{ DOWNLOAD AS PDF }
ABOUT AUTHORS:
Patel Vishakha. D.*, Raj Hasumati, Gheewala Nirav
Department of Quality Assurance,
Shree Dhanvantary Pharmacy College, Kim, Surat
vishuk7293@gmail.com
ABSTRACT
Ranolazine is a piperazine derivative is a new anti-ischemic drug for the treatment of angina.
Ranolazine is to inhibit late INa thus preventing sodium overload of the cell. As a consequence, ranolazine prevents reverse mode sodium–calcium exchange and thus diastolic accumulation of calcium possibly resulting in improved diastolic tone and improved coronary blood flow.
This review article represent the various analytical methods which has been reported for estimation of Ranolazine in synthetic mixture. The spectrophotometric techniques like fluorescent assay and area under curve spectroscopy; Chromatogrraphic methods like HPLC, HPTLC and RP HPLC, GC, LC-MS, LC-MS/MS were reported.
INTRODUCTION(1):
Ranolazine is -(2,6-dimethylphenyl)-2{4-[2-hydroxy-3-(2-methoxyphenoxy)propyl piprazine-1-yl}acetamideis piprazine derivative appears as white to off white crystalline powder. The drug is freely soluble in Methanol. Ranolazine is a strong base with pKa values of 13.6,Six-membered Piprazine Ring. Ranolazine melts at 122-124 degree C.
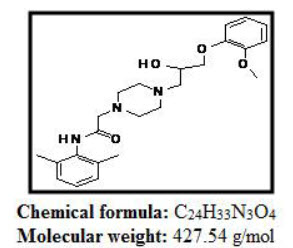
Figure:1 Structure of Ranolazine
MECHANISM OF ACTION(2)
Ranolazine a piperazine derivative is a new anti-ischemic drug for the treatment of angina.Ranolazine is to inhibit late INa thus preventing sodium overload of the cell. As a consequence, ranolazine prevents reverse mode sodium–calcium exchange and thus diastolic accumulation of calcium possibly resulting in improved diastolic tone and improved coronary blood flow.
As a late INa inhibitor, ranolazine was also shown to increase action potential duration and thus modestly QT interval by 2–5 ms. This effect, however, is not heart rate-dependent and cannot be exaggerated during bradycardia. Furthermore, ranolazine does not induce early after depolarization and does not increase dispersion of repolarization across the left ventricular wall.(2)
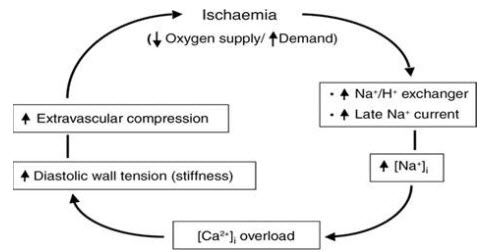
Figure 2: Mechanism of Ischaemia
It is act via selective inhibition of the late inward sodium current (INa) in cardiac muscle cells. This reduces intracellular sodium accumulation and calcium overload, and consequently improves myocardial relaxation and decreases left ventricular diastolic stiffness.
Ranolazine is administered orally and metabolize by CYP3A and excreted in intestine (5%) and in urine
1. Analytical Method
A. CompendialMethod:
Ranolazine is not official in Pharmacopoeia.
B. Reported Method:
I. Chromatographic Methods:
The high-pressure liquid chromatography (HPLC)for Ranolazine estimation. GC method for residual solvent determination in Ranolazine. HPTLC method are widely used chromatographic methods in the analysis of Ranolazine in Formulation. LC-MS/MS, LC-MS and UHPLC use for estimation of Ranolazine in Plasma. RP HPLC method also developed for determination of concentration of Ranolazine in human serum and also for simultaneous determination of Ranolazine and Dronederone.
Table No.1: Summary of Chromatographic Method of Ranolazine
|
Title |
Method |
Mobile phase |
Stationary phase |
Wave Length |
Reference |
|
Ranolazine in bulk & marketed formulation |
HPLC & UV |
Methanol : 0.5% tri ethyl amine pH 6 with orthophosphoric acid (75:25) |
- |
271 |
3 |
|
Estimation of Ranolazine HCL in Tablet Dosage Form |
RP-HPLC |
Buffer : Acetonitrile(60:40),(pH adjust with triethylamine |
Inertsil ODS C18 |
224 nm
|
4 |
|
Determination of Ranolazine HCL in bulk and dosage form |
LC |
Methanol : water (99:1 %,V/V) |
HiQ Sil C18HS |
273 nm |
5 |
|
Quantitation of Ranolazine in rat plasma |
LC |
Acetonitrile : water : formic acid : 10% n-butylamine (70:30:0.5:0.08, v/v/v/v) |
Nova-Pak C18 column
|
- |
6 |
|
Determination of Ranolazine in human plasma |
HPLC |
Acetonitrile: 0.1% formic acid(90?10) |
Agilent-ZORBAX C18 column |
- |
7 |
|
Estimation of Ranolazine in Human Plasma |
LC |
methanol–10mM ammonium acetate (60:40 v/v, pH 4.0) |
Zorbax extend C18 column
|
- |
8 |
|
Ranolazine HCL in bulk and tablet dosage form |
HPTLC |
Chloroform: methanol : toluene (5 : 1 : 1 v/v/v) |
silica gel aluminium plate 60 F – 254 |
273 nm |
9 |
|
Determination of residual solvents in Ranolazine |
GC |
- |
HP-INNOWAX column |
- |
10 |
II. UV spectroscopic method
First order derivative spectroscopy and Area Under curve spectroscopic technique was developed for simultaneous determination of Ranolazine was developed.
Table No.2: Summary of UV spectroscopic method
|
Title |
Method |
Wavelength |
Linearity and R2 |
Recovery |
REF. |
|
Estimation of Ranolazine in bulk drug and pharmaceutical formulation |
UV method |
272 nm |
10-100 µg/ml |
99.77-100.33 % |
11 |
|
Estimation of Ranolazine in bulk and pharmaceutical dosage form |
First order derivative spectroscopic method |
263 nm and 282 nm |
10-35 µg/ml and 0.9992 |
- |
12 |
|
Estimation of Ranolazine in API and tablet formulation |
Area under curve method |
261nm and 281 nm |
75-200 µg/mland 0.998 |
99.42-99.97 % |
13 |
Table No.3: HPLC Method for simultaneous estimation of Ranolazine and Dronederone
|
Title |
Method |
Mobile phase |
Stationary phase |
Wave length |
Ref. |
|
Simultaneous estimation of Ranolazine and Dronederone in bulk and pharmaceutical dosage forms. |
HPLC |
0.02N NH2PO4 buffer (pH 4) : Acetonitrile (50 :50 V/V) |
ODS column |
282 nm
|
14 |
Discussion
Presented systematic review covers the current analytical methods for the determination of Ranolazine and its combination in pharmaceutical and biological samples like serum and plasma. HPLC method were found to be most widely use for Ranolazine. Various chromatographic conditions are presented in table.
Conclusion
The sensitivity, specificity, and better separation efficiency enable HPLC to be used frequently for simultaneous qualitative and quantitative determination of Ranolazine. The presented information is useful for the future study for researcher involved in formulation development and quality control of Ranolazine.
