About Authors:
Shalini Sharma, Manoj Prashar
Manav Bharti University,
Solan
ABSTRACT
Stomach specific mucoadhesive drug delivery system(MADDS) is one of the most prominent and latest system which sustain drug blood concentration and controlling the rate of drug delivery to the target tissue. These systems interact with mucin molecule and mucous layer covering the mucosal epithelial surface and prolong the residence time of the dosage form at the site of application or absorption and thus contribute to improve or better therapeutic performance of drug. This review article also present the polymer used for preparation of mucoadhesive tablet, mechanism of mucoadhesion, factor affecting mucoadhesion and recent developments or techniques in formulation of mucoadhesive tablet and invitro and invivo evaluation of mucoadhesive tablets.
[adsense:336x280:8701650588]
Reference ID: PHARMATUTOR-ART-1212
Introduction: Oral drug delivery is most widely utilized route of administration among all the routes that have been explored. An ideal drug delivery system should deliver an appropriate amount of drug to the desired site with a desired rate to optimize the drug therapy. Due to the considerable therapeutic advantages over conventional oral drug delivery dosage forms, various oral controlled release (CR) dosage forms (DFs) have been developed.1
Advantages of controlled drug delivery system:
1. Improved patient compliance and convenience due to lesser administration of drug.
2. Maintain steady state levels and therefore and reduced chances of local or systemic side effect and better control of disease conditions.
3. Due to better control of plasma levels safety margin of potent drugs increased.
4. The total amount of dose administered is reduced and less frequency of dosing.
5. Shorter treatment period is required from the conventional dosage form
Limitation occurs with a conventional dosage form: 2
1. In conventional dosage form chances of missing the dose of a drug is increased for which frequent administration is necessary
2. The attainment of steady state Condition is difficult due to a typical peak plasma concentration time profile.
3. The chances of under medication or over medication are more due to unavoidable fluctuation in the drug concentration.
By using the concepts and technique of controlled and targeted drug delivery system the above problems can be overcome. In controlled drug delivery system the drug release locally or systemically at a predetermined rate for a predetermined period of time.
Various problems are faced in designing controlled release systems for better absorption and improved bioavailability. One of such problems is the inability to keep the dosage form in the desired area of the gastrointestinal tract. Gastrointestinal tract drug absorption t is a complex process and is subject to many variables drug. The contact time of drug with the small intestinal mucosa tells the extent of gastrointestinal tract absorption .3The most important approaches for getting a prolonged and predetermined drug delivery profile in the GI tract is to control the gastric residence time (GRT) using gastro retentive dosage forms (GRDFs). GRDF prolong the GRT of the drugs by remaining in the gastric region for several hours. Prolonged gastric retention improves solubility of drugs that are less soluble in a high pH environment, the bioavailability and reduces drug waste. . It has applications also for local drug delivery to proximal small intestines and the stomach. 4
The controlled gastric retention dosage forms design and developed by the mechanisms of mucoadhesion1,5,6 expansion , 7 sedimentation, 8 flotation ,9,10 modified shape systems,11,12 both high and low-density drug delivery systems have been suggested as possible approaches to extend the transit time but the results of exploratory studies area equivocal.13,14
Overview on stomach: The main function of the stomach is to store the food temporarily, grind it and then release it slowly into the duodenum. Due to its small surface area, very little absorption takes place from the stomach. It provides a barrier to the delivery of drugs to the small intestine. The stomach is located in the upper left hand portion of the abdomen just below the diaphragm. The stomach on the basis of anatomy can be divided into three parts: fundus (uppermost part), the body (central part) and antrum (lowermost part). 38, 39, 40
Mucoadhesion: The attachment of a synthetic or natural macromolecule to biological surface known as Bioadhesion 15. The process of attachment of a synthetic or natural macromolecule to the mucosal tissue is known as ‘mucoadhesion’.
The design of dosage forms for mucoadhesive drug delivery should be acceptable for patients and should not cause irritation. Other ideal characteristics of a mucoadhesive dosage form include smooth surface, high drug loading capacity, tastelessness, controlled drug release (preferably unidirectional release), good mucoadhesive properties, and convenient application. Erodible formulations can be beneficial because they do not require system retrieval at the end of desired dosing interval. A numerous mucoadhesive dosage forms have been developed for a different type of drugs. Various peptides, including insulin, thyrotropin-releasing hormone (TRH), leuprolide, octreotideand, oxytocin, have been delivered through the mucosal route.16
Mucoadhesive Dosage Forms:
PatchesPatches are covered by protective layer of an impermeable backing material, a mucoadhesive surface for mucosal attachment and a drug-containing reservoir layer from which the drug is released in a controlled way. Solvent casting and direct milling are two methods used to prepare adhesive patches. In the first method, the intermediate sheet is prepared by casting the solution of the drug and polymer(s) onto a backing layer sheet, and subsequently allowing the solvent(s) to evaporate. In the second method, formulation constituents are homogeneously mixed and compressed to the required thickness, and patches of predetermined shape and size are then cut out. An impermeable backing layer may also be applied to prevent drug loss, control the direction of drug release, and reduced disintegration and deformation of the device during the application period.17, 18
Gels and ointments
Such type of semisolid dosage forms, have the advantage of easy dispersion throughout the oral mucosa. However, drug delivery from semisolid dosage forms may not be as accurate as from patches, tablets, or films. Less retention of the gels at the site of application has been improved by using mucoadhesive formulations. Some mucoadhesive polymers, for example, sodium carboxymethylcellulose, 19carbopol,20 hyaluronic acid, 21 and xanthan gum, 22 are used to change the liquid phase formulation to semisolid formulation. This change improves the viscosity, which helpful in sustained and controlled release of drugs. Hydrogels are used for buccal drug delivery. They are formed from polymers that are hydrated in an aqueous condition and drug molecules entrap for subsequent slow release by diffusion. 23 The application of mucoadhesive gels provides an extended retention time in the oral cavity, adequate drug penetration, as well as high efficacy and patient acceptability. In case of the local delivery of medicinal agents for the treatment of periodontitis, adhesive gels have the major application which is an infectious and inflammatory disease that causes formation of pockets between the gum and the tooth, and can cause loss of teeth. When mucoadhesive polymers incorporated in antimicrobial-containing formulations then these are useful for periodontitis therapy and easily introduced into the periodontal pocket with a syringe. 24,25,26
Tablets
Tablets are small, oval and flat, with a diameter of about 5-8 mm. 27 Mucoadhesive tablets allow for speaking and drinking without major discomfort. They adhere to the mucosa, and are remained in position until dissolution or release is complete. A Mucoadhesive tablet has additional advantages, for example, it facilitate absorption and improve bioavailability of the drugs due to a high surface to volume ratio and facilitates a much more intimate contact with the mucus layer. Mucoadhesive tablets provides the possibilities of localized as well as systemic controlled release of drugs.. They release the drug for a prolonged period so that Mucoadhesive tablets are widely used and reduce frequency of drug administration. The lack of physical flexibility is the drawback of mucoadhesive tablets, leading to poor patient compliance for long-term use. 28, 29, 30
Films
In terms of flexibility and comfort mucoadhesive films may be preferred over adhesive tablets. An ideal film should be soft, elastic, flexible and yet adequately strong to withstand breakage due to stress from mouth movements. It must also possess enough mucoadhesive strength in order to be attached in the mouth for the desired duration of action. 31
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
MUCOADHESIVE POLYMER 32: Two broad classes of mucoadhesive polymers: hydrophilic polymer and hydrogels. In the large classes of hydrophilic polymers the best mucoadhesive properties exhibited by those containing carboxylic group.33,34 Hydrogels are the class of polymeric biomaterial that exhibit the basic characteristics of an hydrogels to swell by absorbing water interacting by means of adhesion with the mucus that covers epithelia
i.e.
Cationic group- For example: Chitosan and its derivatives
Anionic group- For example: Carbopol35, Polyacrylates and their cross linked modifications
Neutral group- For example: Eudragit- NE30D etc.
Ideal Characteristics of a Mucoadhesive Polymer 36:
1. The polymer and its degradation products should be no absorbable from the GI tract and should be non-toxic.
2 It should not cause irritation to the mucus membrane.
3. It should form a strong non covalent bond with the epithelial cell surfaces- mucin.
4. It should not offer any hindrance to the drug release.
5. The polymers must not decompose on storage.
6. The cost of polymer should not be high.
Mechanisms of Mucoadhesion: The mechanism of mucoadhesion is generally divided into two steps: the contact stage and the consolidation stage. The first stage involves an intimate contact between the mucoadhesive and the mucus membrane, with wetting, spreading and swelling of the formulation, initiating its deep contact with the mucus layer. 37
In the consolidation step, thepenetration of the bioadhesive into the crevices of the tissue surface takes place.The diffusion and the dehydration theory are used to explain this step.
Theories of Mucoadhesion:
Mucoadhesion is a complex process and numerous theories exist to explain the bioadhesion process. Each theoretical model explains a limited number of interactions that constitute in the particular bioadhesive bond. 41
A. Electrostatic Theory of Mucoadhesion
According to electrostatic theory, transfer of electrons exhibit through the adhesive interface and adhering surface. The electron transfer between the mucus and the mucoadhesive results in the formation of double layer of electrical charges at the mucus and mucoadhesive interface and for maintaining contact between the two layers a series of attractive forces are responsible. 42
B. Wetting Theory of Mucoadhesion
The wetting theory is best applied to liquid or low-viscosity bioadhesive. It explains the penetration of adhesive agents into surface irregularities of the substrate and ultimately hardens, producing many adhesive anchors. Spreading of the adhesive on the surface of the substrate means that it must overcome any surface tension effects present at the interface. 43 For determination of affinity of mucoadhesion the wetting theory calculates the contact angle and the thermodynamic work of adhesion.
The work done is related to the surface tension exhibit between the adhesive and the substrate, as given by Dupre's equation;44
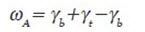
Where ωA is the specific thermodynamic work of adhesion and γb, γ t and γbt represent, respectively, the surface tensions of the bioadhesive polymer, the substrate, and the interfacial tension. The sum of the surface tensions of the two adherent phases is equal to the adhesive work done, less the interfacial tensions apparent between both phases. 45
Horizontal resolution of the forces gives the Young equation:

Where θ is the angle of contact, γbt is the surface tension between the tissue and polymer, γba is the surface tension between polymer and air, and γta is the surface tension between tissue and air. The wetting will be incomplete if the angle of contact is greater than zero.
If θ will approach to zero then wetting will be complete. In other words, bioadhesion is favored by large value γta or by small values of γbt and γba.
C. Diffusion Theory of Mucoadhesion
Diffusion theory describes that interpenetration of both polymer and mucin chains and reach a sufficient depth within the opposite matrix to allow formation of a semi permanent bond. 46
For good bioadhesive bonds the exact depth needed is not clear, but is estimated to be in the range of 0.2-0.5 ?m. 47 the mean diffusional depth of the bioadhesive polymer segments, s, may be represented by following equation:

Where D-- Is the diffusion coefficient t --is the contact time.
Duchene 48 adapted Equation 5 to give Equation 6, which can be used to calculate the time t, to bioadhesion of a particular polymer:

Where
l-- Represents the interpenetrating depth
Db-- represents the diffusion coefficient of a bioadhesive through the substrate.
Reinhart and Pappas 49 reported that the diffusion coefficient depended on the molecular weight of the polymer strand and with increasing cross-linking density is decreased
D. Adsorption Theory of Mucoadhesion
According to the adsorption theory, after an initial contact between adhesive polymer and mucus substrate surfaces, the materials adhere because of surface forces acting between the chemical structures at the two surfaces. 50 When polar molecules or groups are reoriented at the interface. Chemisorption can occur when adhesion is particularly strong. The theory consider that adherence to tissue is due to the net result of one or more secondary forces (hydrogen bonding, hydrophobic bonding and van der Waal's forces,). 51, 52, 53
E. Fracture Theory of Adhesion
This theory analysis the force required for the separation of two surfaces after adhesion. The fracture strength is determined by the following equation.
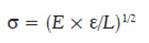
Where
s- is the fracture strength,
e -fracture energy,
E -young modulus of elasticity, and
L -the critical crack length [54]
Factors affecting mucoadhesion:
Several factors affect the mucoadhesion, including molecular weight, cross-linking, hydrophilicity, pH, swelling and the concentration of the active polymer. 46, 50, 55
1. Molecular weight: The low-molecular-weight polymers favor the interpenetration whereas entanglements are favored by higher molecular weights polymers. The optimum molecular weight required for the maximum mucoadhesion is different for different type of polymer. 56
2. Hydrophilicity: Bioadhesive polymers contain numerous hydrophilic functional groups which show hydrogen bonding with the substrate, swelling in aqueous media, thereby allowing maximal exposure to the sites. In addition, swollen polymers have the efficient penetration to the substrate
3. Cross-linking and swelling: To achieve a high degree of swelling, a lightly cross-linked polymer is favored. The degree of swelling is inversely proportional to the cross-link density. 57 The lower the cross-link density, the higher the flexibility and hydration rate; the larger the surface area of the polymer, the better the mucoadhesion. However, too great degree of swelling is not so beneficial. 58 The mucoadhesion of cross-linked polymers can be improved by the inclusion in the formulation of adhesion promoters. 55
4. Drug/Excipient concentrations: Drug/Excipient concentration may affect the mucoadhesion. BlancoFuente 60 analyses the effect of propranolol hydrochloride to Carbopol® (a lightly cross-linked poly (acrylic acid) polymer) hydrogels adhesion. Increasing toluidine blue O (TBO) concentration in mucoadhesive patches based on Gantrez® (poly(methylvinylether/maleic acid) significantly improve mucoadhesion to porcine cheek tissue. 61 This was added to increased internal cohesion within the patches due to electrostatic interactions between the anionic copolymer and cationic drug.
5. Other Factors Affecting Mucoadhesion: Mucoadhesion may be affected by the primary force of application. 62 the greater the initial contact time, the greater The swelling and interpenetration of polymer chains become more when the contact is large. 63Physiological variables can also affect mucoadhesion. The rate of mucus turnover can be affected by the presence of a bioadhesive device and also by disease states.64
Evaluation parameters of Mucoadhesion: Mucoadhesive polymers can be characterized by testing their adhesion strength by in vitro and in vivo tests. These tests are necessary not only for screening a large number of candidates for mucoadhesive, but also to study their mechanisms. The various methods reported are as follows.
1. In vitro / Ex vivo methods:
In vitro tests were initially designed to screen potential bioadhesive with a view to in vivo testing, if successful. Presently, more emphasis is being placed on elucidating the precise mechanisms of bioadhesion because; an evaluation of bioadhesive properties is fundamental to the development of new bioadhesive. The most commonly employed in vitro techniques are:
I. Methods based on measurement of shear strength.65
II. Methods based on measurement of tensile strengths.66, 67 other in vitro methods are: 68-74
III. Fluorescent probe method.
IV. Adhesion weight method
V. Mechanical spectroscopic method.
VI. Falling liquid film method.
VII. Flow channel method.
VIII. Viscometric method.
IX. Colloidal gold staining method.75
X. Adhesion number.
XI. Electrical conductance.
XII. Thumb method
2. In vivo methods:
The most common in vivo techniques to monitor bioadhesion include:
I. Use of gamma scintigraphy .76, 77
II. Use of pharmaco scintigraphy.78
III. Use of radioisotopes.
IV. Isolated loop technique.
V. Use of Electron paramagnetic resonance (EPR) oximetry.79
VI. X-ray studies.80
References:
1. A Hoffman Pharmacodynamic aspects of sustained release preparations. Adv Drug Del Rev 1998, 33, 185–99.
2. DM. Bramankar SB Jaiswal. .Biopharmaceutics and Pharmacokinetics A treatise.1995, 337-371.
3. Hirtz J. The git absorption of drugs in man: a review of current concepts and methods of investigation. Br J ClinPharmacol. 1985;19:77S-83S
4. Arora S, Ali J, Ahuja A, Khar RK, Baboota S. Floating drug delivery systems: A review. AAPS PharmaSciTech 2005;6:E372-90.
5. AA Deshpande, CT Rhodes, NH Shah, AW Malick. Controlled-release drug delivery systems for prolonged gastric residence: an overview. Drug Dev Ind Pharm 1996, 22, 31–39.
6. BN Singh, KH Kim. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Cont Rel
7. AHoffman,D Stepensky. Pharmacodynamic aspects of modes of drug administration for optimization of drug therapy. Crit Rev Ther
8. SJ Hwang, H Park, K Park. Gastric retentive drug-delivery systems. Crit Rev Ther Drug Carrier Sys 1998, 15, 243–84.
9. AJ Moes. Gastroretentive dosage forms. Crit Rev Ther Drug Carrier Syst. 1993, 10, 143–95.
10. JK Vasir, K Tambwekar, S Garg. Mucoadhesive tablets as a controlled drug delivery system. International Journal of Pharmaceutics 2003, 255, 13-32.
11. AF Stockwell, SS Davis, SE Walker. In-vitro evaluation of alginate gel systems. J.Control.release 1986, 3, 167 – 175.
12. J Timmermanns, A Moes . How well do floating dosage forms float?. Int.J.Pharm. 1990, 62(3), 207 – 216.
13. PG Yeole, S Khan, VF Patel. Floating drug delivery systems: Need and development. Indian.J.Pharm.Sci. 2005, 67(3), 265 – 272.
14. R Groning G Henu. Dosage forms with controlled gastrointestinal passage-studies on the absorption of nitrofurantoin Int J Pharm .1989, 56, 111-116.
15. Bernkop A, Hornof M, Zoidl T. Thiolated polymers—thiomers: synthesis and in vitro
evaluation of chitosan- 2-iminothiolane conjugates. Int J Pharm 2003; 260: 229– 237.
16. Veuillez F, Kalia YN, Jacques Y, Deshusses J, Buri P. Factors and strategies for improving buccal absorption of peptides. Eur J Pharm Biopharm 2001;51:93-109.
17. Biswajit B, Kevin G, Thimmasetty J. Formulation and evaluation of pimozide buccal mucoadhesive patches. Int J Pharm Sci Nanotechnol 2010;2:32-41
18. Wong CF, Yuen KH, Peh KK. Formulation and evaluation of controlled release Eudragit buccal patches. Int J Pharm 1999;178:11-22.
19. kumar S, Haglund BO, Himmelstein KJ. In situ-forming gels for ophthalmic drug delivery. J Ocul Pharmacol 1994;10:47-56.
20.Ishida M, Nambu N, Nagai T. Highly viscous gel ointment containing carbopol for application to the oral mucosa. Chem Pharm Bull 1983;31:4561-4.
21.Gurny R, Ryser JE, Tabatabay C, Martenet M, Edman P, Camber O. Precorneal residence time in humans of sodium hyaluronate as measured by gamma scintigraphy. Graefes Arch Clin Exp Ophthalmol 1990;228:510-2.
22.Meseguer G, Gurny R, Buri P. Gamma scintigraphic evaluation of precorneal clearance in human volunteers and in rabbits. Eur J Drug Metab Pharmacokinet 1993;18:190-4.
23.Martin L, Wilson CG, Koosha F, Uchegbu IF. Sustained buccal delivery of the hydrophobic drug denbufylline using physically cross-linked palmitoyl glycol chitosan hydrogels. Eur J Pharm Biopharm 2003;55:35-45.
24.Jones DS, Woolfson AD, Brown AF, Coulter WA, McClelland C, Irwin CR. Design, characterisation and preliminary clinical evaluation of a novel mucoadhesive topical formulation containing tetracycline for the treatment of periodontal disease. J Control Release 2000;67:357-68.
25.Vinholis AH, De Figueiredo LC, Marcantonio E, Marcantonio RA, Salvador SL, Goissis G. Subgingival utilization of a 1% chlorhexidine collagen gel for the treatment of periodontal pockets. A clinical and microbiological study. Braz Dent J 2001;12:209-13.
26.Ikinci G, Kenel SS, AkVncVbay H, Kas S, Ercis S, Wilson CG, et al. Effect of chitosan on a periodontal pathogen Porphyromonas gingivalis. Int J Pharm 2002;235:121-7.
27.Schn?rch AB. Mucoadhesive systems in oral drug delivery. Drug Discov Today Technol 2005;2:83-7.
28.Rathbone MJ, Drummond BK, Tucker G. The oral cavity as a site for systemic drug delivery. Adv Drug Deliv Rev 1994;13:1-22.
29.Rajput GC, Majmudar FD, Patel JK, Patel KN, Thakor RS, Patel BP, et al. Stomach specific mucoadhesive tablets as controlled drug delivery system: A review work. Int J Pharm Biol Res 2010;1:30-41.
30.Remeth D, Sfurti S, Kailas M. In-vitro absorption studies of mucoadhesive tablets of acyclovir. Indian J Pharm Educ Res 2010;44:183-8.
31. Shah D, Gaud RS, Misra AN, Parikh R. Formulation of a water soluble mucoadhesive film of lycopene for treatment of leukoplakia. Int J Pharm Sci Rev Res 2010;12:6-11.
32. NK .Jain. Controlled release and Novel Drug Delivery. 1st edition.CBS publishers and Distributors New Delhi.1997, 353-370
31. H. W. Hui and J. R. Robinson, “Ocular delivery of progesterone using bioadhesive polymer”, Int. J. Pharm., 1985, 26, pp. 203-213.
34. A Ahuja, RK Khar, J Ali. Mucoadhesive drug delivery systems, Drug Development and industrial pharmacy, 1997, 23 5, 489-515.
35. D Chickering, J Jacob, E Mathiowitz.. Poly(fumaric-cosebacic) microspheres as oral drug delivery systems. Biotechnol. Bioeng. 1996,52, 96–101
36. Leon Lachman, Herbert A.Lieberman,Josephl.,Kangi. The Theory and Practice of Industrial Pharmacy, 1991, 296-302.
37.Hδgerstrφm H, Edsman K, Strψmme M. Low-frequency dielectric spectroscopy as a tool for studying the compatibility between pharmaceutical gels and mucus tissue. J Pharm Sci 2003;92:1869-81.
38. Tortora GJ, Grabowski SR. Principles of anatomy and physiology. 10 th ed. USA: John Wiley and Sons Inc; 2002.
39 Wilson KJ, Waugh A. Anatony and physiology in health and illness. 8 th ed. USA: Churchill Livingstone; 1996.
40. Guyton AC, Hall JE. Textbook of medical physiology. 9 th ed. Philadelphia: W B Squnders Company; 1996.
41. Longer MA, Robinson JR. Fundamental aspects of bioadhesion. Pharmacy Int 1986;7:114-7.
42. Deraguin BV, Smilga VP. Adhesion: Fundamentals and Practice. London: McLaren; 1969.
43 McBain JW, Hopkins DG. On adhesives and adhesive action. J Phys Chem 1925;29:188-204.
44. Pritchard WH. In: (3rd edition ed.),D. Alder, Editor, Aspects of adhesion 6, London University Press, London (1970), pp. ll-23.
45. Wake WC. Adhesion and the Formulation of Adhesives. London: Applied Science Publishers; 1982.
46.Jimenez-Castellanos MR, Zia, H, Rhodes CT. Mucoadhesive drug delivery systems. Drug Dev Ind Pharm 1993;19:143-94.
47.Duchene D, Touchard F, Peppas NA. Pharmaceutical and medical aspects of bioadhesive systems for drug administration. Drug Dev Ind Pharm 1988;14:283-18.
48.Peppas NA, Buri PA. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J Control Release 1985;2:257-75.
49.Reinhart CP, Peppas NA. Solute diffusion in swollen membranes ii. influence of crosslinking on diffusion properties. J Memb Sci 1984;18:227-39.
50.Ahuja A, Khar RK, Ali J. Mucoadhesive drug delivery systems. Drug Dev Ind Pharm 1997;23:489-515.
51.Huntsberger JR. Mechanisms of adhesion. J Pain Technol 1967;39:199-211.
52.Kinloch AJ. The science of adhesion I. Surface and interfacial aspects. J Material Sci 1980;15:2141.
53.Yang X, Robinson JR. Bioadhesion in Mucosal Drug Delivery. In: Okano T, editor. Biorelated Polymers and Gels. London: Academic Press; 1998.
54.Gu JM, Robinson JR, Leung SH. Binding of acrylic polymers to mucin/epithelial surfaces: Structure-property relationships. Crit Rev Ther Drug Carrier Syst 1988;5:21-67.
55.Peppas NA, Little MD, Huang Y. Bioadhesive Controlled Release Systems. In: Wise DL, editor. Handbook of pharmaceutical controlled release technology. New York: Marcel Dekker; 2000. p. 255-69.
56.Gurny R, Meyer JM, Peppas NA. Bioadhesive intraoral release systems: Design, testing and analysis. Biomaterials 1984;5:336-40.
57.Gudeman L, Peppas NA. Preparation and characterisation of ph- sensitive, interpenetrating networks of poly(vinyl alcohol) and poly(acrylic acid). J Appl Polym Sci 1995;55:919-28.
58.McCarron PA, Woolfson AD, Donnelly RF, Andrews GP, Zawislak A, Price JH. Influence of plasticiser type and storage conditions on the properties of poly(methyl vinyl ether-co-maleic anhydride) bioadhesive films. J Appl Polym Sci 2004;91:1576-89.
59.Park H, Robinson JR. Physicochemical properties of water soluble polymers important to mucin/epithelium adhesion. J Control Release 1985;2:47-7.
60.Blanco Fuente H, AnguianoIgea S, OteroEspinar FJ, BlancoMendez J. In-vitro bioadhesion of carbopol hydrogels. Int J Pharm 1996;142:169-74.
61.Donnelly RF, McCarron PA, Tunney MM, Woolfson AD. Potential of photodynamic therapy in treatment of fungal infections of the mouth. design and characterisation of a mucoadhesive patch containing toluidine Blue O. J Photochem Photobiol B 2007;86:59-69.
62.Smart JD. An in vitro assessment of some mucoadhesive dosage forms. Int J Pharm 1991;73:69-74.
63.Kamath KR, Park K. Mucosal Adhesive Preparations. In: Swarbrick J, Boylan JC, editors. Encyclopedia of Pharmaceutical Technology. New York: Marcel Dekker; 1992. p.133.
64. Lehr CM, Poelma FG. An Estimate of turnover time of intestinal mucus gel layer in the Rat in situ Loop. Int J Pharm 1991;70:235.
65. Smart JD. An in vitro assessment of some mucosa-adhesive dosage forms. Int J Pharm. 1991;73:69- 74.
66. Sam AP, Van Dan Heuij JT, Tukker J. Mucoadhesion of both film-forming and non- film forming polymeric materials as evaluated with the Wilhelmy plate method. Int J Pharm. 1989;53:97-105.
67. Gupta A, Garg S, Khar RK. Measurement of bioadhesive strenghs of mucoadhesive buccal tablets: Design of in-vitro assembly. Indian Drugs.1992;30(4):152-155
68. Smart JD, Kellaway IW. Pharmaceutical factors influencing the rate of gastrointestinal transit in an animal model. Int J Pharm. 1989;53:79-86.
69. Mortazavi SA, Carpenter BG, Smart JD. Investigation of the rheological behaviour of the mucoadhesive/mucosal interface. Int J Pharm. 1992;83:221-225.
70. Mortazavi SA, Carpenter BG, Smart JD. Comparative study on the role played by mucous glycoproteins in the rheological behaviour of the mucoadhesive/mucosal interface. Int J Pharm. 1993;94:195-201.
71. Mortazavi SA, Carpenter BG, Smart JD. Factors influencing gel strengthening at the mucoadhesive-mucous interface. J Pharm Pharmacol. 1994;46:86-91.
72. Ho NF, Day JS, Barsuhn CL, Burton PS, Raub TJ. Biophysical model approaches to mechanistic transepithelial studies of the peptides. J Control Release. 1990;11:3-10.
73. Rao KVR, Buri P. Novel in situ method to test polymers and coated microparticles for bioadhesion. Int J Pharm. 1989; 52:265-270.
74. Hassan EE, Gallo JM. Simple rheological method for the in vitro assessment of mucin-polymer bioadhesive bond strength. Pharm Res. 1990;7:491-498.
75. Park K. A new approach to study mucoadhesion: colloidal gold staining. Int J Pharm. 1989;53:209-217.
76. DavisSS, Hardy JG, Taylor MJ, Stockwell A, Whalley DR, Wilson CG. The in vivo evaluation of an osmotic device (osmet) Using gamma scintography. J Pharm Pharmacol.1984;36:740-742.
77. Krishnaiah YSR, Kondala Rao B, Rama Prasad YV, Satyanarayana S. Gamma scintigraphy : an imaging technique for non-invasive in vivo evaluation of oral dosage forms. Indian Drugs. 1998; 35(7):387-398.
78. Singh AK, Bhardwaj N, Bhatnagar A. Pharmacoscintigraphy: An unexplored modality in India. Ind J Pharm Sci. 2004;66(1):18-25.
79. Petelin M, Pavlica Z, Bizimoska S, Sentjure M. In vivo study of different ointments for drug delivery in to oral mucosa EPR oximetry. Int J Pharm.2004; 270:83-91.
80. R. Bala Ramesha Chary, G. Vani, Y. Madhusudan Rao, In Vitro and In Vivo Adhesion Testing of Mucoadhesive Drug Delivery Systems. Drug Dev Ind Pharm.,1999, 25(5), 685-690.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









