About Authors:
Rajender Kumar1*,Dr.Sukhbir L.Khokara2,
1Department of Pharmacy, Manav Bharti University, Solan (H.P.) 173229
2Institute of pharmaceutical sciences, Kurukshetra University,
Kurukshetra, Harayana-136119
*rajkaushal13@gmail.com
ABSTRACT
Butenolides (furanones) consist of unsaturated γ- lactone which occur in plants as glycosides. There is a group of steroids which contain α,β unsaturated butenolide ring in the side chain such steroids are known as cardenolides. The presence of γ - lactone ring contain natural products like Goniobutenolides A & B ,Digitoxigenin and lignans. These compound have biological properties like antibiotic, antitumor, anticonvulsant, antimicrobial and medicinal uses like fungicides, bactericides and allergic inhibitor.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1401
1.INTRODUCTION
Butenolides derivatives are a group of important compounds containing inique carbon skeleton of 2(5H) & 2(3H) furanone and may be regarded as furan dv. i.e. 2,3 and 2,5 di hydro furan-2-one(l-3) (I) and (II) and the term “butenolide” was first time used by Klobb(4).
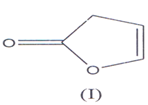
They are also known as butenolactones / crotonolactones / (α,β)-angelica lactones(5’6). They are also represented by the term “Furanones”(7) hence?β,γ-butenolides are 2(3H)-furanones and ?α,β-
They are also known as butenolactones / crotonolactones / (α,β)-angelica lactones(5’6). They are 2(5H)-furanones. Hydrolysis of these compoimds & Hydrogenation of the double bond in their structure, results the loss of activity of these compounds. There is a group of steroids which contain α,β unsaturated butenolide ring in the side chain such steroids are known as cardenolides(8-10).
2.AVAILABILITY IN NATURE
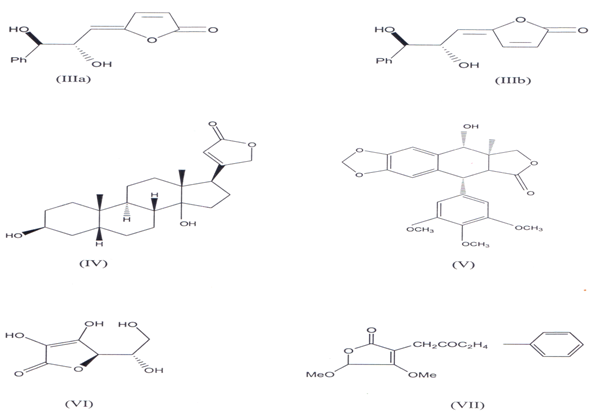
Butenolides consist of unsaturated γ- lactone which is widely present in many natural products(11)Some of them occur in plants as glycosides. The γ - lactone ring is a part of varity of natural products like Goniobutenolides A & B (llla, IIIb) (l2`14) Digitoxigenin (IV), lignans like Podophyllotoxin W), Ascorbic acid (VI), Sesquiterpene, antibiotic like patulin(l5). They also exist naturally like fissohamione (VII) from Fissistigma oldhamii(16)
3.METHODS OF PREPARATION
3.1.Synthesis of ?β,r-butenolides [2(3H) furanones]
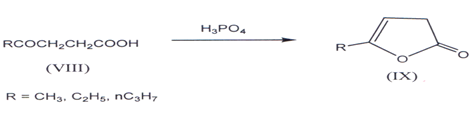
I. γ-keto acid such as levulinic acid give ?β,γ- butenolides(17’18). Succinic anhydride with dichloroalurninoethyl (C2H5A1Cl2) gives compound (VIII) (R=C2H5)(l9,20), which on cyclization by heating with orthophosphoric acid gives ?β,γ - butenolides(21) (IX).
γ –Aryl-?β,γ -butenolides can be prepared by acetic anhydride cyclisation of β-aroylpropionic acid(22'25) while cyclization of β -benzoylpropionic acid give γ -phenyl-?β,γ - butenolides which has been studied extensively (26).
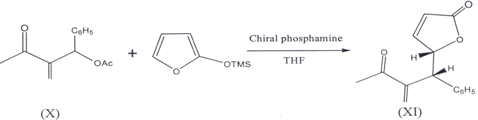
II. 2-Trimethylsilyloxy furan and Morita- Baylis- Hillman acetate (X) in the presence of chiral phosphines (cata1yst)(27) and THF as a solvent results in corresponding syn-γ-butenolides (XI) (28).
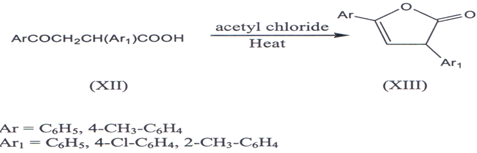
III. α-Aryl-β-aroyl propoinic acid (XII) on heating with acetyl chloride(29) or acetic anhydride(30) above their melting points reported to yield α-y-diaryl ?β,γb butenolides(XIII).
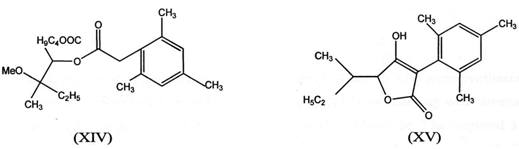
IV. Cyclization of alkanoate ester (XIV) in the presence of bases gives furanone (3l)(XV)
Za
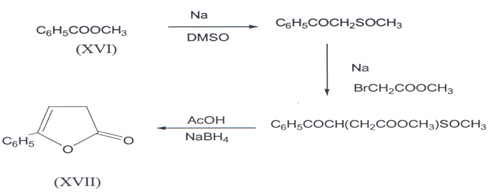
A multistep synthesis of butenolides (XVII) starting from methyl benzoate (XVI) has been reported(32).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
3.2. Synthesis of α-arylidene (alkylidene)-γaryl(alkyl)-?β,γ butenolides
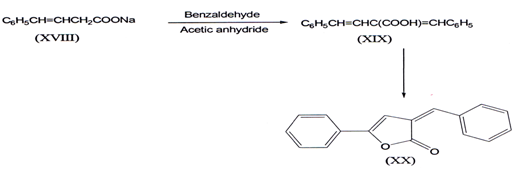
I. The earliest reported synthesis of α-benzylidene-y-phenyl-?β,γ- butenolides(XX) was from dibenzylidene propoinic acid (XIX). latter it was prepared from benzaldehyde by heating with sodium phenyl isocrotone (XVIII) and acetic anhydridem(33).
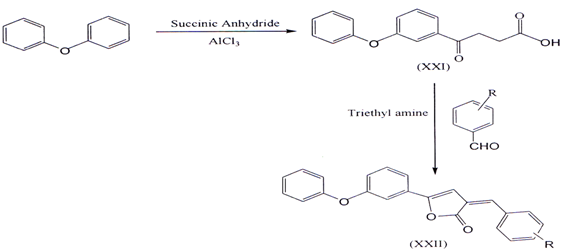
II. 2-Arylidene-4-(4-phenoxy-phenyl) but-3-en-4-olides (XXII) were synthesized from 3-(4-phenoxy-benzoyl) propionic acid (XXI) by reacting with aromaticaldehydes in presence of triethylamine in acetic anhydride. The required 3-(4phenoxy-benzoyl) propionic acid was prepared by condensing diphenyl ether with succinic anhydride in presence of anhydrous ahuninium chl.
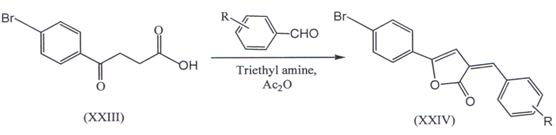
III. 3-(4-bromo-benzoyl) propionic acid (XXIII) were obtained from dry bromobenzene by the method reported earlier(34’35). A solution of (XXIII) and aromatic aldehyde in acetic anhydride with 23 drops of triethylamine was refluxed for four hour under anhydrous conditions and we got 3-Ary1idene-5-(4-bromophenyl-3(3H)ZQSH)-furanone(XXIV)(36)
IV. 3-Ary1idene-5-(4-methylphenyl)-2(3H)-furanone were prepared from 3-(4- methyl-benzoyl) propanoic aid and several aromatic aldehydes(37)
3.3. Synthesis of ?β,γ Butenolides [2(5H) furanone]
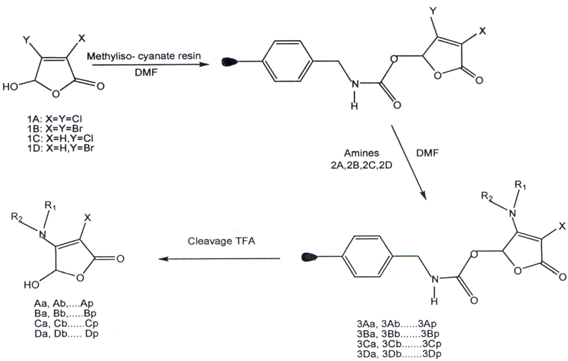
I. Butenolides prepared by using solid phase synthesis. Using the four reactive butenolide building blocks lA, lB, lC, 1D with 16 diverse amines in acombinatorial manner, a library of 64 individual compounds was afforded in tota1(38)
Compound (XXV) with resin supported in organic solvent in the presence of base and pd catalyst at 60-800Cfollowed by decomposition with Lewis acid gives furanone(XXVI)(39)

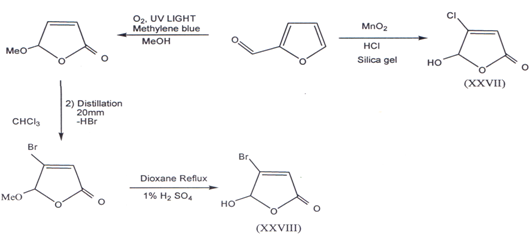
II. Furfural, a versatile aromatic aldehyde was used as the initial starting materialin the construction of the required butenolides building blocks. Mucochloricacid and Mucobromic acid can be synthesized from furfural(38). 4-Chloro-5-hydroxy-furanone (XXVII)(40) and 4-bromo-5-hydroxy-2(5H) furanone(XXVIII) was prepared(41) according to following method.
III. When cyclobutenolides reacts withh lithium trimethyl silyacetylene we report unusual transformation of cyclobutenediones into butenolides(42).
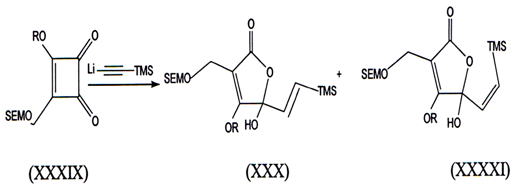
When lithium trirnethylsilylacetylene was treated w1th 3-methoxy-4-[trimethylsilyl ethoxy methoxy methyl]-3 cyclobuten- 1, 2 dione (SEMdione) (XXIX). The butenolides (XXX) and (XXXI) were isolated 1n 26% yleld.
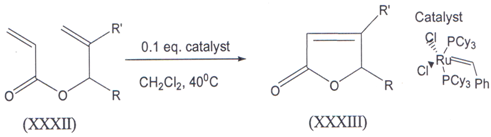
IV. The ruthenium catalyzed rlng closing metathesis of methallyl acrylates(XXXII) gave 4- Methyl 5- alkyl 2(5H) furanones (XXXIII)(43) in good to hlgh yields. Desplte the electron deficiency of both double bonds in the starting acrylates, the first generation Grubbs, catalyst proved to be an effective catalyst for the ring closure.
V.
VI. The facile iodolactonisation of ethyl 2,3-allenoates (XXXIV) with I2 inaqueous MeCN gave 4-iodofuran-2(5H)-ones O(XXV) in moderate to high yields(44).
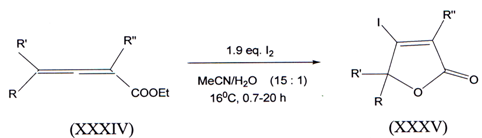
l) R=H, R’=C7H15, R”=H 2) R=H, R,=C7H15, R”=CH3 3) R=H, R’=Ph, R”=Ph
VII. A one-pot, convenient and general access to 5-sp2-substituted and 5, 5-disubstituted tetronic acids embodies two consecutive chemical events: a Michael addition of pyrrolidine on a secondary or tertiary γ-hydroxy-α,β-alkynyl ester derivative to give the corresponding enamine, and a subsequentacid-catalyzed hydrolysis lactonization(45). The product obtained by this method and percentage yield is given below.
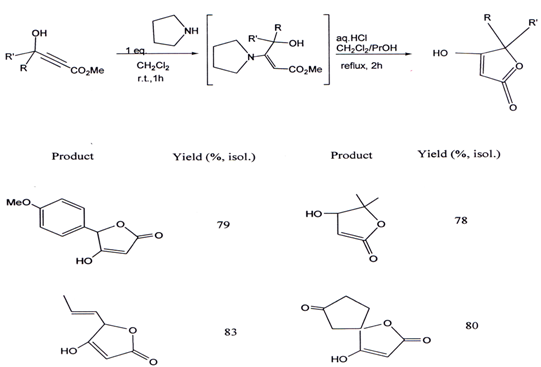
3.4. Synthesis of γ- alkylidene ?β,γ butenolide
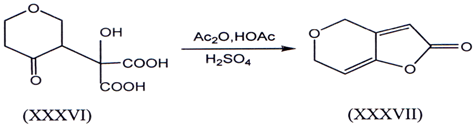
I. Lactonization of γ -ketoaeid and is one of the method for the synthesis of γ-alkylidene butenolides. Conversion of ketol (XXXVI) into des-oxypatulin(XXXVI)I(46) represent some of the earliest example.Such an example are mention here.
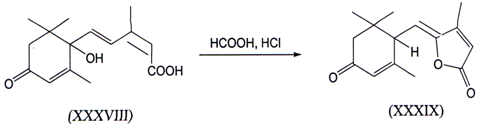
II. γ-Hydroxyacids are known to readily cyclize to give γ - lactones. In caseswhere such γ- lactones contain a suitable functional group, such as an allyl,halogen, oxygen, or sulfur group that can participate in elimination reactions, γ -alkylidenebutenolides can be obtained(47). One example is given below where γ -alkylidenebutenolide (XXXIX) is prepared from abscisic acid(XXXVIII)(48).
3.5. Synthesis of γ-aminomethyl-?-α,β butenolides from methylene-amine equivalents in the presence of a Lewis acid (Mannich reaction)
Mannich reaction is a well known method to synthesis wide range of nitrogen containing molecules(49’50) Which involves iminium ions (Mannich bases) derived from amine and aldehyde Lmder acidic condition to yield the aminomethylated products. These reactions which involves N-methylene amine (monomeric formaldehyde imine) is very limited from a synthetic viewpoint because N- methylene amine is difficult to generate. These reaction with various nucleophiles yield aminomethylated products at on position of amines(51).
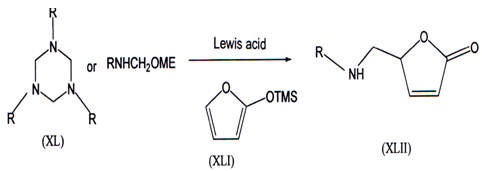
Heterocyclic nucleophile 2-trimethylsilyloxyfuran (XLI) reacted with the N-methyleneamine equivalents generated either from 1, 3, 5-trisubstituted hexa-hydro-l,3,5-triazines (XL) or N-methoxymethylamines in presence of catalyticamount Ti(Oi-Pr)4 to yield 5-aminomerhyl-2,5-dihydrofuran-2-ones (XLII)(52).N-Methyleneamine is only known in the gas phase through flash vaccum thermolysis from the corresponding on-amino nitriles G{NHCH2CI\D(53). Synthetic efforts enabled to generate N-methylene amine as an intermediate fromN-chloroalkylamine(RNClMe)(54,55),N-tosylmethylaminelw, _ N-methoxy-methy1amine(57’58).
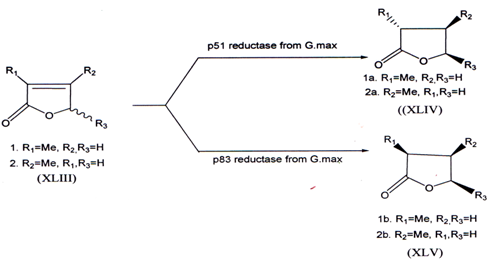
3.6.Stereoselective reduction of 2-butenolides by two reductases from cultured cells of Glycine max
The stereoselective reduction of 2-butenolides by two reductases has been investigated. The reduction of 2-methyl-2-butenolides (XLIII) by p51 reductase produced (R)-2-methylbutenolides (XLIV), whereas the reduction by p83 reductase gave (S)-2-methylbutenolide (XLV)(59).
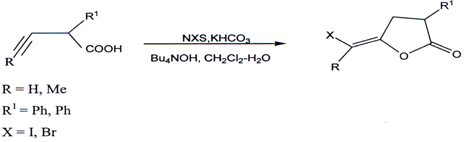
3.7. Halolactonization of 4-alkynoic acid`
Treatment of 4-alkynoic acids with N-halosuccinimide (NXS) containing I, Br,or C1 in the presence of KHCO3 and Bu4NOH in CH2C12-H20 provides stereoslecetively the corresponding anti-halolactonization products(60).
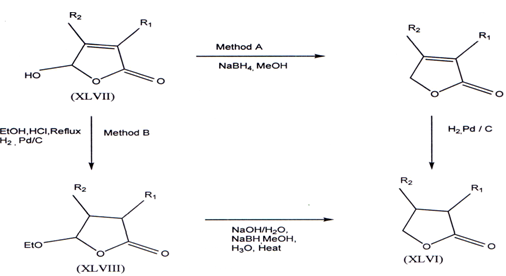
3.8. Synthesis of γ-butyrolactones
The non-commercially available Y-butyrolactones (XLVI) can be prepared from a sequenceof NaBH4(61) and catalytic reduction applied to 4-hydroxy-?2 -butenolides (XLVII)(62-64) (method A) OR from a NaBH4 reduction of the 4-ethoxy butenolides (XLVIII)(62) (method B).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
4.BIOLOGICAL ACTIVITY
4.1.Antibiotic property
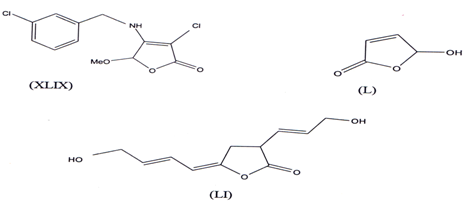
Butenolides containing substituted o-chloro-benzylamine (XLIX) have been recently identified as antibiotic agent against multiresistant Staphylococcus aureus(81). Narthogenine, 5Hydroxy-2-(5H)-furanone(L)(82) was found to have good Antibiotic properties and recently, a butenolide antibiotic named lissodinolide (LI) has been synthesized and was found to have good antibiotic activity(83).
4.2.Anti-tumor Activity
Basidalin represents an 4-amino 2 (5H)-furanone (LII) has shown Anti-tumor activity(82).
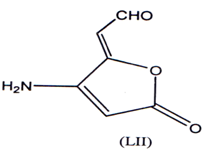
Biscyc1ic-3.4-dihalogenated- 2 (5H) furanone were found to have anti-cancer/anti- tumor activity(84). A number of sesquiteipine lactones having antitumour activity have been reported(85). The presence of a α-methylene lactones is essential for their activity(87). A series of α-methylene-γ-butyrolactones were synthesized and evaluated for their cytotoxic activity(87) with significant results.
The tumour inhibitory α-methylene-lactones were shown to inhibit the sulfhydryl enzyme phosphofructokinase probably by reaction with sulfhydryl groups of the enzyme(88’89).
4.3.Cardiotonic activity
Steroids which contains a α,β unsaturated butenolides ring in side chain exert a specific and power full action on the cardiac muscles of man & animals & therefore have been named as cardinolides(90). Large number of [3-substituted?β,γ -butenolides have been examined for their cardiotonic activity but potency is less as compared to cardiac glycosides(9l`97). Cardiac glycosides like that of digitalis contains unsaturated 7-lactone group substituted by a steroidal residue on the β-carbon(98).
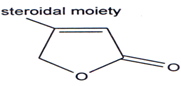
Three new cardiac glycosides were isolated from seeds of Corchorus olitorius L(99). The cardiac effects of 4-(3', 4'-dimethoxy-phenyl)-3-acetyl-4-butenolides were investigated in dogs(100). The drug had a specific and long lasting cardio stimulating action.
4.4.Anti-inflammatory activity
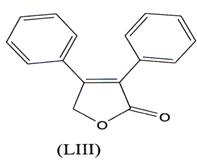
Butenolides exhibit a good anti-inflammatory activity. 3,4 diphenyl- 2,5 dihydro- 2- ftuanone (LIII), a novel compound exhibited good anti- inflammatory activity, selectively COX-2 with little effect on COX-1(101).
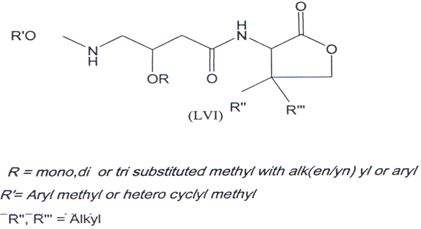
Compound (LVI) is used in disorders such as allergy, inflammation and autoimmune diseases(102).
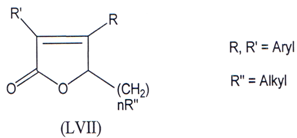
Compounds which selectively inhibit COX-2 are better antiinflammatory agents and devoid of gastro-intestinal problem(103) and in search of this a series of diary1-5- alkyl-5-methyl-2(5H)-furanones (LVII) were synthesized and few of them were found to be effective COX-2 inhibitors.
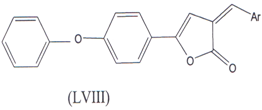
2-arylidene-4-(4-phenoxy phenyl) but-3-en-4-olides (LVIII) are also used as a anti-inflammatory Agents which is COX-2 inhibitor(36).
α-Butyl-β-hydroxy-?β,γ -butenolide & α -benzyl-β-hydroxy-?β,γ -butenolide were synthesized and used in combination with aminopyrine as analgesic, antipyretic and antiphlogistic agent(104’l05).
Kunes, J. and coworkers have synthesized & evaluated anti-fungal activity of (-) analogues of incrustoporine so a series of 5-alkyl-3-hetaryl-2,5-dilfydrofuranones (LIX) with hetaryl (thienyl, furyl and thiazolyl) moieties at C-3 were synthesized by the replacement of phenyl moiety(106) . The anti-fungal efficiency of derivatives was lower in comparison with analogues substituted with phenyl at C-3(106).
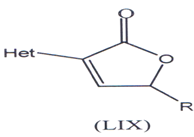
Het = 2, 3-thienyl, 4-thiazolyl, 4-oxazolyl, 2-furyl
R = CH3, CZH5
4.5.Anti-convulsant activity
γ-Butyrolactones (GBL) are known to be neuropharmacologically active(107’108). Studies have shown that the hydrolysis product of GBL is γ-hydroxybutyrolactones (GHB) which is a part of brain tissue in several mammalian species(l09’1l0). GHB was first studied in 1960 by Laborit and his co-worker as an isostere of γ-amino-butyric acid (GABA) able to cross the blood-brain barrier and was proposed as an hypnotic and general anesthesic(111). GHB modulates dopaminergic activitymz) and plays a role in sleep regulation and in specific seizure state. Klunk & co-worker(113-116) have synthesized several alkyl substituted GBL and studied their anti-convulsant activity.
4.6.Antimicrobial activity
Macrolides, hydroxylated macrocyclic lactones represent important class of anti- microbials. Recently, halogenated furanones and related haloalkenones were discovered to have anti-microbial activity through inhibition of two component signal transduction system(117).
Fimbrolides a furanone, inhibits biofilm formation by inhibiting Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicansm(118).
Nowadays various coatings contain furanones(119) to prevent microbial attack.
Synthetic analogs of natural (-) incrustroporine, 3-(substitutedphenyl)-5-acyl-oxy- methyl-2H, 5H-furan-2-ones are found to have anti-fungal activity(120).
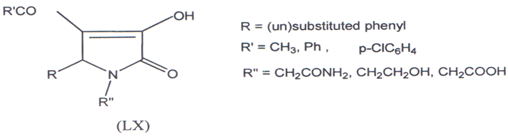
Gein and co-workers(121) have synthesized 1-substituted-5-aryl-4-acyl-3- hydroxypyrrolin-2-ones (LX) and found that these compounds were effective as bacteriostatic agents.
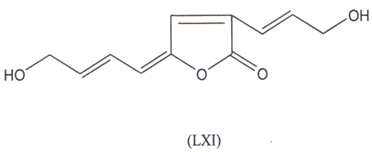
Kozminykh and co-workers(122,123) have synthesized 2-hydroxy-3(2H) pyrrolinones and 5-aryl-furan-2,3-diones which exihibted good Anti- microbial activity. Recently, a butenolide antibiotic named lissodinolide (LXI) has been synthesized and was found to have good activity(l24).
A new γ-arylidene bicyclic butenolide named cochinolide has been recently reported to posses potent anti-viral activity(125).
A series of 3-halogen-4-amino-5-alkoxy-2(5H)-furanones has been synthesized and evaluated for antimicrobial activity. The 4-benzylamino-2-(SH)-furanone shows excellent antibacterial activity against multi-resistant Staphylococcus aureus(126).
4.7.Miscellaneous activity
Acepsenolides: lst bisbutenolides lipid isolated from marine organism, (The Gorgonian perogorgia anceps) this genus is noted for its ability to biosynthesize a unique class of interesting C2 symmetric long chain fatty acid dilactone dv. The importance of its gamma lactone unit as a generic inhibitor of enzymes(127), and other biological & biomedical propertiesm(128).
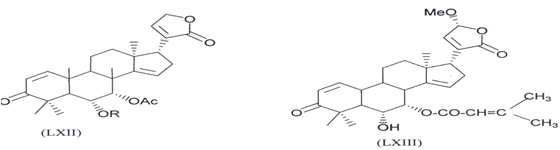
Two new triterpenoids 23-O-methylnimocinolide [7 α -acetoxy-23 -methoxy-3- OXO 24,25,26,27 tetrarnoraportirucal1a(apoeupha)-1,14,20(22)-trieno-21,23-lactone] (LXII) and7-O-deacetyl-23-O-methy-7α-O-senecioynimo cinolide [6a-hydroxy-23s- methoy-3Oxo-7α-senecioyloxy-24,25,26,27-tetranocapotirucalla(apocupha)- ,l420(22)-trieno-21,23-(actone) (LXIII) have been isolated from methanolic extract of the fresh leaves ofAZADIRACHTA INDICA show the insect growth resulating effect on mosquitoes (Aedes aegypti)with LC50 53ppm and 2.14ppm respectively (129).
Butenolides can be used as cockroach attractant(130) and repellants for white flies (131). Furanone derivatives and their pharmaceutically acceptable salts are claimed for prevention and treatment of nephritis from NSAIDs, imaging agents, anti-tumour agents etc(132). They can also be used against chronic nephritic syndrome, diabetic nephropathy, gouty nephropathy.
3-Hydroxy-4, 5-dimethyl-2(5H)-turanone and 3-hydroxy-4-methyl-5-ethyl-2(5H)- furanone were found to be tyrosinase inhibitor suitable for use in skin preparation or as food discoloration preventive agentm(128). Two 2(5H)-furanone in association with medium chain fatty acid released by Lactobacillus helveticus play an important role in cheese technology(l33). Recently, a novel class of synthetic γ-hydroxybutenolide has been discovered as a orally active nonpeptide endothelin antagonist(134), closely related to snake venom toxins having potent renal, pulmonary, neuro-endocrine action used in wide variety of human diseases including ischaemia, stroke renal failure, hypertension, pulmonary hypertension and restenosis .
Four compounds belonging to 4-hydroxy-3 (2H)-furanone series specially 4-hydroxy- 2, 5-dimethyl-3(2H)-furanone and 2-ethyl-4-hydroxy-5 -methyl-3 (ZH)-lixranones have been found to inhibit cataract fonnationm(135).
Recently a series of pinusolide derivatives were found to possess potent in vitro platelet inhibitory activity (136).
In the search of phospholipase A2 inhibitors, Evans and co-vvorkers(137) have synthesized Cinatrin C1 (LXIV) and Cinatrin C2 (LXV) as a successful candidates.

NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
5.MEDICINAL USES
Butenolides exhibit a variety of biological properties and have been considered as potential anti-tumor agents, fungicides, bactericides, insecticides, antibiotics, anti- intlammatory, allergy inhibitor(80) and so on.
References
1. Chia, Y.; Chang, F.; Wu, Y., T etrahedren Lett., 40, 7513 (1999).
2. Branchadell, V.; Ort., J.; Ortuno, R.M.; Font, J., .£Org. Chem., 56, 2190 (1991).
3. Fan, X.E., Huang, M.; Huang, H., Chen, Q.H., Chinese .1 Org. Chem, 22, 1359- 1365 (2004).
4. Klobb, T., C. R., Acad. Sci., 130, 1254 (1900).
5. Haynes LJ.; Plimmer, J.R., Quart Rev., 14, 292 (1960).
6. Jones, E.R.H.; W11iting, M.C., .I Chem. Soc., 1419 (1949).
7. Jones, E.R.H.; Whiting, M.C., J Chem. Soc., 343 (1951).
8. Klingsberg, E., Chem. Rev., 54, 59 (1954).
9. Iwakura, Y.; Magakubo, K., .1 Chem. Soc., 59, 476 (1956).
10. Watton, B.; Creiger, J., .Z Am. Chem. Soc., 67, 1121 (1945).
11. Ito, N., Pure Appl. Chem., 13, 63 (1991).
12. Shing, T.K.M.;Tsui, H.C.; Zhou, Z.H., .L Org. Chem., 60, 3121 (1995).
13. Mukai, C.; Hirai, S.; Kim, I. J.; Kido, M.; Hanaoka, M., Tetrahedron Lett., 52, 6547 (1996).
14. Smivet, J.P;; Vatele, J .M., T etrahedron Lett., 37, 4373 (1996).
15. Haynes, L.J.; Quart. Rev., 2, 46 (1948).
16. Yi-Chen, C.; Fang-Rong, C.; Yang-Chang, W., Tetrahedron Lett., 40, 7513-7514 (1999); C_A., 132, 2037k (2000). }
17. Gilman, H.; Franz, Ra. A.; Hewlett, A.P.; Wright, G.F., .Z Am. Chem. Soc., 3, 72, (1950).
18 .Walton, E., .I Chem. Soc., 438 (1940).
19. Reinheckel, H.; Haage K., .Z Organomet Chem., 10, 29 (1967).
20. Reinheckel, H.; Haage, K., German Ojfen., 1285, 554 (1969); C.A., 70,77356Y(1969).
21. Lukes, R., Collection Czech. Chem. Commun., 1, 461 (1929).
22;. Swain, G.; Todd, A.R.; Waaring, W.S., .I Chem. So., 548 (1944).
23. Zymolkosky, F., Arch. Farm, 284, 292 (1951).
24. Fieser, L.F.; Daudy, W.H., .Z Am. Chem. Soc., 63, 782 (1971).s
25. Ramachandran, R .; Chiron, R.; Graff, Y., Bull. Soc. Chem. Fr., 103 (1972).
26. Rao, Y.S., Chem. Rev., 625 (1976).
27. Fang, Y.Q.; J aobsen, EfN.; .Z Am. Chem. Soc.,130, 5660 (2008).
28. Jiang, Y.-Q.; Shi, Y. L.; Shi, M.; .L Am. Chem. Soc.,130, 7202-7203, (2008). 29. Fuson, R.C.; Bannister, R.G., J Am. Chem. Soc., 74, 1629 (1952).
30. Sammour, A.; Elhashash, M., .L Prakt. Chem., 314, 906 (1972).
31. Yuji, A.; Kiyoshi, T.; Isamu, N.; Tatsuo, O.; Kazuyuki, N.; Akira, N., Jpn. Kokai Tokkyo Koho JP, 86, 649 (2000); C.A., 132, 222436e (2000).
32. Kurono, M.; Imaki, K.; Tanigawa, C.; Kida, J ., Japanese Patent I 7405961(1974); C.A., 80, 120328m (1974).
33. Dalpozza, A.; Dansi, A.; Mariotti, V.; Meneghini, E., Boll. Chim. Pharm., 111, 342 (1972); C.A., 77, 14795v (1972).
34. Husain, A.; Khan, M.S.Y.; Hasan, S.M.; Alam, M.M.; Eur. J Med. Chem., 40, 1394-1404 (2005). ’
35. Husain, A.; Hasan, S.M.; Lal, S.; Alam, M.M.; Indian .I Pharm. Sci.,68, 536 (2006)
36. Husain, A.; Alam, M.M.; Hasan, S.M.; Yar, M.S.; Acta Poloniae Pharm.-Drug Research, 66, 2, 173-180 (2009). f
37. Husain, A.;A1am, M.M.; Siddiqui, N.; .L Ser. Chem. Soc.,74, 2, 103-115 (2009)f
38. Arayarat, P.; Singh H.; Lattmann, E.; ScienceAsia, 27,121-125 (2001) 39. Shengming, M.; Dehui, D., Faming Zhuanli Shenqing Gongkai Shuomingshu CN, 1, 273, 245; C.A., 135, 61222b (2001).
40. Hachihama, Y.; Shono, T.; Ikeda, S.; .I Org. Chem., 29, 137 (1964)
41. Lattmann, E.; Billington, D.C.; Langley, A.; Drug Design and Discovery, 16, 237-42 (1999)
42. Lee, K. H.; Moore, H. W.; J Korean Chem. Soc.,47, 229-36 (2003)
43. Bassetti, M. A.; Annibale, D.; Fanfoni, A.; Minissi, F.; Org. Lett., 7, 1805-1808 (2005). _
44. Fu,C.; Ma, S.; Eur. .Z0rg. Chem..24, 942-945 (2005)
45. Tejedor, D.; Exposito, A. S.; Tellado, F. G.; Synlett, 10, 1607-1609 (2006)
46. Woodward, R.B.; Singh, G.; J Am. Chem. Soc., 71, 758 (1949).
47. Negishi, E.; Kotora, M.; T etrahedron, 53(20), 6707-6738 (1997).
48. Mallaby, R.; Ryback, G.; .I Chem. Soc., Perkin Trans II, 919 (1972).
49. Tramontini, M.; Angiolini, L., Mannich Bases, Chemistry and Uses,’ CRC Press, Boca Raton, FL, (1994).
50. Arend, M.; Westennann, B.; Risch, N., Angew. Chem. Int. Ed. Engl., 37, 1045 (1998).
51. Ha, H.-J.; Won, K.L., Heterocycles, 57(8), 1525-1538 (2002).
52. Ha, H.-J.; Kang, K.-H.; Ahn, A.-G.; OH, S.-J., Heterocycles, 45, 277 (1997). 53. Kawamori, T.; Tanaka, T.; Hirose, Y.; Satoh, K.; Hara, Y.; Torihara, M.; Yamahara, J .; Tamai, J.; Mori, S., Cancer Lett., 92, 159 (1995).
54. Guillemin, J.-C.; Denis, J.-M., T etrahedron, 44, 4431 (1998).
55. Ha, H.-J.; Choi, C.-J.; Ahn, Y.-G.; Yun, H.; Dong, Y.; Lee, W.K., J Org. Chem., 65, 8384 (2000).
56. Akiyama, T.; Suzuki, M.; Kagoshima, H., Heterocycles, 52,529 (2000).
57. Kinoshita, H.; Inomata, K.; Kondoh, T., Chem. Lett., 1033 (1986).
58. Barluenga, J .; Bayon, A.M.; Asensio, G., .L Chem. Soc., 427 (1984).
59. Shimoda, K.; Kubota, N.; Hirata, T.; Kondo, Y.; Tetrahedron Letters, 48, 1345- 1347 (2007).
60. Krafft, G. A.; Katzenellenbogen, J. A.; J Am. Chem. Soc., 103, 5459 (1981). 61. Sum, F. W.; Weiler, L.; .L Org. Chem., 44, 1012 (1979).
62. Bourguignon, J. J.; Wermuth, C. G., J. Org. Chem., 46, 4889 (1981).
63. Brugel, W.; Stengel, G.; Reichender, F.; Suter, H., Angew. Chem., 68, 440(1956); C.A., 51, 102391(1957).
64. Dauben, W.G.; Hance, P.D., .1 Am. chem. Soc., 7, 3352 (1953).
65. Wiley, P.F.; Weaver, O., J Am. Chem. Soc., 78, 808 (1956).
66. Baddar, F.G.; Sherif, S., .1 Chem. Soc., 2309 (1960).
67. Davey, W., Tivey, D.J., .L Chem. Soc., 1230 (1958).
68. Filler, R.; Leipold, H.A., .L Org. Chem., 27, 4440 (1962).
69. Khattab, S.A.; Shawali, S.A.; Farag, A.M., .I Chem. Engng. Data, 22, 104 (1967).
70. Awad, W.I.; Omran, S. M. A.; Hashem, A.I., .L Chem. U A. R., 10, 287 (1967). 71. Reusch, W.; Starkey, R., .L Org. Chem., 32, 931 (1967).
72. Filler, R.; Hebron, L.M., .Z Am. Chem. Soc., 81, 391 (1959).
73. Hanna, C.; Schuler, F.W., .Z Am. Chem. Soc., 75, 741 (1953).
74. Freeman, R., Mol. Phys., 5, 499 (1962).
75. Wells, P.R., Australian .L Chem., 16, 165 (1963).
76. Porter, Q.N.; Baldas, J., Mass Spectrometry of Heterocyclic Compd., Wiley Interscience, New York (1971).
77. Loughran, E.D.; Wewerka, E.M.; Hammons, GJ., .lf Heterocycl. Chem., 9, 57 (1972).
78. Wewerka, E.M., .L Polym. Sci., 9, 2703 (1971).
79. Pei, W.; Wang, P.; He, M., Rapid Commun. Mass Spectrum., 14, 1 (2000).
80. Otsuka, H.; Kotani, K.; Dando, M.; Kido, M.; Takuda, Y.; Chem. Pharm. Bull., 46, 1180 (1998).
81. Larodk, R. C.; Riefling, B.; Fellows, C.A.J.; Org. Chem., 43, 113 (1978).
82. Marshall, J.A.; Bartley, G.S.; Wallace, E.M.; .Z Org. Chem., 61, 5729 (1996) 83. Ishikawa, T.; Nishigaya, K.; Uchikoshi, H.; Chang, I.S., .L Nat. Prod., 61, 534 (1998).
84. Hartwell, J .L.; Abbot, BJ., Adv. Pharmacol. Chemother., 7117 (1969).
85. Kupchan, S.M.; Eakin, M.A.; Thomas, A.M., J Med. Chem., 14, 1147 (1971). 86. Satomi,Y.; Nishino, H.; Iwashima, A.; Posrihara,M.; Tama1,Y., Anticancer Drug Des., 7, 169 (1972).
87. Dickens, F., Brit. Med. Bull., 20, 96 (1964).
88. Dickens, F.; Cooke, J., Cancer, 19, 404 (1965).
89. Kawamori, T.; Tanaka, T.; Susui, M.; Okamoto, K.; Taqmai, Y.; Torihara, M.;Yamahara, J.; Mo1i,H., Carcinogenesis 16, 795 (1995).
90. Lea K. H.; Huang, B.R.; Tzeng, C. C.; Bioorg Med Ch€l)1.L€flf, 93.41 §\999\
91. Prigent, A.F.; Grouiller, A; Pacheco, H.; Cier,A., Chim. Ther., 7, 329 (1972); C.A., 78, 37903h (1973).
92. Prigent, A.F.; Grouiller, A.; Pacheco, H.R.; Cier, A.A., Ger. Offen., 2, 228, 948, 32 (1972); C.A., 78, 72532h (1973).
93. Kanevskaya, S.I.; Znaeva, K.I., J Gen. Chem., 21, 1726 (1951); C.A., 46, 4520b (1952).
94. El-Said, F., Linnell, W.H., Acta Pharm. Intern., 2, 413 (1951).
95. Chemnitius, K.H., Artch. Exptl. Pathol. Pharmakol., 236, 292(l959).
96. Sontag, K.H., Acta Biol. Et. Med. Ger., 6, 110 (1961); C.A., 55, 13679c (1961). 97. Chanh, P.H.; Schwadrohn, G.; Couquelet, J .M.; Chanh, A.P.H.; Nguyen, V.T.M., Ann. Pharm. Fr., 39, 215 (1981).
98. Fieser, L.F.; Fieser, M.,"steroid", Reinhold Publishing Co., New York, 727 (1959).
99. Nakamura, T.; Goda,Y.; Sakai, S.; Kondo, K.; Akaiyama, S.; Toyoda, M., Phytochemistry, 49, 2097 (1998).
100. Eric, L.; Derek, K.; Harjit, S.; Isidro, M.; Adiba, B.; Washington, A.; Micheal, JIT, Pharm. Pharmacol. Lett., 11, 5-8
101. Andrew, F.; Elizabeth, F.; Anne-Geraldine, G., PCT Int. Appl. W0, 01, 44, 221; C.A., 135, 46078r (2001).
102. Cryer, B.; Dubois, A., Prostaglandins Other Lqnid Mediators, 56, 341 (1998). 103. Boltze, K.H., Reihe, 545(1955); C.A., 50, 15912e (1956).
104. Hohnann, H.; Henning, K., Reihe, 5S1(1955); C.A., 50, 15912f (1955).
105. Saytzcfi, A., Justus Liebigs Ann. Chem., 171, 258 (1874).
106. Kunes, J .; Balsanek, V.; Pour, M., Collection of Czechoslovak Chemical Communications, 66(12), 1809-1830 (2001).
107. 1Rubin, B.A.; Giarman, N.J., .Z Biol. Med., 19, 1017 (1947).
108. Roth, R.H.; Giarman, N.J., Biochem. Pharmacol., 19, 1087 (1970).
109. Doherty, J .D.; Hattox, S.E.; Snead, O.C.; Roth, R.H., J. Pharmacol. Exp. Ther., 207, 130 (1978).
110. Klunk, W.E.; Covey, D.F.; Ferrendelli, J .A., Mol. Pharmacol., 22, 431 (1982). 111. Laborit, H.; Jouany, J .;Gerard, J .; Fabiani,P.; Neuro-Psycho -pharmacol., Proc., 2,490 (1961).
112. Gessa, G.L.; Vargia, L.; Crabai, F.; Boero, G.C.; Caboni,F.; Camba, R .; Ly’e sci. 5,1921 (1966) '
113. Klunk, W.E.; Covey, D.F.; Ferrendelli, J .A., Mol. Pharmacol., 22, 438 (1982). 114. Klunk, W.E.; Covey, D.F.; Ferrendelli , J .A., Mol. Pharmacol., 22, 444 (1982). 115. Klunk, W.E.; Mckeon, A.C.; Covey, D.F.; Ferrendelli, J.A., Science, 217, 1040 (1982).
116. Peterson, E.M.; Xu, K.; Holland, K.D.; Mckeon, A.C.; Fen'ende1li, J .A.; Covey, D.F.; Rothman, S.M., J Med. Chem., 37, 275 (1984).
117. Naresh, K.‘,Roger, WR., PCT Int. Appl. WO, 02, 00,639; C.A., 136 , 69697b (2002).
118. Roger, R.; Naresh, K.; Mark, W.; Hua, Z,; Hans, G.; Ben, M.; Helmut, T.; Tim, H., PCT Inc. Appl. WO, 01, 76, 594; C.A., 135, 308957Z (2001).
119. Milan, P.; Marcel, S.; Vladimir, B.; Petra, K.; Marie, V.; Vladimir, W.; Helena, F.; Pter, K.; Hana, P.; Radan, S., .L Med. Chem., 44, 2701-2706 (2001); C.A., 135, 257083b (2001).
120. Gein,V.L.; Gein, L.F.; Porseva,N.Y.; Voronina, E.V.; Vakhrin, M.I.; Potemkin, K.D.; Ko11a,V.E.; Drovosekova, L.P.; Milyutin, A.V.; Schukalina, N.S.; Veikhman, G.A., Khim. Farm. Zh., 32, 23 (1998); C.A., 130, 153527e (1990).
121. Kozminykh, V.O.; Igidov, N.M.; Kozminykh, E.N.; Aliev, Z.G., Pharmazie, 48, 99 (1993).
122. Kozminykh, V.O.; Igidov, N.M.; Kozrninykh, E.N.; Semenova, Z.N.; Andreichikov,Y., Pharmazie, 47, 261(1992).
123. Caiding, X.; Ei-Ichi, N., T etrahedron Lett., 40, 431 (1999).
124. Ishikawa, T.; Nishigaya, K.; Uchikoshi, H.; Chang, I.S., .L Nat. Prod., 61, 534 (1998).
125. Lattmann,E.; Billinton, D.C.; Langley, C.A., Drug Des. Discov. 16,243 (1999). 126. Manojit, P.; Koteswar, R.Y.; Rajagoplan, R.; Parimal, M.; Prem, K.M.; Seshagiri, R.C., PCTInt. Appl. WO, 01, 90, 097; C.A., 136, 5893e (2002).
127. Konaklieva, M.I.; Plotkin, B.J.; Mini- Rev. Med Chem., 73-95 (2005)
128. Lorenzo, M.; Brito, I.;Cueto,M.; Organic Letters, 8, 22, 5001-5004 (2006)
129. Siddiqui, B.S.; Afshan, F.; Faizi, S.; Taeiq, R.M.; .L Chem. Soc Perkin Trans, 1, 2367-2370 (1999).
130. Michiyoshi, M.; Masahiro, K.; Mikio, O., Jpn. Kokai T okkyo Koho JP, 278, 708 (2001); C.A., 135, 268773c (2001).
131. Reiner, F.; Thomas, B.; Christoph, E., Ger. 0/Yen. DE, 10, 008, 510; C.A., 135, 191667m (2001).
132. Keisuke, S.; Yutaka, K.; Akio, H., Jpn. Kokai T okkyo Koho JP, 163, 719(2oo1); C.A., 135, sossvw (2001).
133. Ndagijimana, M.; Vallicelli, M.; Cappa, F.; Lanciotti, R.; Appl Environ Mzcrobzo 72, 9, 6053-6061 (2006).
134. Tsutomo, S.; Jun, Y.; Lakoto, S.; Kouichi, K.; Takanao, M.; Mamoru, K.; Takuro, K.; Kenji, M., Biosci. Biotechnol. biochem., 62, 1865 (1998).
135. Han, B.H.; Yang, H.O.; Kang, Y.H.; Suh, D.Y.; Go, H.J.; Song, W.J.; Kin, Y.C.; Park, M.K.; Farmaco, .I Med. Chem., 41, 2626 (1998).
136. Evans, D.A.; Trotter, B.W.; Borrow, J .C., Tetrahedron, 53, 8779 (1997).
137. Rucker, G.; Hostettmann, K.; Gajewski, W.; Lobbert, M.; Boken, P.,Arch. Pharm. (Weinheim), 326, 941 (1993).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