REFERENCES:
1. Ranolazine Drug Info.(database available on internet):Drug Bank. Available from: drugbank.ca/drugs/DB00243 ran Ranolazine Drug Info.(database available on internet):Chemical Book. Available from: pubchem.ncbi.nlm.nih.gov/compound/ranolazine
Ranolazine Drug Info.(database available on internet):Chemical Book. Available from: scbt.com/datasheet-212769-ranolazine.html
2. Hasenfuss G, Maier L.S, “Mechanism of action of the new anti-ischemia drug ranolazine.” Clinical Research Cardiology, 2008, 97(4), 222-226
3. Parvathareddy S, Desam N, Nuthalapati M, B.T, “Development and Validation of Hplc and UV methods for estimation of Ranolazine in Bulk and Marketed formulation.” International journal of Innovative Pharmaceutical Sciences and Research, 2014, 2(5), 1042-1058
4. Patel R.C, Rathod D.K, Patel P.R, Patel V.S, “Estimation of Ranolazine Hydrochloride by Spectrophotometric and RP-HPLC in tablet dosage forms.” International Journal of Pharmaceutical and Appiled Sciences, 2010, 1(2), 79-83
5. Sharma T, Moitra S.K, Si.S.C, Sankar D.G, “Stability indicating LC method for the determination of Ranolazine Hydrochloride in the bulk drug and in pharmaceutical dosage form.” International Journal of Pharmacy and Pharmaceutical Sciences, 2011, 3(4), 327-332
6. Lei T, Juanjuan J, Yiling H, Lu H, Hong L, Yishi L, “Sensitive quantification of Ranolazine in human plasma by liquid chromatography-tandem mass spectrometry with positive electrospray ionization.” Journal of Chromatography B, 2007, 846(1-2), 346-350
7. Bai S, Gao H, Qu H, Liang Y, Li Y, Zheng Z, Wang X, Hao G, “Quantitative determination of Ranolazine in human plasma by high performance liquid chromatography-tandem mass spectrometry.” Northwest Pharmaceutical Journal, 2011
8. Zhao L, Li H, Jiang Y, Piao R, Li P, Gu J , “Determination of Ranolazine in human plasma by liquid chromatographic-tandem Mass Spectrometric assay.”Journal of Chromatographic Science, 2008, 46(8), 697-700
9. Khedkar A.N, Veer S.U, Rakh M.S, Rao J.R, “Stability indicating method development and validationof Ranolazine Hydrochloride in bulk and tablet dosage form by HPTLC.” International Journal of Pharmaceutical and Clinical Research, 2015, 7(1), 77-83
10. Ye YL, Yang XM, “Determination of residual solvent in Ranolazine by headspace gas chromatography.” Nan Fang Yi Ke Da Xue Bao, 2008, 28(1), 134-135
11. J.K, K.R, R.K, N.S, “Method development and Validation for the Estimation of Ranolazine in Bulk and in Pharmaceutical Dosage Form by UV-Spectrophotometry.”Annals of Pharma Research , 2013, 1(1), 4-7
12. Ugale J. B, Mulgund S. V, “Development and Validation of UV Spectrophotometric Area under cure method for Quantitative Estimation of Ranolazine in API and Tablet Formulation.”World journal of Pharmaceutical Research, 2015,4(5), 2665-2672
13. Guada M, Imbuluzqueta E, Estella-Hermoso de Mendoza A, Lana H, Dios-vieitez M.C, Blanco-Prieto M.J, “Ultra high performance liquid chromatography-tandem mass spectrometry method for cyclosporine a quantification in biological samples and lipid nanosystems.”J Chromatogr B Analyt Technol Biome Life Sci., 2013, 927, 164-172
14. Nahid A, Pavani H, “Development and Validation of HPLC method for simultaneous dtermination of Ranolazine and Dronederone in bulk and pharmaceutical dosage forms.” Indian Journal of research in Pharmacy and Biotechnology, 2015, 2(6), 1524-1528
REFERENCE ID: PHARMATUTOR-ART-2399
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 4, Issue 4 Received On: 11/10/2015; Accepted On: 21/10/2015; Published On: 01/04/2016 How to cite this article: Patel VD, Raj H, Gheewala N; A Review on Analytical Methods for Ranolazine determination in synthetic mixture; PharmaTutor; 2016; 4(4); 28-31 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









