ABOUT AUTHORS:
ABOUT AUTHORS:
Edukondalu.Vanka*, M. Jhansi Rani, A.M.Sudhakar Babu, P.Venkatesawara Rao
Department Of Pharmaceutics,
A.M. Reddy Memorial College of Pharmacy,
Narasaraopet, Guntur (district), Andhra Pradesh
7yedu7@gmail.com
{ DOWNLOAD AS PDF }
ABSTRACT:
Novel Oral Drug Delivery technologies have emerged and expanded into different drug delivery System. Among them Pulsatile Drug Delivery Systems are gaining a lot of interest as they deliver the drug at the right place at the right time and in the right amount, thus providing spatial and temporal delivery and increasing patient compliance. The drug delivery is designed according to the circadian rhythm of body. System is suit for the drugs which shows high first pass effect. A pulse has to be designed in such a way that a complete and rapid drug release is achieved after the lag time. Various systems like Time controlled pulsatile release systems, Stimuli-induced pulsatile release system, Externally regulated pulsatile release system, Multiparticulate regulated pulsatile release system have been dealt with in the article. These delivery occurs at predetermined lag time. These systems are beneficial for the drugs having chronopharmacological behavior where night time dosing is required, such as anti-arhythmic and anti-asthmatic.
REFERENCE ID: PHARMATUTOR-ART-2043
Introduction
Oral controlled drug delivery systems represent the most popular form of controlled drug delivery systems for the accessible advantages of oral route of drug administration. Such systems shows either constant or variable drug release rates. The oral controlled release system shows nearly stable plasma drug level without much fluctuations, reduction in dose of drug, reduced dosage frequency, among them drug concentration is maintained in the therapeutic window for a prolonged period of time, termed as sustained therapeutic action1.
And those drug release which maintain plasma drug level within therapeutic range Are termed as Pulsatile release dosage forms shows lag time. i.e., Chronopharmacotherapy of diseases which shows Circadian rhythms in their pathophysiology. Several disease states have been proven to follow biological rhythms, expressed by short, intermediate, and long-period oscillations.2.3

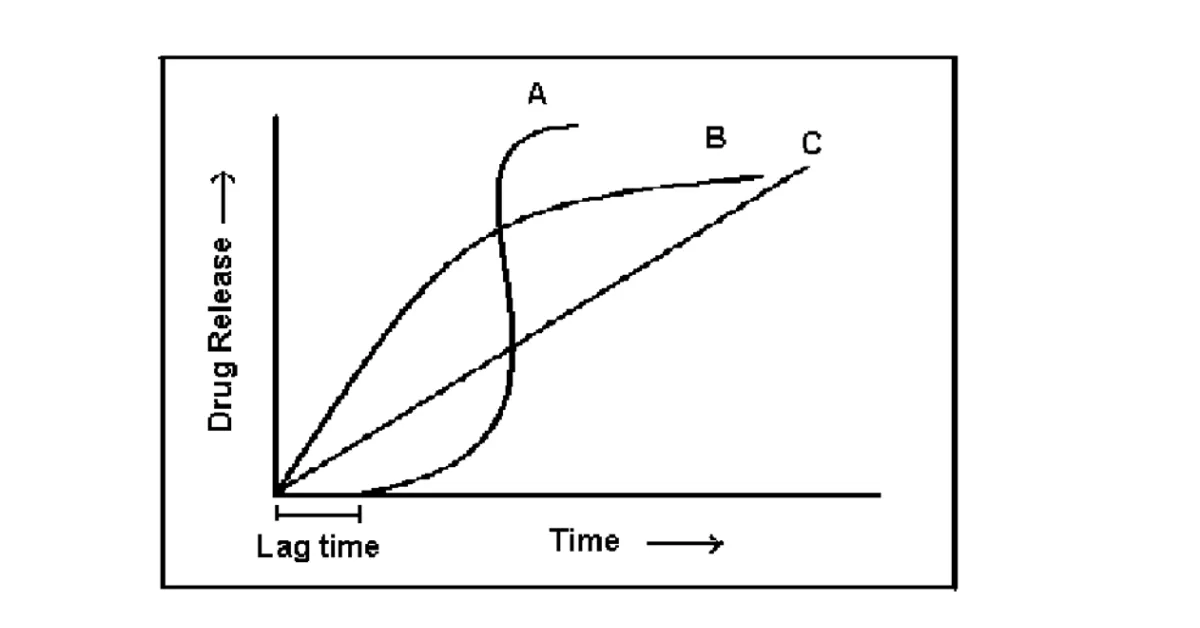
Figure: 1. shows Schematic representation of different drug delivery systems where (A) sigmoidal release after lag time (B) delayed release after lag time (C) sustained release after lag time (D) extended release without lag time.
[adsense:468x15:2204050025]
PDDS gaining importance in following situations:4.5
a) circadian rhythm which fallows body functions i.e..alter in their release e.g: Secretion of hormones, acid secretion in stomach, gastric emptying, and gastrointestinal blood transfusion.
b) Circadian rhythms shows in diseases, some of these disease worst conditions in morning , others in nights pathophysiology like bronchial asthma, myocardial infarction, angina pectoris, rheumatic disease, ulcer, and hypertension.
c) Drugs that produce biological tolerance demand for a system that will prevent their continuous presence at the biophase as this tends to reduce their therapeutic effect.
d)The lag time is essential for the drugs that undergo degradation in gastric acidic medium (e.g.: peptide drugs) and irritate the gastric mucosa or induce nausea and vomiting.
e) Targeting a drug to distal organs of gastro-intestinal tract (GIT) like the colon requires that the drug release is prevented in the upper two-third portion of the GIT.
f) The drugs that undergo first-pass metabolism resulting in reduced bioavailability, altered steady state levels of 109 drug and metabolite, and potential food drug interactions require delayed release of the drug to the extent possible. All of these conditions demand for a time controlled therapeutic scheme releasing the right amount of drug at the right time. This requirement is fulfilled by Pulsatile Drug Delivery Systems.6
ADVANTAGES of PDDS:
1. Nearly constant drug levels at the site of action.
2. Avoidance of undesirable side effects.
3. Reduced dose.
4. Improved patience compliance.
5. Used for drugs with chronopharmacologcal behavior.
6. No risk of dose dumping.
7. Improved bioavailability, tolerability and reduces side effects.7.8
LIMITATION:
1. Lack of manufacturing reproducibility and efficacy.
2. Large number of process variables.
3. Multiple formulation steps.
4. Higher cost of production.
5. Need of advanced technology.
6. Trained/ skilled personal needed for manufacturing.
Diseases Requiring Pulsatile Delivery
Recent studies have revealed that diseases have predictable cyclic rhythms and that the timing of medication regimens can improve outcome in selected chronic conditions [9.10]. The list of diseases which are required pulsatile release given in table.
Table.1.Diseases which follows Chronological behavior
|
Chronological behavior |
Drugs used |
Diseases |
|
Acid secretion is high in the afternoon and at night |
H2 blockers |
Peptic ulcer |
|
Precipitation of attacks during night or at early morning |
β2 agonist, Antihistamines |
Asthma |
|
BP is at its lowest during the sleep cycle and rises steeply during the early morning |
Nitroglycerin, calcium channel blocker, ACE inhibitors |
Cardiovascular diseases |
|
Pain in the morning and more pain at night |
NSAIDs, Glucocorticoids |
Arthritis |
|
Increase in blood sugar level after meal |
Sulfonylureas Insulin, pioglitazone |
Diabetes mellitus |
|
Cholesterol synthesis is generally higher during night than day time |
HMG CoA reductase inhibitors Hypercholesterolemia |
Hypercholesterolemia |
Classification of Pulsatile Drug Delivery Systems
A. Time controlled pulsatile release systems.
B. Stimuli-induced pulsatile release system.
C. Externally regulated pulsatile release system.
D. Multiparticulate regulated pulsatile release system.
A. Time controlled pulsatile release systems.
In time controlled drug delivery systems pulsatile release is obtained after specific time interval in order to mimic the circadian rhythm. Such type of pulsatile drug delivery system contains two components: One is of immediate release type and other one is a pulsed release type. Various methodologies that can used for time controlled pulsatile release systems are:
Pulsed release by erodible coating layer.
a) Bulk erosion
In type the polymer and sample rate of degradation is faster due to incises water . after a while rate of degradation is regulated due to attaining critical molecular weight of polymer sample.
Due this time lag occurs, this critical molecular weight provides an sufficient porous and hydrated to polymer sample. This leads to drug release in lag time. the core containing drug is coated is coated with erosion or soluble polymers and drug released is controlled by dissolution or erosion of outer coat.Time dependent release of drug can be obtained by obtimizing the thickness of outer coat.
b) Surface eroding system.
In this type of system, the core containing drug is coated is coated with erosion or soluble polymers and drug released is controlled by dissolution or erosion of outer coat. Time dependent release of drug can be obtained by optimizing the thickness of outer coat.
Time clock system is another example of surface eroding system, these systems are coated with lipid barriers like bee wax along with surfactants, as by this combination of coating produces emulsified layer when it contacts water. This layer erodes after lag time
Pulsed release system with rupturable coating layer.
These systems are dependent on disintegration of coating for release of drug. The pressure necessary for rupturable for coating can be achieved by swelling, disintegration, effervescent excipients, or osmotic pressure. Water permeation and mechanical resistance of the outer membrane are two important factors affecting the lag time.
These systems show the puls drug release by disintegration of coating layer. It needs pressure to rupture either using excipients like effervescent, swelling agents or by osmotic pressure .An effervescent mixture of citric acid and sodium bicarbonate has been reported where in the mixture was incorporated in a tablet core coated with ethyl cellulose. The carbon dioxide developed after penetration of water into the core resulted in a pulsatile release of drug after rupture of the coating. The release may depend on the mechanical properties of the coating layer. It is reported that the weak and non-flexible ethyl cellulose film ruptured adequately as compared with more flexible films. The lag time increases with increasing coating thickness and hardness of the core table.15,16 The highly swellable agents called superdisintegrants, were used to design a capsule based system comprising a drug, swelling agent and rupturable polymer layer.12-14
Capsule shaped system provided with release controlling plug.
Several single unit pulsatile dosage forms with a capsular design have been developed. Most of them consist of an insoluble capsule body, containing the drug, and a plug, which gets removed after a predetermined lag time because of swelling erosion or dissolution. e.g. Pulsincap system and port system.
The pulsincap system consist of a water- insoluble capsule body (exposing the body to formaldehyde vapour which may be produced by the addition of trioxy methylene tablets or potassium permanganate to formalin or any other method), filled with the drug formulation and plugged with a swellable hydrogel at the open end. Upon contact with dissolution media or gastrointestinal fluid, the plug swells and comes out of the capsule after a lag time, followed by a rapid release of the contents. The lag time prior to the drug release can be controlled by the dimension and position of the plug. In order to overcome the potential problem of variable gastric residence time of a single unit dosage forms, the pulsincap system was coated with an enteric layer, which dissolved upon reaching the higher pH- regions of the small intestine. The plug consists of Swellable materials coated with but permeable polymer (polymethacrylates).
* Erodible compressed polymer (HPMC, polyvinyl alcohol).
* Congealed melted polymer (glyceryl mono oleate).
* Enzymatically controlled erodible polymer (pectin).
B. Stimuli-induced pulsatile release system.
These systems drug release and respond to biological rhythms like temperature, or any other chemical stimuli. Based on the basis of stimulus. These systems are further classified into Temperature Induced system and chemical stimulated Induced system on the basis of stimulus.
These are of following types.15.16,20,21
* Temperature induced Pulsatile Release
* Themoresponsive hydrogel system
* Thermoresponsive polymeric micelle system
* Glucose responsive insulin release Devices lik Pulsincap system
* pH sensitive Drug delivery system
* Inflammation induced Pulsatile release
a) Thermo-responsive pulsatile release.
In these hydrogels are response to temperature changes and undergo reversible volume known to be themosensitive gels. These are claimed to be carriers in stimuli-responsive drug delivery. These are cross linked semi- synthetic polymers. Hydrogels are made at lower critical temperature by gels shrinks.
One of the common characteristics of temperature-sensitive polymers is the presence of hydrophobic groups, such as methyl, ethyl and propyl groups. From the many temperature-sensitive polymers, poly(N-isopropylacrylamide) is probably the most extensively used. PINPA crosslinked gels have shown thermoresponsive, discontinuous swelling/ deswelling phases; swelling, for example at temperatures below 32°CThe pulsatile release of acetaminophen occurred due to pulsatile change in temperature between 35°C and 40°C.
b) Chemical stimuli-induced pulsatile release.
The development of stimuli-sensitive delivery system has been the latest topic of interest. These systems release therapeutic agents in presence of any biological factor like enzyme, pH or any other chemical stimuli. One prominent application of this technology has been development of a system that can automatically release insulin in response to elevated blood glucose levels. Kazunori, et al.30 developed a gel composed of Pnipaam with phenylboronic acid moieties that showed a remarkable change in the swelling induced by glucose. This type of glyco-sensitive gel may have potential utilitiy in self-regulated drug releasing systems as well as in other applications, such as actuators, regulators and separation systems with glyco-sensitivity.17
C. Externally regulated pulsatile release system.
a) Electro responsive pulsatile release.
An electric field as an external stimulus hasadvantages, such as availability of equipment, which allow precisecontrol with regards to the magnitude of the current, duration ofelectric pulses, interval between pulses etc. Electrically responsivedelivery systems are prepared from polyelectrolytes and are thuspH-responsive as well as electro responsive. Under the influence of electric field, electro responsive hydrogels generally deswell, swell or erode. Poly(2-acrlamide-2-methylpropanesulfonic acidco- butyl methacrylate) (P(AMPS-co-BMA)) hydrogels were used for electric stimuli-induced drug delivery system.33,34 Kwon et al. exploited cross-linked poly(2-acrylamide-2-methylpropanesulfonicacid-co-butyl methacrylate) (P(AMPS-co-BMA)) hydrogels for electric stimuli-induced drug delivery.35 The mechanisms of drug release include expulsion of drug from the gel as the fluid phase syneresis out, drug diffusion along a concentration gradient and electrophoresis of charged drug toward an oppositely charged electrode and release of the entrapped drug as the gel complex erodes with regards to the magnitude of the current.
b) Ultrasonically stimulated.
Ultrasound is mostly used as an enhancer for the improvement of drug permeation through a biological barrier, such as skin, lungs, intestinal wall and blood vessels.There are several reports describing the effect of ultrasound on controlled drug delivery. Kost and coworkers depicted an ultrasound-enhanced polymer. Miyazaki et al. used ultrasound to achieve up to a 27-fold increase in the release of 5-fluorouracil from an ethylene and vinyl acetate (EVAc) matrix. Increasing the strength of the ultrasound resulted in a proportional increase in the amount of 5-fluorouracil released.18
c) Magnetically induced pulsatile release.
Use of an oscillating magnetic to regulate the drug delivery from a polymer matrix was one of the first methodologies investigated to develop an externally controlled drug delivery system. Magnetic carriers receive a response to a magnetic field from incorporated materials such as magnetite, iron, nickel, cobalt, etc. For biomedical applications, magnetic carriers must be water-based, biocompatible, non-toxic and non-immunogenic. Basically the mechanistic approach behind the strategy is based on the slowing down the movement of oral drugs in the gastrointestinal system through magnetic attraction. This is possible by filling an additional magnetic component into capsules or tablets. The speed of travel through the stomach and intestines can then be slowed down at specific positions by an external magnet, thus changing the timing and or extent of drug absorption into stomach or intestines.19-20
D) Multiparticulate System:
These system are reservoir type system with either rupturable or altered permeability coating and generally housed in Capsular body.A rupturable pulsate,e drug delivery system consist of (a) drug core (b) swelling layer comprising of a superdisintegrant and a binder (c) an insoluble water permeable polymeric coating.21
Conclusion
Therefore the pulsatile drug delivery systems have prominent advancement in the drug delivery which are exhibiting chronopharmacological behavior, and offer an solution for these chornothepathic drugs which shows, extensive first-pass metabolism, necessity of night-time dosing, or absorption window in GIT. Pulsatile drug delivery is one such system that, by delivering drug at the right time, right place. A variety of systems like Time, Stimuli, Externally regulated Multiparticulateregulated pulstaile Thus designing of proper pulsatile drug delivery will enhances the patient compliance, optimum drug delivery to the target site and minimizes the undesired effects. The approaches in this article represent attempts conducted over the past decade to achieve pulsatile release.
REFERENCE:
1.Yoshida R, Sakai K, Okano T, Sakurai. Pulsatile drug delivery systems using hydrogels. Advanced Drug Delivery Review 1993; 11: 85-108.
2.Kikuchi A, Okano T. Pulsatile drug release control using hydrogels. Advanced Drug Delivery Review 2002; 54: 53-77.
3.Gazzaniga A, Maroni A, Sangalli ME, Zema . Timecontrolled oral delivery systems for colon targeting. ExptOpin Drug Del 2006; 3: 583-597
4.Peppas NA, Leobandung W.Stimuli-sensitive hydrogels: idealcarriers for chronobiology and chronotherapy. J Biomat Sci Polym Ed 2004; 15: 125-144.
5.Stubbe BG, De Smedt SC, Demeester J. Programmed polymeric devices for pulsed drug delivery.Pharm Res2004; 21: 1732-1740.
6.Gazzaniga A, Palugan L, Foppoli A, Sangalli ME. Oral pulsatile delivery systems based on swellable hydrophilic polymers, Eur J Pharm Biopharm 2008; 68: 11-18.
7.Abraham A and Mathew T.S., Formulation and Evaluation of Enteric coated time released Press coated tablets of Theophylline for chronopharmacotherapy.Scholars Research Library, 2012; 4(2):599-606.
8.Survase S and Kumar N. Pulsatile drug delivery system :current scenario .CRIPS Vol. 8 No. 2 2007;106-108.
9.Gurny R, Junginger HE,Peppas. Pulsatile Drug Delivery,Current Application and Future Trends. Stuttgart, Germany, Wissenschaftliche Verlagsgesellschaft;1993.
10.Lemmer B. Chronopharmakokinetics.”Implications for Drug treatment. J Pharm Pharmacol.1999;51;887-890
11.Bhargavi R,A. Comprehensive Review of PulsatileDrugs, International Research Journal of Pharmacy2012; 3(3) 106-108.
12.Arora S,Ahuja A. Pulsatile Drug Delivery System .Indian Journal of Pharmaceutical Sciences 2006;Vol 68,295-300.
13.Kyatanwar U.A. Pulsatile Drug Delivery System. Journal of Phamacy Research 2010;Vol 3, No 1,Aug 15.
14.Gazzaniga A, Paluga L, Foppoli A. et al. Eur. J. Pharm. and Biopharm 2007(In Press).
15.Krogel I and Bodmeier R. Int. J. Pharm1999;187:175- 184.
16.Sungthongjeen S, Puttipipatkhachorn S, PaeratakulO, et al. J. Control. Rel2004. 95:147-159.
17.Jimoh AG, Wise DL, Gresser JD et al. J.Control.Rel 1995; 34:87-95.
18. Kost J and Langer R. Adv. Drug Del Rev 2001;46:125-148.
19. Sangalli ME, Maroni A, Foppoli A.European Journal of Pharmaceutical Sciences 2004; 22:469-476.
20.Bae YH, Okano T, Hsu R, Makromol.Chem.1987; 8:481-485.
21.Kataoka K, Harada A and Nagasaki Y.Adv.Drug Del. Rev 2001; 47:113-131.
|
PharmaTutor (ISSN: 2347 - 7881) Volume 1, Issue 2 Received On: 29/10/2013; Accepted On: 03/11/2013; Published On: 20/12/2013 How to cite this article: Vanka E., Rani MJ, Babu AMS, Rao PV, An Overview on Pulsatile Drug Delivery System, PharmaTutor, 2013, 1(2), 17-22 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE