About Authors:
Kalpen N. Patel*, Maulika S. Patel, Divya Thakkar, Manan Patel, Kaushal Raval
Shree Krishna Institute of Pharmacy, Shankhalpur,
Bechraji, Mahesana, Gujarat, India.
*kalpenpharma@gmail.com
ABSTRACT
Sustained releases tablets have been used for reduced the dosing frequency and maintain the plasma drug concentration level within narrow therapeutic range. Aspirin used as antiplatelate agent and Atorvastatin is HMG-CoA reductase inhibitor which lowers the plasma concentration of cholesterol. Here in present study sustained release tablets of Aspirin and Atorvastatin prepared by using cellulose acetate phthalate (CAP) and microcrystalline cellulose (MCC) as a polymers. The sustain release tablet of Aspirin And Atorvastatin were prepared by wet granulation method and were substituted for film coating to mask the spotting from Atorvastatin and for protection from light. From the dissolution profile of F2B2 gives controlling the release up to 12 hrs with required value i.e. - 55.85 % for Aspirin and 54.78 % for Atorvastatin in 4 hrs respectively and 100.70 % for Aspirin and 100.60 % for Atorvastatin in 12 hrs respectively. The result of stability studies of batch F2B2 indicate that it is stable at 400C / 75 % ±0.5 % relative humidity as there was no significant differences observe for dissolution and average drug content data after two months.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1373
INTRODUCTION
Oral drug delivery has been known for decades as the most widely utilized route of administered among all the routes that have been employed for the systemic delivery of drug via various pharmaceutical products of different dosage forms. The reasons that the oral route achieved such popularity may be in part attributed to its ease of administration and the belief that oral administration of the drug is well absorbed1.
All the pharmaceutical products formulated for systemic delivery via the oral route of administration irrespective of the mode of delivery (immediate sustained or controlled release) and the design of dosage forms (either solid dispersion or liquid) , must be developed within the intrinsic characteristics of GI physiology, pharmacokinetics , pharmacodynamics and formulation design is essential to achieve a systemic approach to the successful development of an oral pharmaceutical dosage form.2,3
The basic rational for controlled drug delivery is to alter the pharmacokinetic and pharmacodynamics of pharmacological active moieties by using novel drug delivery system or by modifying the molecular structure and physiological parameters inherent in the selected route of administration. It is desirable that the duration of drug action becomes more a design property of a rate controlled dosage form and less or not at all a property of the drug molecules properties, inherent kinetics. Thus optional design of controlled release systems necessitates a thorough understanding of the pharmacokinetics and pharmacodynamics of the drugs.
The goal in designing sustained or controlled delivery system is to reduce the frequency of the dosing or to increase effectiveness of the drug by localization at the site of action, reducing the dose required or providing uniform drug delivery. So sustained release dosage form is a dosage form that release one or more drugs continuously in predetermined pattern for a fixed period of time, either systematically or to a specified target organ.
[adsense:468x15:2204050025]
MATERIALS AND METHODS:
Collection of Materials:
Aspirin was collected from Siddharth Cargo Chem. product Ltd. Mumbai, Atorvastatin was collected from Dr. Reddy’s Lab., Hyderabad, Microcrystalline cellulose and Cellulose acetate phthalate were collected from Reliance cellulose Pvt. Ltd., Polyethylene glycol 8000, Corn starch, Talcum, Acetone, Methylene chloride, Hydroxy propyl methyl cellulose, Polyethylene glycol 6000, Isopropyl alcohol were collected from Loba chemi and Titanium dioxide was collected from Berje, Chemical, Mumbai.
Preparation of Sustained Release Tablets of Atorvastatin and Aspirin 4, 5, 6
Preparation Procedure for Granules of Aspirin 7, 8, 9
The Aspirin avicel and cornstarch were blended and placed in Hobart mixer. And passed through 40 # sieve. Cellulose acetate phthalate dissolved in acetone. And corbowax 8000, dissolved in methylene chloride are blended until uniform viscous mixture is obtained. The cap and PEG mixture was slowly added to aspirin blend with constant stirring until uniform with granular mass was obtained. The material was discharged on to trays and dried at 1250 F. for 1-2 hrs. The dried run rate was screen through 20 # sieve the formulation of Aspirin granules are described in Table no. 1.
Table No. 1: Formulation of Granules of Aspirin According To MCC: CAP Different Ratio
|
Ingredients (mg) |
Formulations |
|||||||
|
B1 |
B2 |
B3 |
B4 |
B5 |
B6 |
B7 |
B8 |
|
|
Aspirin |
225 |
225 |
225 |
225 |
225 |
225 |
225 |
225 |
|
MCC |
8 |
8 |
8 |
8 |
4 |
8 |
16 |
24 |
|
CAP |
4 |
8 |
16 |
24 |
8 |
8 |
8 |
8 |
|
PEG |
8 |
7 |
3 |
1 |
8 |
7 |
3 |
1 |
|
Cornstarch |
14 |
11 |
6 |
2 |
14 |
11 |
6 |
2 |
|
Methylene chloride |
Q.s |
--- |
--- |
--- |
--- |
---- |
--- |
--- |
|
Acetone |
Q.s |
--- |
--- |
--- |
--- |
--- |
--- |
---- |
Preparation Procedure for Granules of Atorvastatin10, 11
The atorvastatin avicel and cornstarch were blended and placed in Hobart mixer. And passed through 40 # sieve. Cellulose acetate phthalate dissolved in acetone. And corbo waxes 8000, dissolved in methylene chloride are blended until uniform viscous mixture is obtained. The cap and PEG mixture was slowly added to atorvastatin blend with constant stirring until uniform with granular mass was obtained. The material was discharged on to trays and dried at 1250 F. For 1-2 hrs.
For Tablet preparation equivalent quantity of Atorvastatin and ENT enteric coated granules of aspirin were mixed (13% and 87% respectively) and blended with 1% talc and compressed on rotary press, the formulation of Atorvastatin granules are described in Table no. 2.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table No. 2: Formulation of Granules of Atorvastatin According To MCC: CAP Different Ratio
|
Ingredients (mg) |
Formulations |
|||||||
|
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
|
|
Atorvastatin |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
30 |
|
MCC |
2 |
2 |
2 |
2 |
1 |
2 |
4 |
6 |
|
CAP |
1 |
2 |
4 |
6 |
2 |
2 |
2 |
2 |
|
PEG |
2 |
1 |
1 |
1 |
2 |
1 |
1 |
1 |
|
Cornstarch |
4 |
4 |
2 |
1 |
4 |
4 |
2 |
1 |
|
Methylene chloride |
Q.s |
--- |
--- |
--- |
--- |
--- |
--- |
--- |
|
Acetone |
Q.s |
---- |
--- |
--- |
--- |
--- |
--- |
--- |
Preparation of Film Coating Solution11, 12
Dissolved polyethylene glycol 6000, hydroxypropyl methyl cellulose in a mixture of isopropyl alcohol and methylene chloride. Dispersed talc, Titanium dioxide in a mixture of IPA. With methylene chloride and pass through a colloidal mill. And mix for ten mins. Step -2 added to step-1 mix 5 mins. Coating solution was taken to coat the tablet.
Table No. 3: Formulation of Film Coating Solution
|
Sr.No |
Ingredients |
Quantity/Tablet |
|
1. |
Hydroxy Propyl Methyl Cellulose (CPS) |
3.75mg |
|
2. |
Poly Ethylene Glycol 6000 |
0.75 mg |
|
3. |
Iso Propyl alcohol +Methylene chloride |
q.s |
|
4. |
Talc |
0.8mg |
|
5. |
Titanium Dioxide |
0.95mg |
RESULTS & DISCUSSION:
Evaluation of Granules 13, 14, 15
Determination of Bulk Density and Tapped Density
The bulk density, and tapped density were calculated using the following formulas.
Bulk density = W/V0
Tapped density = W/Vf
Where
V0 = initial volume
Vf = final volume
Compressibility index
Þ tapped - Þ bulk
Compressibility index = ------------------------------ X 100
Þ tapped
Þ tapped
Hausner ratio = ------------
Þ Bulk
Loss on drying: Determination of loss on drying of granules is important drying time during granulation was optimized depending LOD value. LOD of each batches were tested at 105o c for 2.5 minutes by using “Sartorious” electronic LOD apparatus.
Angle of repose: Angle of repose is defined as the maximum angle possible between the surface of a pile of the powder and the horizontal plane.
tan q=h/r
q= tan-1 h/r
These all parameter results are described in Table no. 4 and 5 respectively for Aspirin Tablet and Atorvastatin Tablet.
Table No. 4: Characterization of Aspirin Granules:
|
Batch No. |
Bulk density |
Tapped density |
Loss on Drying |
Compressibility Index |
Hausner Ratio |
Angle of Repose(0) |
|
B1 |
0.523 |
0.602 |
2.2 % |
13.12 |
1.15 |
35o |
|
B2 |
0.499 |
0.564 |
1.8 % |
11.52 |
1.13 |
32 o |
|
B3 |
0.488 |
0.526 |
2.1 % |
7.22 |
1.08 |
27 o |
|
B4 |
0.486 |
0.556 |
1.2 % |
12.59 |
1.14 |
31 o |
|
B5 |
0.502 |
0.581 |
0.9% |
13.60 |
1.16 |
30 o |
|
B6 |
0.512 |
0.574 |
1.4% |
10.80 |
1.12 |
28 o |
|
B7 |
0.442 |
0.506 |
1.7% |
12.65 |
1.14 |
34 o |
|
B8 |
0.491 |
0.566 |
1.6% |
13.25 |
1.15 |
31 o |
Table No. 5: Characterization of Atorvastatin Granules:
|
Batch No. |
Bulk density |
Tapped density |
Loss on Drying |
Compressibility Index |
Hausner Ratio |
Angle of Repose(0) |
|
F1 |
0.521 |
0.598 |
2.2 % |
13.12 |
1.15 |
33o |
|
F2 |
0.489 |
0.563 |
1.8 % |
11.52 |
1.13 |
31 o |
|
F3 |
0.482 |
0.516 |
2.1 % |
7.22 |
1.08 |
26 o |
|
F4 |
0.478 |
0.564 |
1.2 % |
12.59 |
1.14 |
29 o |
|
F5 |
0.499 |
0.576 |
0.9% |
13.60 |
1.16 |
33 o |
|
F6 |
0.508 |
0.563 |
1.4% |
10.80 |
1.12 |
25 o |
|
F7 |
0.436 |
0.489 |
1.7% |
12.65 |
1.14 |
30 o |
|
F8 |
0.487 |
0.555 |
1.6% |
13.25 |
1.15 |
28 o |
EVALUATION OF TABLET
All the prepared Sustained release tablets were evaluated for following official and unofficial parameters and there results are described in Table no. 6.
Table No. 6: Physical Parameters of tablets of each batch
|
Batch No |
Weight variation (mm)* |
Diameter (mm)* |
Thickness (mm)* |
Hardness (N)* |
Friability (%) |
Drug content of ASP (%) |
Drug content of ATR (%) |
|
F1B1 |
308±2.15 |
9.59±0.03 |
4.6±0.03 |
136±2015 |
0.53 |
96.89 |
95.04 |
|
F2B2 |
303±3014 |
9.57±0.02 |
4.6±0.08 |
143±3.42 |
0.67 |
96.42 |
97.75 |
|
F3B3 |
307±1.18 |
9.58±0.01 |
4.5±0.07 |
157±3.25 |
0.54 |
96.82 |
93.67 |
|
F4B4 |
306±1.68 |
9.60±0.04 |
4.4±0.06 |
144±1.86 |
0.62 |
98.22 |
97.14 |
|
F5B5 |
301±4.45 |
9.57±0.03 |
4.7±0.04 |
133±2.23 |
0.51 |
98.77 |
95.32 |
|
F6B6 |
304±3.01 |
9.58±0.04 |
4.7±0.07 |
166±1.94 |
0.49 |
100.83 |
98.23 |
|
F7B7 |
309±1.97 |
9.56±0.02 |
4.5±0.08 |
143±2.56 |
0.62 |
101.65 |
96.86 |
|
F8B8 |
308±2.23 |
9.58±0.03 |
4.4±0.09 |
165±3.67 |
0.59 |
99.01 |
98.21 |
*Each value represents the mean + standard deviation (n=10)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Drug Assay of Aspirin16
Triturate the 20 tablets and take equivalent to 225mg Aspirin For rapid estimation of Aspirin in combined tablet formulation, method is based on measurement of change in absorbance at 302 nm due to formation of salicylic acid during hydrolysis of aspirin in 0.1 N NaOH. Change in absorbance follows relationship with Aspirin in the range 10 to 100 µg/ml.
Drug Content of Atorvastatin
The assay of atorvastatin in pharmaceutical formulations.
Triturates the 20 tablets and take equivalent to 30mg atorvastatin. Examined drug and bromocresol green producing ion-pair complexes which can be measured at the optimum wavelengths. The optimization of the reaction conditions is investigated. Beer's law is obeyed in the concentration ranges 1.0-25.0 μg mL-1with bromocresol green. The molar absorptivities and percent recoveries are evaluated. On the other hands, atorvastatin was determined by measurement of its first derivative signals at 246 nm, Calibration graph was established for 2-14 μg mL-1 of atorvastatin by spectrometric ally.
Dissolution
Medium: 6.8 pH phosphate buffer
Volume: 900 ml
Apparatus: Basket
Rotation: 150
Time: 12 hrs.
Detection: UV, 246 nm for ATR and 302 nm for ASP.
Table No. 7: Dissolution Profile of F1b1–F8b8 in 6.8 Ph Phosphate Buffer for Aspirin at 302 Nm.
|
Batch no. |
Time in hours ( Cumulative % Drug release of aspirin) |
||||||
|
TIME |
1 |
2 |
4 |
6 |
8 |
10 |
12 |
|
F1 B1 |
46.97 |
62.23 |
81.01 |
89.03 |
100.04 |
--- |
--- |
|
F2 B2 |
27.27 |
39.13 |
55.85 |
68.31 |
80.16 |
93.01 |
100.70 |
|
F3 B3 |
25.65 |
43.09 |
49.94 |
63.54 |
72.27 |
78.99 |
91.81 |
|
F4 B4 |
24.10 |
30.73 |
43.66 |
56.41 |
70.83 |
78.87 |
89.82 |
|
F5 B5 |
44.91 |
64.21 |
82.20 |
88.13 |
100.02 |
---- |
------ |
|
F6 B6 |
25.17 |
37.13 |
51.73 |
66.21 |
80.16 |
91.20 |
99.89 |
|
F7 B7 |
24.78 |
43.11 |
47.53 |
65.01 |
74.29 |
79.91 |
93.71 |
|
F8 B8 |
25.77 |
31.37 |
42.13 |
54.53 |
71.58 |
77.82 |
92.98 |
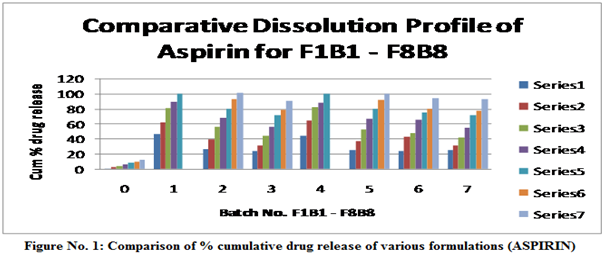
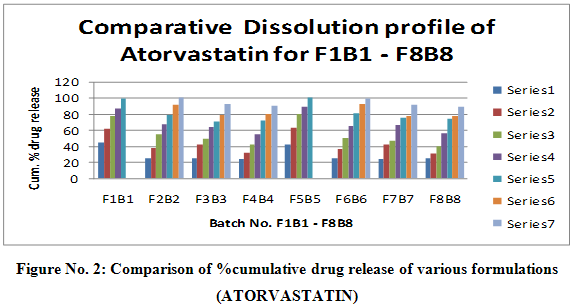
Table No. 8: Dissolution profile of F1B1 – F8B8 IN 6.8 pH phosphate buffer For ATORAVASTATIN at 246 nm.
|
Batch No. |
Time In Hours ( Cumulative % Drug release of Atorvastatin) |
||||||
|
TIME |
1 |
2 |
4 |
6 |
8 |
10 |
12 |
|
F1 B1 |
44.80 |
61.21 |
78.12 |
87.01 |
99.92 |
--- |
--- |
|
F2 B2 |
25.27 |
38.14 |
54.78 |
67.21 |
78.77 |
91.02 |
100.60 |
|
F3 B3 |
24.70 |
42.01 |
48.96 |
63.54 |
71.28 |
79.02 |
92.82 |
|
F4 B4 |
23.90 |
31.63 |
42.61 |
55.40 |
72.12 |
79.87 |
90.81 |
|
F5 B5 |
41.90 |
63.11 |
80.10 |
89.03 |
100.16 |
---- |
------ |
|
F6 B6 |
24.72 |
37.13 |
49.72 |
65.21 |
81.28 |
92.30 |
99.98 |
|
F7 B7 |
23.78 |
42.12 |
46.54 |
66.20 |
75.28 |
78.12 |
91.17 |
|
F8 B8 |
24.72 |
30.73 |
40.03 |
55.57 |
73.85 |
78.28 |
89.12 |
Stability Study of Tablets of Batch F2B2
The batch F2B2 was selected as an optimum batch and the stability study was carried out at accelerated condition of 40oC/75 % RH condition for a period of two month.
Method:
Ten tablets were individually wrapped using aluminum foil and packed in ambered color screw cap bottle and put at above specified condition in incubator for 2 months. After two month tablets were evaluated for content uniformly and in-vitro release.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table No. 9: Drug Content of Batch F2B2 Kept For Stability
|
Time |
Drug content for aspirin (%) |
|
Zero month |
96.42 |
|
Two Month |
95.78 |
Table No 10: Drug Content of Batch F2B2 Kept For Stability
|
Time |
Drug content for atorvastatin (%) |
|
Zero month |
97.75 |
|
Two Month |
96.85 |
Table No 11: Dissolution Profile of Aspirin for Batch F2B2 Kept For Stability
|
Sr.No. |
Time (hrs) |
Cumulative drug release (%) |
|
|
|
|
Initial |
Two Month |
|
1 |
0 |
0 |
0 |
|
2 |
1 |
27.27 |
26.78 |
|
3 |
2 |
39.13 |
37.03 |
|
4 |
4 |
55.85 |
54.65 |
|
5 |
6 |
68.31 |
67.21 |
|
6 |
8 |
80.16 |
78.06 |
|
7 |
10 |
93.01 |
91.00 |
|
8 |
12 |
96.42 |
95.85 |
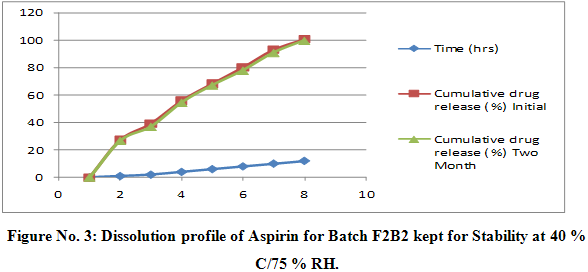
Conclusion:
The result of stability studies of batch F2B2 indicate that it is table at 40 0 C / 75 % ±0.5 % relative humidity as there was no significant difference observe for dissolution and average drug content data after two months. From the above discussed parameters it is concluded that formulation of sustained release tablet of Aspirin and Atorvastatin 1 : 1 ratio of CAP : MCC gives ideal optimized formulation i.e. batch F2B2 for twelve hour release and the study encouraged for the further clinical trials on this formulation.
References:
1. Chien Y. W., “Novel Drug Delivery System” (IInd Edn), 1992, Revised and expanded, 1-2,139-140.
2. Remington, “The Science and Practice of pharmacy”, 20th Edn, vol.I, .903-913.
3. Brahmankar D.M. and Jaiswal S.B., “Biopharmaceutics and Pharmacokinetics”, “A. Treatise” Vallabh Prakashan, 1995 1st edn, 347-352.
4. Aartholomeaeus, “sustained release drug formulation containing tramadol salt “U.S. patent no. Us00/5601842, (1997). www.patentstorm.com
5. Eddy, K.R. Mutalik S, Reddy S, “Once daily sustained release matrix tablets of Nicorandil, formulation and invitro evaluation “American Association of Pharmaceutical Science (2003) 4(4),61.
6. Aririan I, Ghaffari A, Mohammad M., “formulation of controlled released matrix tablets of Isobirbide Dinitrate “Indian Journal of Pharmaceutical Science, 2001, 63(1), 24-29.
7. Oyashi T, Kanbe H, Okado M. Suzuki M, Sonobe T, “formulation study and drug release mechanism of a new theophylline sustained Release preparation “International Journal of Pharmacy, 2005,304, 91-101.
8. Cox P. J. Kham K.A. Munday D.L., Areevath J. “Development and evaluation of multiple unit oral Sustained release dosage form for Ibuprofen, Preparation and release kinetics “International Jouornal Pharmacy, 1999,193, 73-84.
9. Olinis M.A. Cruz Y. De. Gascon A, Calvo B, Pedraz J.L “Release of New Diffusion Studies “International Journal Pharmacy, 2002, 239, 61-68.
10. Nagesh C, Setty C, ghosh B. “Development and invitro evaluation of extended release formulation of diltiazem HCL” Indian Drugs, 1999, 36(10), p.no.657-660.
11. Okar RS, Eichie FE, Effect of protective coating of aspirin tablets with acrylatemethacrylate copolymers on tablet disintegration times and dissolution rates,Indian Journal of Pharmaceutical S 2000, Vol. 69, 591
12. Louders Ochoa, Manuela Igatua , Rosa M a , Hernandez , Alicia R. Gascon, Jose Luis Pedraz, “preparation of sustained release hydrophilic matrices by melt granulation in high-shear mixer “J. Pharm Pharmaceut Sci.2005, 8(2), 132-140.
13. Silvina A. Bravo,Maria C. Lamas Claudio J. Saloman , “In Vitro Studies of Diclofenac Sodium Controlled –release from Biopolymeric Hydrophillic Matrices “J. Pharm Pharmaceut Science.2002, 5(3), 213-219.
14. Saleh M. Al.-Saidan, Yellela S.R. Krishnaiah, Srinivas S. patro, and Vemulapalli Satyanarayana “In Vitro and In Vivo Evaluation of Guar Gum Matrix Tablets for Oral Controlled Release of Diltiazem Hydrochloride” American Association of Pharmaceutical Sciences Tech, 2005, 6(1) Article 5.
15. Hman M, Poddar S.S. Shahjahan “A. Development of Matrix and Coatedunitsfro pH-independent release of a weakly basic drug” Kundnani College of Pharmacy, Mumbai, Indian Journal of Pharmaceutical S, 2000, Vol.67, P.no.132.
16. Document title Department of Analytical Chemistry, Faculty of Pharmacy, Ankara University, Ankara, Turquie Analytical letters ISSN 0003-2719 Coden Analbp Source, 2003, vol. 36, no.12, 2699-2711 [13 page(s) (article)] (17 ref.).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









