ABOUT AUTHORS:
Vishwanadham Yerragunta*, Prathima Patil, V.Anusha, T.Kumara Swamy, D.Suman, T.Samhitha.
Department of Pharmaceutical Chemistry,
Malla Reddy College of Pharmacy,
Maisammaguda, secunderabad-14. A.P.
Vishwanadham.y@gmail.com
{ DOWNLOAD AS PDF }
ABSTRACT:
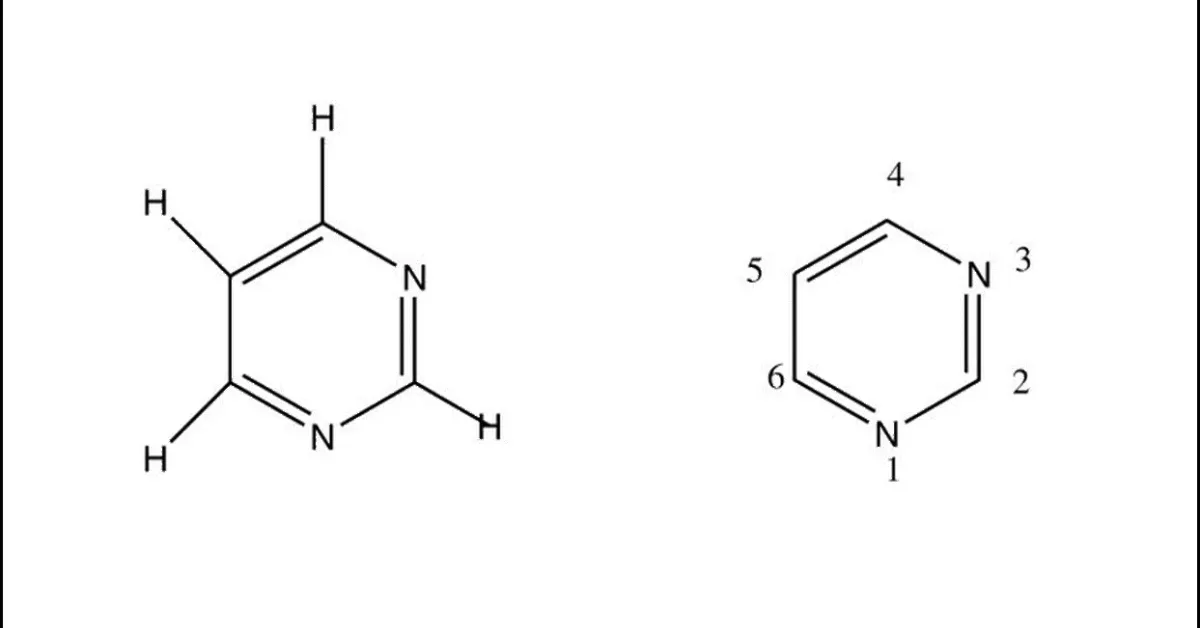
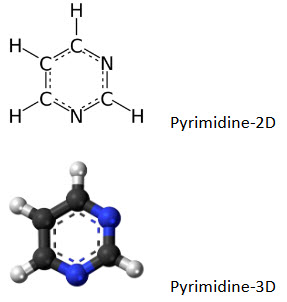
Pyrimidine is a heterocyclic aromatic organic compound containing two nitrogen atoms at positions 1 and 3 of the six- member ring shows wide range of biological activities. Pyrimidine posses wide spectrum of biological activities like including anti-tubercular, anti-bacterial, anti-fungal, anti-viral, anti-inflammatory, Anti-malarial activity, anti-cancer and anti-neoplastic activity, anti-hiv activity. The present reviews attempted to gather the various biological activities of pyrimidine derivatives and is marketed drugs.
REFERENCE ID: PHARMATUTOR-ART-2066
INTRODUCTION:
Pyrimidineis an aromatic heterocyclic organic compound similar to pyridine.[1] The pyrimidine ring system has wide occurrence in nature[2] as substituted and ring fused compounds and derivatives, including the nucleotides, thiamine (vitaminB1) and alloxan. It is also found in many synthetic compounds such as barbiturates and the HIV drug, zidovudine. Although pyrimidine derivatives such as uric acid and alloxan were known in the early 19th century,
Pyrimidine nucleus exhibited remarkable pharmacological activities. Pyrimidine derivatives form a component in a number of useful drugs and are associated with many biological and therapeutical activities[3]. Nitrogen containing heterocyclic ring such as pyrimidine is a promising structural moiety for drug design. Condensed pyrimidine derivatives have been reported as anti-microbial[4], analgesic, anti-viral, anti-inflammatory[5], anti-HIV[6], anti-tubercular[7], anti-tumour[8], anti-neoplastic[9], anti-malarial[10], diuretic[11], cardiovascular[12] agents. Pyrimidine compounds are also used as hypnotic drugs for the nervous system [13].
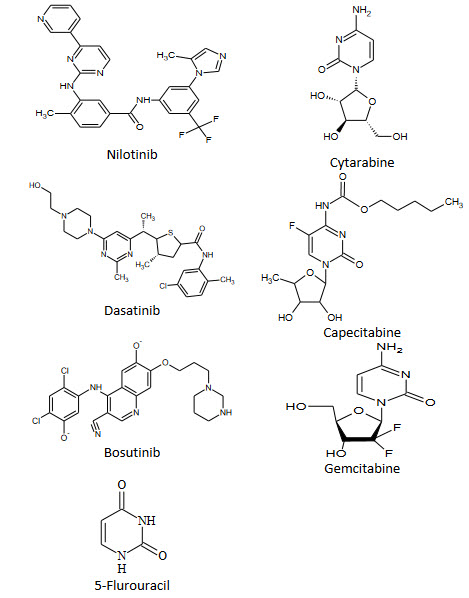
In medicinal chemistry pyrimidine derivatives have been very well known for their therapeutic applications. The presence of a pyrimidine base in thymine, cytosine and uracil, which are the essential binding blocks of nucleic acids, DNA and RNA is one possible reason for their activity. The literature indicated that the compounds having pyrimidine nucleus possess broad range of biological activities. Like 5-fluorouracil as anticancer; idoxuridine and trifluoridine as antiviral; zidovudine and stavudine as anti-HIV, trimethoprim, sulphamethiazine and sulphadiazine as antibacterial; sulphadoxin as antimalarial and antibacterial; minoxidil and prazosin as antihypertensive; barbiturates e.g. phenobarbitone as sedative, hypnotics and anticonvulsant; propylthiouracil as antihyroid; thionzylamine as H1-antihistamine; and toxoflavin and fervennuline as antibiotics.
Most drugs in the pyrimidine series fall in to four categories; the barbiturates, the sulphonamide; the antimicrobials and antitumor agents.
Frederick C et al [14]., synthesized a series of inhibitors of mammalian target of Rapomycin (mTOR) kinase based on a quaternary-substituted dihydrofuropyrimidine by cyclocondensation of β-keto ester. The compound with 4-acetamido pyrazole moiety was found to be most potent.(I)
Barbituric acid was made conveniently from diethyl malonate and urea in ethanolic sodium ethoxide[15] and it has a variety of biological properties. Luminol (II) was prepared in 1904 but used as a long active CNS depressant only from 1912 until the present day.
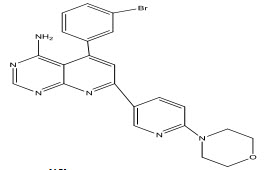
Lee et al[16] synthesized and studied some 6-substituted pyridopyrimidine analogous as potential AK inhibitors, led to the identification of 4-amino-5-(3-bromophenyl)-7-(6-morpholinopyridin-3-yl)pyrido[2,3-d]-pyrimidine (III), a novel and potent non-nucleoside AK inhibitor with oral activity in animal models of pain and inflammation The ABT-702 was further studied in details by Boyle et al[17] to evaluate its potential utility in chronic inflammation.
Bruno et al[18] synthesized two different series N-methyl-N-pyrimidin-2-yl glycine and N-5H-[1]benzopyrano[4,3-d]pyrimidin-2-yl (IV) substituted amino acids and tested for anti-inflammatory activity. All the compounds showed significant anti-inflammatory activity.
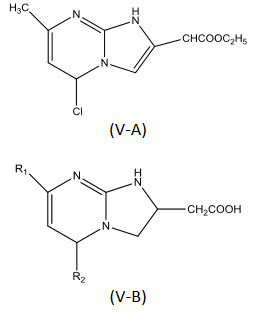
Sacchi et al[19] synthesized a series of imidazo[1,2-a] pyrimidine 2-carboxylic acid and 20 acetic acid analogs (V-A, V-B) and tested them for anti-inflammatory activity. Almost all the carboxylic acid derivatives showed aremarkable anti-inflammatory activity.
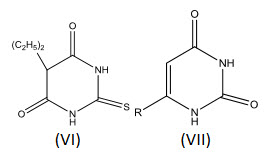
Hyperthyroidism may be treated in several ways. One of these is interference with the synthesis of the thyroid hormones, possibly or by removal of iodine. Thiouracil (VI) and thiobarbital (VII) are effective thyroid drugs. Compound (VI) is widely used probably because it has fewer side effects than the others[20].
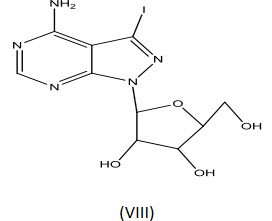
Cottom et al[21] were synthesized several pyrazolo[3,4-d] pyrimidine derivatives as potential inhibitor of adenosine kinase. One of the compounds (VIII) was found to display good anti-inflammatory activity.
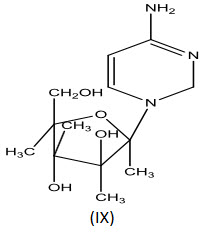
Cytosine arabinoside (IX) is established drug for the treatment of acute leukemia's of childhood and adult granulocytic. It has also incidental antiviral activity against herpes and herpes zaster types[22].
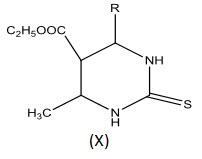
El-Gaby, Adel-Hamide and Gharab[23] prepared some new pyrimidine-2-thiones (X). Some of these compounds were tested for in vitro anticancer activity against Ehrlich Ascites Carcinoma Cells.
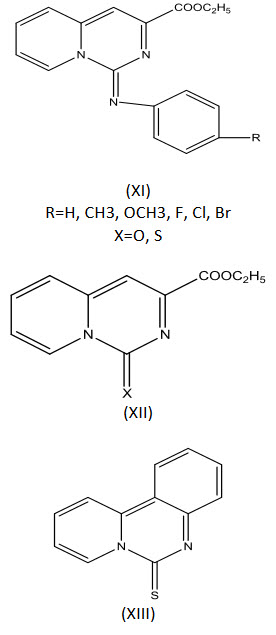
Molina et al[24] synthesized a number of pyrido[1,2-C] pyrimidines (XI-XIII) and tested for effects on leukocyte function in vitro and anti-inflammatory activity.
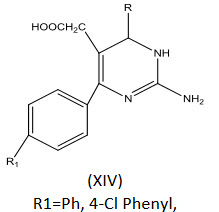
Bahekar et al[25] reported the synthesis of some [2-amino-6-(4-substituted aryl)-4-(4-substituted phenyl)-1,6-dihydropyrimidin-5-yl]acetic acid derivatives (XIV) and evaluated for anti-inflammatory activity. Only few of them showed remarkable anti-inflammatory activity.
