular weight, which determine the color of mammalian skin and hair. Quinone can also react with amino acids and proteins and thus enhance the development of brown color.
CONCLUSION
In summary, chalcones act as an excellent and universally accepted pharmacophore for development and design of novel medicine for treatment of pathological conditions. Ease of synthesis, surplus literature data, less time consumption, handy methodology, and good yield of final product makes the same as an attractive backbone for future research.
REFERENCES
1 Kyoko Nakagawa Goto, Koji Yamada, et al.,Synthesis and evaluation of Dietary antioxidant, Bio-organic & Medicinal chemistry Letters, 2007, 5204-5209.
2. Gorden E, Barrett R.W, Dower W. J, Folder S.P., Applications of combinatorial technologies to drug discover, J. Medchem., 1994, 1485.
3. Nielsen S.B, Christensen S. F, Cruciani G, Kharazmi A., Journal of Medchem.,1998, 4819.
4. Wu, J.Z.; Cheng, C.C.; Shen, L.L.; Wang, Z.K.; Wu, S.B.; Li, W.L.; Chen, S.H.; Zhou, R.P.;Qiu, P.H. Synthetic chalcones with potent antioxidant ability on H2O2-induced apoptosis in PC12 cells. Int. J. Mol. Sci. 2014, 18525–18539.
5. Kumar, C.S.C.; Loh, W.S.; Ooi, C.W.; Quah, C.K.; Fun, H.K. Heteroarylchalcones: Design, synthesis, X-ray crystal structures and biological evaluation. Molecules 2013, 12707–12724.
6. Nguyen, T.T.N.; Do, T.H.; Huynh, T.N.P.; Tran, C.D.T.; Thai, K.M. Synthesis and antibacterial activity of some heterocyclic chalcone analogues alone and in combination with antibiotics.Molecules 2012, 6684–6696.
7. Hassan, S.Y. Synthesis, antibacterial and antifungal activity of some new pyrazoline and pyrazole derivatives. Molecules 2013, 2683–2711.
8. Kang, J.E.; Cho, J.K.; Curtis-Long, M.J.; Ryu, H.W.; Kim, J.H.; Kim, H.J.; Yuk, H.J.;Kim, D.W.; Yuk, H.J.; Kim, D.W.; et al. Preparation of substituted pyridines and pyridazines with angiogenesis inhibiting activity for pharmaceutical use as antitumor agents. Molecules 2013, 140–153.
9. Solomon, V.R.; Lee, H. Anti-breast cancer activity of heteroarylchalcone derivatives.Biomed. Pharmacother. 2012, 213–220.
10. Kumar, D.; Kumar, N.M.; Akamatsu, K.; Kusaka, E.; Harada, H.; Ito, T. Synthesis and biological evaluation of indolylchalcones as antitumor agents. Bioorg. Med. Chem. Lett. 2010, 3916–3919.
11. Domýngueza, J.N.; Charris, J.E.; Loboa, G.; de Domýnguezb, N.G.; Moreno, M.M.; Riggione, F.;Sanchez, E.; Olson, J.; Rosenthal, P.J. Synthesis of quinolinylchalcones and evaluation of their antimalarial activity. Eur. J. Med. Chem. 2001, 555–560.
12. Hayat, F.; Moseley, E.; Salahuddin, A.; Zyl, R.L.V.; Azam, A. Antiprotozoal activity of chloro-quinoline based chalcones. Eur. J. Med. Chem. 2011, 1897–1905.
13. Kotra, V.; Ganapathy, S.; Adapa, S.R. Synthesis of new quinolinylchalcones as anticancer and anti-inflammatory agents. Ind. J. Chem. 2010, 1109–1116.
14. Rizvi, S.U.F.; Siddiqui, H.L.; Johns, M.; Detorio, M.; Schinazi, R.F. Anti-HIV-1 and cytotoxicity studies of piperidyl-thienylchalcones and their 2-pyrazoline derivatives. Med. Chem. Res. 2012, 3741–3749.
15. Stevens, J. F.; Page, J. E. Phytochemistry 2004, 1317.
16.. Lee, J.-H.; Jung, H. S.; Giang, P. M.; Jin, X.; Lee, S.; Son, P. T.; Lee, D.; Hong, Y.-S.;Lee, K.; Lee, J. J. J. Pharmacol. Exp. Ther. 2006, 316, 271.
17. Ahmad, S.; Israf, D. A.; Lajis, N. H.; Shaari, K.; Mohamed, H.; Wahab, A. A.; Ariffin, K. T.; Hoo, W. Y.; Aziz, N. A.; Kadir, A. A.; Sulaiman, M. R.; Somchit, M. N. Eur. J. Pharmacol. 2006, 538, 188.
18. S. V. Kostanecki and Tambor, J. Chem Ber., 1921 (1899).
19. M. A. El.Hashah; M. El-Kady; M. A. Saiyed and A. A. Elaswy, Egypt. J.Chem., 715 (1985)
20. L. S. Crawley and W. J. Fanshawe, J. Heterocyclic chem., 531 (1977)
21. E. C. Taylor and R. W. Morrison, J. Org. Chem., 2379 (1967)
22. P. S. Utale, P. B. Raghuvanshi and A. G. Doshi, Asian J. Chem., 597(1998)23.
23. Dhar, D.N. The chemistry of chalcones and related compounds, Wiley Interscience, New York (1981
24. Machodo, T.B., Leal, I.C.R., Kuster, R.M., Amaral, A.C.F. and Santos, K.R.N., Phytother. Res., 519 (2005).25. Prasad, Y. R., Ravi Kumar, P., Asha Deepthi, Ch. and Venkata Ramana M., Asian J. Chem.,
4799 (2007).
26. Nielsen, S.F., Thomas, B., Larsen, M., Kristian, S. and Kromann, H., Bioorg. Med. Chem., 3047 (2004).
27. Prasad, Y. R., Ravi Kumar, P., Asha Deepthi, Ch. and Venkata Ramana, M., E- Journal of Chem., 236 (2006).
28. Rao, N. R., Rao, G. S. and Mukkanti, P., The Pharma Review, 117 (2004).
29. Nowakowaska, Z., Kedzia, B. and Schroeder, G., Eur. J. Med. Chem., 707 (2008).30. Okunade, A.L., Hufford, D.C., Clark, A.L. and Lentz, D., Phytother. Res., 142 (1997).
31. Boeck, P., Leal, P.C., Yunes, R.A. and Zacchino, S., Archiv der Pharmazie, 338, (2005).
32. Sohly, H.N.E., Joshi, A.S., Nimrod, A.C. and Clark, A.M., Planta Med., 67, 87 (2001).
33. Stevaz, L., Tapia, S.N., Lopez, R.L.E., Furlan, E., Petenatti, R. and Zacchino S.A., J.Agrc. Food Chem., 3297 (2004).
34.Tsuchiya, H., Sato, M., Akagiri, M., Takagi, N., Tanaka, T. and Linuma, M., Japan Pharmazie, , 756 (1994).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