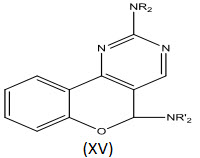
Bruno et al[26] reported the synthesis of some new 2,5-cycloamino-5H - benzopyrano[4,3-d]pyrimidines (XV) and screened them for anti-inflammatory, analgesic and antipyretic activities and in vitro antiplatelet activity. All the compounds failed to exhibit anti-inflammatory, analgesic and antipyretic activities but they showed an interesting antiplatelet activity
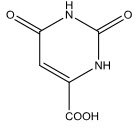
Orotic acid[27], a simple pyrimidine derivative and its mineral forms are used in metabolic therapy, especially for cardiovascular patients to prevent heart failure in car- diomyopathy. Oroate is needed as a key intermediate in biosynthesis of pyrimidine nucleotides, which are building blocks for DNA and RNA required for the final protein synthesis.
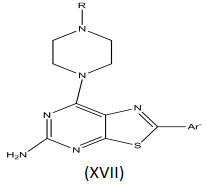
Yuga O et al[28] synthesized a series of pyrrolo[3,2-d] pyrimidine derivatives and evaluated their application as type-II inhibitors of vascular endothelial growth factor receptor 2 (VEGFR2) kinase. Incorporation of diphenylurea moiety at C 4-position of pyrrolo [3, 2-d] pyrimidine core via an oxygen linker (Fig. 23) resulted in potent inhibitors.
Marketed Drugs Containing Pyrimidine Nucleus:-
Anti-inflammatory & Analgesic agents:
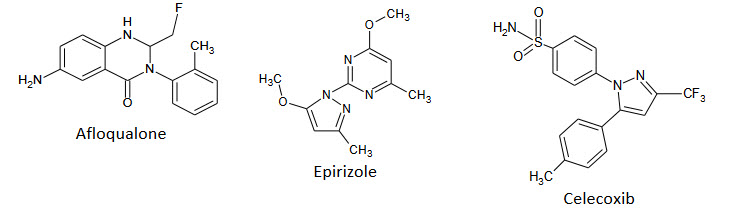
Marketed Drugs Containing Pyrimidine Nucleus:-
Anticancer Agents:-
Prazosine: a sympatholytic drug used to treat high blood pressure and anxiety, PTSD, and panic disorder.
CONCLUSON:
The reviewed pyrimidine moiety has shown a wide spectrum of biological activities. The various substituted pyrimidine derivatives have been reported as anti-microbial, analgesic, anti-viral, anti-inflammatory, anti-HIV, anti-tubercular, anti-tumour, anti-neoplastic, anti-malarial, diuretic, cardiovascular agents and also used as hypnotic drugs for the nervous system. Now a day’s Pharmaceutical companies are produced pyrimidine derivatives drugs, such as marketed drugs are mentioned above. The versatile synthetic applicability and biological activity of these heterocycles compounds will help the medicinal chemists to plan, organize and implement new approaches towards discovery of novel drugs.
REFERNCES:
1. Gilchrist, Thomas Lonsdale; Gilchrist, T. L. Heterocyclic chemistry. New York: Longman. (1997).
2. Lagoja, Irene M.. "Pyrimidine as Constituent of Natural Biologically Active Compounds". Chemistry and Biodiversity 2 (1): 1–50, (2007).
3. Patel, R.; Desai, K.; Chikhalia, K. J. Ind. Chem. Soc. 80 , 138(2003).
4. Desai, K.; Patel, R.; Chikhalia, K. J. Ind. Chem. 45 (B), 773(2006).
5. Amr, A.E., Nermien, M.S., Abdulla, M.M. Monatsh. Chem. 138, 699. (2007).
6. Fujiwara, N., Nakajima, T., Ueda, Y., Fujita, H., Kawakami, H. Bioorg. Med. Chem. 16, 9804. (2008).
7. Ballell, L., Field, R.A., Chung, G.A.C., Young, R.J. Bioorg. Med. Chem. Lett. 17, 1736. (2007).
8. Wagner, E., Al-Kadasi, K., Zimecki, M., Sawka-Dobrowolska, W. Eur. J. Med. Chem. 43, 2498. (2008).
9. Cordeu, L., Cubedo, E., Bandres, E., Rebollo, A., Saenz, X., Chozas, H., Victoria Domínguez, M., Echeverria, M., Mendivil, B., Sanmartin, C. Bioorg. Med. Chem. 15, 1659. (2007).
10. Gorlitzer, K., Herbig, S, Walter, R.D. Pharmazie 52, 670, (1997).
11. Ukrainets, I.V.; Tugaibei, I.A.; Bereznykova, N.L.; Karvechenko, V.N.; Turov, A.V. Chemistry of Heterocyclic Compounds 5, pg-565 (2008).
12. Kurono, M.; Hayashi, M.; Miura, K.; Isogawa, Y.; Sawai, K. Sanwa Kagaku Kenkyusho Co., Japan , Kokai Tokkyo Koho, Chem. Abstr. (1988).
13. Wang, S.Q.; Fang, L.; Liu, X.J.; Zhao, K. Chinese Chem. Lett. 15, pg-885 (2004).
14. C Frederick, B Philippe, B Elizabeth, KB Krista, C Huifen, GD Antonia, AE Jennifer, FTK Michael, L Kevin, L Cristina, L Lichuan, QL Cuong, M Shiva, N Jim, FO Daniel, P Zhonghua, DR Kirk, S Steve, T Lan, T Tom, W Jiansheng, Z Xianrui, PL Joseph, J. Med. Chem. 54(9), 3426-3435, (2011).
15. J. B. Dickey and A. R. Gary, Org. Syntch. Coll. Vol., 2, 62 (1943).
16. C. H. Lee, M. Jiang, M. Cowart, M. F. Jarvis and S. S. Bhagwat, J. Med. Chem., 44, 2133 (2001).
17. D. L. Boyle, E. A. Kowluk, M. F. Jarvis, C. H. Lee and S. S. Bhagwat, J. Pharmacol. Exp. Ther., 495, 296 (2001).
18. O. Bruno, S. Schenone, A. Ranise, F. Bondavalli, W. Filippelli and F. Mazzeo, II Farmaco, 54, 95 (1999) .
19. A. Sacchi, S. Laneri, F. Arena, E. Luraschi and F. Rossi, Eur. J. Med. Chem., 32, 677 (1997)
20. P. Libert and J. B. Stanbury, Annu. Rev. Pharmacol., 11, 113 (1971).
21. H. B. Cottom, D. B. Wasson, H. C. Shih, G. D. Pasquale and D. A. Carson, J. Med. Chem., 36, 3424 (1993).
22. P. Calabresi and R. E. Parks, Jr. in 'The Pharmacological Basis of Therapeutics ed.’ L. S. Goodman and A. Gilman, Macmillan, New York, 5th ed., 1254 (1975).
23. M. S. El-Gaby, S. G. Abdel-Hamide and M. M. Gharab, Acta. Pharm., 49 (3), 149 (1999); Chem. Abstr, 132, 93278d (2000).
24. P. Molina; E. Aller, A. Lorengo, P. L. Cremadis, I. Rioja and M. J. Alcaraz, J. Med. Chem., 44, 1011 (2001).
25. S. S. Bahekar and D. B. Shinde, Acta Pharm., 53, 223 (2003).
26. O. Bruno, C. Brullo; A. Ranise, S. Schenone, S. Bondavalls, M. Tognolini and M. Impicciatore, Bioorg. Med. Chem. Lett., 11, 1379 (2001).
27. Jones, M. E., Annu. Rev. Biochem., , 49, 233–279, (1980).
28. O Yuga, M Naoki, O Kengo, T Terufumi, I Hidehisa, A Yoshiko, M Hiroshi, H Akira, K Keiji, I Shinichi, Bioorg. Med. Chem.18 (20), 7260-7273 (2010).
|
PharmaTutor (ISSN: 2347 - 7881) Volume 1, Issue 2 Received On: 22/11/2013; Accepted On: 26/11/2013; Published On: 20/12/2013 How to cite this article: Yerragunta V, Patil P, V.Anusha, T.KumaraSwamy, D.Suman, T.Samhitha, Pyrimidine and Its Biological Activity: A Review, PharmaTutor, 2013, 1(2), 39-44 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE