INTRODUCTION
• Spectroscopy is the branch of science dealing with the study of interaction of electromagnetic radiation with matter.
• Infrared spectroscopy is the spectroscopy which is concerned with the study of infrared region of electromagnetic spectrum (i.e. light having a longer wavelength & a lower frequency than visible light), which results in vibrational transitions i.e. Study of interaction between infrared radiations & matter.
• It is also called as vibrational spectroscopy.
• Vibrations in IR spectroscopy also known as fundamental vibrations.
• The IR spectroscopy concept can be generally analysed in 3 ways:
* By measuring reflection
* By measuring emission
* By measuring absorption
• It is an important qualitative analytical technique for determining the structure of both organic & inorganic compounds.
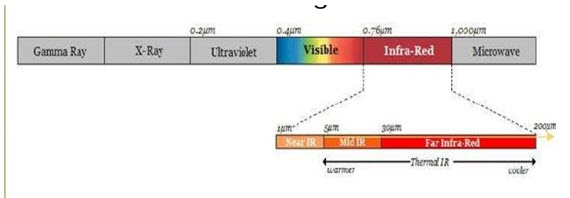
• IR radiations lies in the wavelength range of 0.7-400 μm
• Infrared radiations refers broadly to that part of electromagnetic spectrum between visible & microwave region.
• It is based on the absorption spectroscopy in which molecular vibration is observed due to absorption of IR radiation.
• It measures the bond vibration frequencies in a molecule.
• Energy of radiation is directly proportional to wavenumber & inversely proportion to wavelength.
• IR radiation shows only rotational & vibrational level.
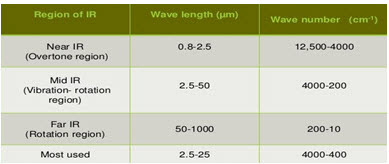
• The infrared region is divided into 3:
* Near
* Mid
* Far
Principle :
• The principle of IR spectroscopy is related to the vibrational & rotational energy of a molecule.
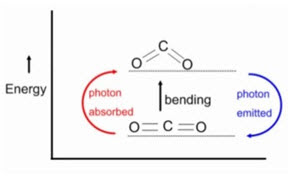
• When the frequency of the IR radiation is equal to the natural frequency of vibration, the molecule absorb IR radiation
• Absorption of IR radiation causes an excitation of molecule from a lower to the higher vibrational level.
• Each vibrational level is associated with a number of closely placed rotational level.
• Therefore the IR spectroscopy is also called as ‘vibrational-rotational spectroscopy’.
• All the bonds in a molecule are not capable of absorbing IR energy but those bonds which are accompanied by a change in dipole moment will absorb in the IR region & such transitions are called IR active transitions.
• The transitions which are not accompanied by a change in dipole moment of the molecule are not directly observed & are considered as IR inactive transitions..
• In IR spectroscopy the changes in the vibrational energy depends upon:
1. Mass of the atoms present in a molecule
2. Strength of the bonds
3. Arrangement of the atoms within the molecule
• No 2 compounds except the enantiomers can have the similar IR spectra.
Theory :
• Molecules are made up of atoms linked by chemical bonds.
• In any molecule, movement of atoms & chemical bond is like spring & balls (vibration).
• This characteristic vibration is called Natural frequency of vibration.
• The energy of molecular vibration is quantized.
• When energy in the form of infrared radiation is applied then it causes the vibration between the atoms of the molecules & when,
Applied infrared frequency= Natural frequency of vibration
• Then, absorption of IR radiation takes place & a peak is observed.
• Different functional groups absorb characteristic frequencies of IR radiation. Hence gives the characteristic peak value.
• Therefore, IR spectrum of a chemical substance is a finger print of a molecule for its identification.
• CRITERIA/ REQUIREMENTS FOR A COMPOUND TO ABSORB IR RADIATION:
1. Correct wavelength of radiation
2. Change in dipole moment
1. Correct wavelength of radiation:
• A molecule absorbs radiation when the natural frequency of vibrations of some part of a molecule is the same as the frequency of incident radiation.
2. Change in dipole moment:
• A molecule can only absorb IR radiations when its absorption causes a change in its electric dipole.
• A polar bond is usually IR active whereas non polar bond in a symmetrical molecule will absorb weakly or not at all.
• A molecule is said to have an electric dipole when there is a slight positive & a slight negative charge on its component of atoms.

II. MODES OF VIBRATIONS IN POLY ATOMIC MOLECULES
• A vibration in a molecule is any change in shape of the molecule-stretching bonds, bending of bonds, or internal rotation around single bonds.
• Molecular vibration should be studied because whenever the interaction between electromagnetic waves & matter occur, change appears in these vibrations.
• Simple diatomic molecules have only one bond, which may stretch.
• Polyatomic molecules have more than one vibrational frequencies.
• In polyatomic molecules, each atom having three degrees of freedom in three directions which are perpendicular to each other. Thus, a molecule of n atoms has 3n degrees of freedom.
• Degree of freedom is the number of variables required to describe the motion of a particle completely.
• There are 2 cases:
1. For linear molecule
2. For non-linear molecule
1. For linear molecule: It is a special case since by definition all the atoms lie on a single straight line. Two degrees of freedom describe rotation & three degrees describe translation.Thus, the number of vibrations for linear molecules is given by (3n-5)
2. For non-linear molecule: For a nonlinear molecule, all rotation degrees of freedom is three (i.e. for rotation & translation). Thus, the number of vibrations for nonlinear molecule is given by (3n-6)
• More complex molecules have more than one bonds & different types of vibrations may occur.
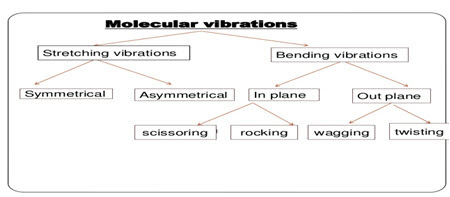
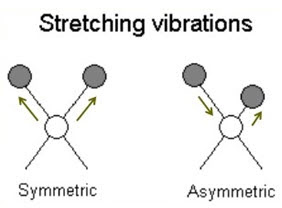
1. Stretching vibration
• It involves continuous change in the inner atomic distance (i.e. distance between 2 atoms increases or decreases) along the axis of bond between 2 atoms such that bond length changes without change in bond angle in regular interval.
• It requires more energy so appear at shorter wavelength.
• It occurs at higher frequency : 4000-1250 cm-1
• It is further classified into 2:
a) Symmetrical stretching
b) Asymmetrical stretching
a) Symmetrical stretching:
• The two atoms either move towards or away from central atom without change in bond angle or bond axis.
• Two bonds increase or decrease simultaneously.
b) Asymmetrical stretching:
• The 2 atoms move with respect to central atom such that one moves away & other moves towards the central atom.
• One bond length is increased & other is decreased.
2. Bending vibration
• They involve movement of atoms which are attached to common central atom.
• These are characterized by change in the angle between two bonds.
• Also known as deformation vibration.
• It requires less energy so appear at longer wavelength.
• It occurs at lower frequency: 1400-666cm-1.
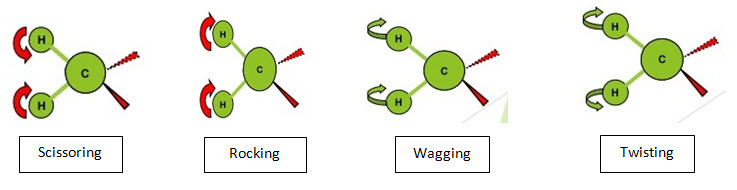
• It is further classified as:
a) In plane bending
i. Scissoring
ii. Rocking
b) Out plane bending
i. Wagging
ii. Twisting
a) In plane bending-
* In these types of vibrations, there is change in bond angle.
* This type of bending takes place within the same plane.
i. Scissoring:
• Two atoms approach each other i.e. they move back & forth.
• Bond angles are decreased.
• Also known as symmetrical bending.
ii. Rocking:
• Movement of atoms takes place in the same direction i.e. they swing back & forth along the central atom.
b) Out plane bending-
This type of bending takes place outside of the plane of molecule.
i. Wagging:
• Two atoms move to one side of the plane i.e. they oscillate back & forth.
• They move up & down of the plane.
ii. Twisting:
• One atom moves above the plane & other move below the plane i.e. they rotate around the bond which joins the central atom of molecule.
III. SAMPLE HANDLING
* IR spectroscopy is used for the characterization of solid, liquid or gas samples.
* Materials containing sample must be transparent to the IR radiation. So, the salts like NaCl, KBr are only used.
* Samples of the same substance shows shift in absorption bands as it pass from solid to gases & hence the samples of different phases have to be treated differently in IR spectroscopy.
1. Sampling of solids:
a) Solids run in solution
b) Mull technique
c) Pressed pellet technique
d) Case film technique
a) Solids run in solution:-
• In this, solid sample is dissolved in non-aqueous solvent provided that there is no chemical interaction with the solvent & the solvent is not absorbed in the range to be studied.
• A drop of this solution is placed in alkali metal disc & solvent is evaporated to dryness leaving a thin film which is then mounted on spectrophotometer.
• E.g. of solvents- acetone, cyclohexane, chloroform, etc.
b) Mull technique:-
• A thick paste (mull) is prepared by grinding 2-5mg of sample in smooth mortar made of marble or agate with a pestle by adding 1 to 2 drops of mulling agent (Nujol) & continues grinding to get homogenous paste.
• Mull is transferred to mull plates & the plates (NaCl plates) are squeezed together to adjust the thickness
• It is then mounted in a path of IR beam & the spectrum is recorded.
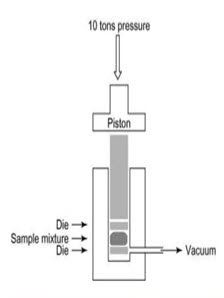
c) Pressed pellet technique:-
• Finely powdered sample is mixed with about 100 times its weight of KBr in a vibrating ball mill & the mixture is then compressed in an evaluated die to form a thin transparent pellet (1-2mm thick & 1cm in diameter) using hydraulic press.
• These pellets are transparent to IR radiation & it is used for analysis.
* Advantages-
i) Eliminates bands which appear due to mulling agent
ii) Pellets can be stored for longer period of time
iii) Concentration of sample can be adjusted.
iv) Resolution of spectrum is superior
* Disadvantages-
i) Not suitable for polymers which are difficult to bind with KBr.
ii) High pressure may change the crystallinity of the sample.
iii) At high pressure polymorphic changes occur
iv) Not useful for polymer which are difficult to grind with KBr
v) Always has a band at 3450cm-1.
d) Case film technique:-
• If the solid is Amorphous in nature then the sample is deposited on the surface of a KBr or NaCl cell evaporation of a solution of the solid & ensured that the film is not too thick to pass the radiation.
2. Sampling of liquid:
• Liquid sample can be sandwiched using liquid sample cells of highly purified alkali halides ( NaCl, KBr, CaF2)
• Aqueous solvents cannot be used because they cannot dissolve alkali halides.
• Organic solvents like chloroform can be used.
• The sample thickness should be selected so that the transmittance lies between 15-20%.
• For most liquids sample cells thickness is 0.01-0.05mm.
• Pressing a small drop of liquid sample between plates produces a film of about 0.1mm thick.
• The plates are held together by capillary action & mounted in a sample beam.
• Plates are cleaned & dried immediately after use by rinsing in suitable solvent such as chloroform because some plates are highly soluble in water.
3. Sampling of gas sample:
• The sample cell is made up of NaCl, KBr, etc. & it is similar to liquid sample cell.
• A gas sample is introduced into an evacuated gas cell.
• Gas cells are available in lengths ranging from a few cm to some meters.
• Usually a sample cell with a long path length of 5-10cm is needed because the gases show relatively weak absorbance.
• The sampling area of standard dispersive IR spectrophotometer is about 10 cm.
• So long path are achieved by using multiple gas cells in which internal mirrors permit the beam to be reflected several times through the sample before it exists the gas cell.
IV. FACTORS AFFECTING VIBRATION
OR
FACTORS AFFECTING VIBRATIONAL FREQUENCIES
OR
FACTORS INFLUENCING ABSORPTION
• Vibrational frequency: A molecular vibration occurs when atoms in a molecule are in periodic motion while the molecule as a whole has constant translational & rotational motion. The frequency of the periodic motion is known as a vibrational frequency.
• The value of vibrational frequency of a bond calculated by Hooke’s Law is not always equal to their observed value. The force constant is changed with the electronic and steric effects caused by other groups present in the surroundings.
• There are number of factors that influence the precise frequency of a molecular vibration:
a) Effect of bond order
b) Vibrational coupling/ Coupled vibrations
c) Hydrogen bonding
d) Fermi resonance
e) Electronic effects
a) Effect of bond order:
• Bond order affects the position of absorption bands. Higher the bond order larger is the band frequency.
• A C-C triple bond is stronger than a C=C bond, so a C-C triple bond has higher stretching frequency than does a C=C bond.
• The C-C bonds show stretching vibrations in the region from 1200-800 cm-1 but these vibrations are weak and of little value in identifying compounds.
• Similarly, a C=O bond stretches at a higher frequency than does a C-O bond and a C-N triple bond stretches at a higher frequency than does a C=N bond which in turn stretches at a higher frequency than does a C-N bond.
b) Coupled vibrations:
• Vibrations which occur at different frequencies of higher wave number are called coupled vibration.
• Strong coupling between stretching vibrations occurs only when the 2 vibrations have a common atom
• Interaction between bending vibrations occurs only when a common bond is present between the vibrating groups.
• Coupling between stretching & bending vibrations occurs. If stretching bond forms one side of angle that varies in bending vibrations
• Interaction is greatest when the coupled groups have individual energies that are approx. equal
• If groups are separated by 2 or more bonds, no interaction occurs
• Coupling occurs when vibrations are of same symmetry species.
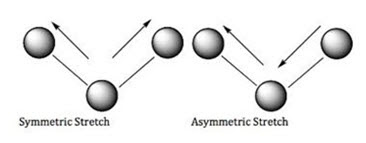
• An isolated C-H bond has only one stretching vibrational frequency whereas methylene group shows 2 stretching vibrations- symmetrical & asymmetrical
• Asymmetric vibrations occur at higher frequencies or wave numbers than symmetric stretching vibrations.
• These are known as coupled vibrations because these vibrations occur at different frequencies than that required for an isolated C-H stretching
• A strong vibrational coupling is present in carboxylic acid anhydrides in which symmetrical & asymmetrical stretching vibrations appear in the region 1720-1825 cm-1.
• The interaction is very effective probably because of the partial double bond character in the carbonyl oxygen bonds due to resonance which also keeps the system planar for effective coupling.
• Asymmetrical stretching band in acyclic anhydride is more intense whereas symmetrical stretching band is more intense in cyclic anhydrides.
• For interaction to occur, the vibrations must be of same symmetry species.
• There must be a common atom between the groups for strong coupling between stretching vibrations.
• For coupling of bending vibrations, a common bond is necessary.
• Interaction is greatest when coupled groups absorb, individually near the same frequency.
• Coupling is negligible when groups are separated by one or more carbon atoms & vibrations are mutually perpendicular.
c) Fermi resonance:
• A vibration of large amplitude produced by a relatively small vibration
• Coupling of 2 fundamental vibration modes produces 2 new modes of vibration, with frequencies higher & lower than that observed in absence of interaction. Interaction can also take place b between fundamental vibrations & overtones or combination tone vibration s & such interactions are known as Fermi Resonance.
• If 2 different vibrational levels, belonging to the same species, have nearly the same energy.
• A mutual perturbation of energy may occur.
• Shifting of one towards lower & other towards higher frequency occur
• A substantial increase in intensity of respective bands occurs.
• In this, a molecule transfers its energy from fundamental vibrational level to overtone or combination tone level & back.
• Resonance pushes the 2 levels apart & mixes their character, consequently each level has partly fundamental & partly overtone or combination tone character.

• E.g.: symmetrical stretching vibration of CO2 in Raman spectrum shows bands at 1337cm-1. The 2 bonding vibrations are equivalent & absorb at the same frequency of 667.3cm-1.
• The first overtone of this is 1334.6cm-1
• Fermi resonance occurs.
• There is mixing of 1337.6cm 1 with intensity ratio 1:0.9 respectively.
d) Hydrogen bonding:
• It occurs in any system containing a proton donor group (X-H) & a proton acceptor.
• The stronger the hydrogen bond, the longer the O-H bond, the lower the vibration frequency & broader & more intense will be absorption band.
• The N-H stretching frequencies of amines are also affected by hydrogen bonding as that of hydroxyl group but frequency shifts for amines are lesser than that for hydroxyl compounds.
• Because nitrogen is less electronegative than oxygen so hydrogen bonding in amines is weaker than that in hydroxyl compounds.
• Intermolecular hydrogen bond gives rise to broad bands, while intramolecular hydrogen bonds give sharp & well defined bands.
• The inter & intra molecular hydrogen bonding can be distinguished by dilution.
• Intramolecular hydrogen bonding remains unaffected on dilution & as a result the absorption band also remains unaffected where as in intermolecular, bonds on dilution & as a result there is decrease in bonded O-H absorption.
• The strength of hydrogen bonding is also affected by:
i) Ring strain
ii) Molecular geometry
iii) Relative acidity & basicity of proton donor & acceptor groups
e) Electronic effect:
• Changes in absorption frequencies for a particular group take place when the substituents in the neighbourhood of that particular group are changed.
• It includes:
i) Inductive effect
ii) Mesomeric effect
iii) Field effect
i) Inductive effect-
* The introduction of alkyl group causes +I effect which results in lengthening or weakening of bond.
* Hence the force constant is lowered & wave number of absorption decreases.
* Let compare wave numbers of (C=O) absorptions for following compounds:
a. Formaldehyde (HCHO)1750cm-1
b. Acetaldehyde (CH3CHO) 1745cm-1
c. Acetone (CH3COCH3) 1715cm-1
* Introduction of an electronegative atom or group causes –I effect which results in bond order to increase
* Hence force constant increases & wave number of absorption rises.
ii) Mesomeric effect-
* It causes lengthening of weakening of a bond leading in lowering of absorption frequency.
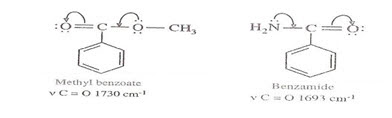
* As nitrogen atom is less electronegative than oxygen atom, the electron pair on nitrogen atom in amide is more labile & participates more in conjugation.
* Due to this greater degree of conjugation, the C=O absorption frequency is much less in amides as compared to that in esters.
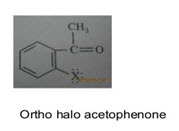
iii) Field effect-
* In ortho substituted compounds, the lone pair of electrons on 2 atoms influences each other through space interactions & changes the vibrational frequencies of both groups.
* This effect is called field effect.
INSTRUMENTATION
1. Sources of radiation
2. Wavelength selectors
3. Detectors
i. Golay cell
ii. Bolometer
iii. Thermocouple
iv. Thermister
v. Pyroelectric detector

1. Sources of radiation:
• IR instruments require a source of radiant energy which emits IR radiation which must be steady, intense enough for detection and extend over the desired wavelength.
• Various sources of IR radiations are as follows.
a) Nernst glower
b) Incandescent lamp
c) Mercury arc
d) Tungsten lamp
e) Globar source
f) Carbon dioxide laser
• The radiation source is composed of an inert solid which is electrically heated to a temperature in the range 1500-2000 K.
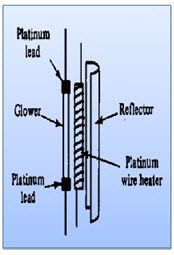
a) Nernst glower
• More intense emitted radiation.
• Constructed from mixture of fused oxides of Zr, Th & Cs.
• Consists of cylinder of rare earth oxides formed into a cylinder of diameter 1-2mm length approx. 20mm.
• Platinum discs are attached at the ends of cylinder to permit electrical connection
• They are heated to a temperature of 1200-2200K where IR radiations are emitted.
• They are good energy output.
• It provides maximum radiation about 7100cm-1.
• Non conducting at room temperature
• Used in near IR region.
• Used for detection of carbohydrates & proteins.
b) Globar source
• It is also made of cylinder.
• It is sintered silicon carbide rod which is about 50mm in length & 5mm in diameter.
• They are self-starter.
• It is heated to about 2000oC.
• It provides maximum radiation of about 5200cm-1.
• Water cooling is needed to cool the metallic electrodes attached to the rod
• Less convenient to use, more expensive & less intense than Nernst Glower.
• Used to detect simple functional groups
c) Incandescent lamp
• This is a tightly wound coil of nichrome wire.
• Electrically heated to 1100 K.
• It produces a lower intensity of radiation than the Nernst or Globar sources, but has a longer working life.
d) Mercury arc
• Used for far IR region (wave no<200cm-1).
• It is a high pressure mercury arc which consist of a quartz jacketed tube containing Hg vapour at P>1atm.
• When current passes through the lamp, mercury is vaporized , excited & ionized, forming a plasma discharge at high pressure.
• In the UV & visible regions, this lamp emits atomic Hg emission lines that are very narrow & discrete, but emits an intense continuous in the far IR region.
• Intensity of radiation is greater.
• Used to detect inorganic complexes.
e) Carbon dioxide laser
• Useful for narrow radiation bands.
f) Tungsten lamp
• It is a black body source.
• Heated up to 3500K.
• Intensity of radiation is mild.
• Used in mid IR region.
• Used in detection of organic functional groups.
2. Wavelength selectors
• Output from a wavelength selector is a band of wavelengths
i. Filters
ii. Monochromators
a. Entrance slit
b. Collimating lens or mirror
c. Dispersion element (prism or grating)
d. Focusing lens or mirror
e. Exit slit
i. Filters:
• They are very simple
• They are sheets of plastic or glass that simply absorbs wavelengths other than those required for the analysis.
• Generally, the range of wavelengths allowed by a filter is relatively wide.
• They separate different parts of the electromagnetic spectrum by absorbing or reflecting certain wavelengths & transmitting other wavelengths.
ii. Monochromators:
• These are far more complicated, & comprise a series of optics inside a lightproof box, which has entry & exit slits which allows the radiation of all wavelengths in & a narrow range of wavelengths out.
• They convert polychromatic light into monochromatic light
• They must be constructed of materials which transmit IR.
• Two types:
a. Prism monochromator
b. Grating monochromator
a) Prism:-
• It is used to isolate different wavelength.
• They are constructed of materials of various metal halide salts (NaCl, KBr, CsBr, lithium fluoride) which transmit in infrared.
• Based on high dispersion.
• Prism may be made of glass or quartz.
• The glass prisms are suitable for radiation essentially in the visible range whereas the quartz prism can cover the ultraviolet spectrum also.
• Dispersion given by glass is about 3 times that of quartz.
• Advantage-
* No chromatic abrasion
* Steady
• Disadvantage-
* Instable
* Water solubility
• Two types of prism:
i. Metal halide prism
ii. NaCl prism
i) Metal halide prism-
* Prisms which are made up of KBr are used in the wavelength region from 12-25 μm.
*Lithium fluoride prisms are used in wavelength region from 0.2-0.6 μm.
* CsBr prisms used in wavelength region from 15-38 μm.
ii) NaCl prism-
• Used in the whole wave length region from 4000-650cm-1.
• They have to be protected above 200 C because of hygroscopic nature.
a) Gratings:-
• It is a device which consists of a series of parallel straight lines cut into a plane surface.
• They offer better resolution at higher frequency than prisms.
• They offer linear dispersion & may be constructed from a wide variety of materials.
• Several gratings, each with different rulings (lines/cm) are necessary to cover the wide wavelength (energy) range associated with IR radiation.
• Dispersion of a grating follows law of diffraction.
• High dispersion can be achieved.
• They offer much better resolution at low frequency, viz. typical rulings are 240 lines per nm for the 4000-1500cm-1 region & 120 lines per nm for the 1500-650cm-1 region.
• In other words, gratings are nothing but rulings made on some materials like glass, quartz or alkyl halides depending upon the instrument.
• The mechanism is that diffraction produces reinforcement
• The rays which are incident upon the gratings get reinforced with the reflected rays.
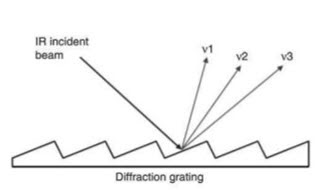
* Advantages over prism-
Made with materials like aluminium which are not attacked by moisture.
* Used over considerable wavelength range.
* Sturdy & long lasting.
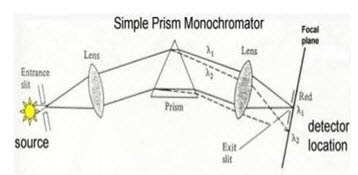
• Entrance slit allows source radiation to illuminate the first lens which collimates the light spreading it across the face of the prism.
• Prism disperses radiation into component wavelengths & the second lens focuses the spectrum at the focal plane.
• An exit slit selects the band of radiation to reach the detector.
3. Detectors:
• An infrared detector is a detector that reacts to infrared radiation.
• It is simply a transducer of radiant energy.
• Detectors are used to measure the intensity of unabsorbed infrared radiation.

Types of detectors
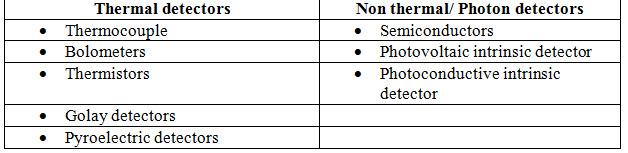
1. Thermal detectors:
• Can be used for wide range of wavelength.
• Operate at room temperature.
• Slow response time & lower sensitivity hence not use for FTIR.
• Thermal detectors are better choice except in the near infrared where photoconductivity cells are generally used.
• Requirements for thermal detectors-
i. Short responsive time
ii. Absorption heat must be lost rapidly
a. Thermocouple-
• Made up of 2 dissimilar metal like bismuth & antimony coated by metal oxides which produce small voltage proportional to temperature of junction.
• If wires of 2 dissimilar metals joined head to tail, then a difference in temperature between head & tail causes a current to flow in the wire.
• This current is proportional to the intensity of radiation.
• These are also called as thermopile detectors.
• Materials should be thermally active.
• These are used in dispersive instruments.
• Response time is 30 milliseconds.
• They give response for all frequencies.
• The potential difference (voltage) between the junctions changes according to the difference in temperature between the junctions.
• Heating capacity of absorbing element is small as to detect the change in temperature.
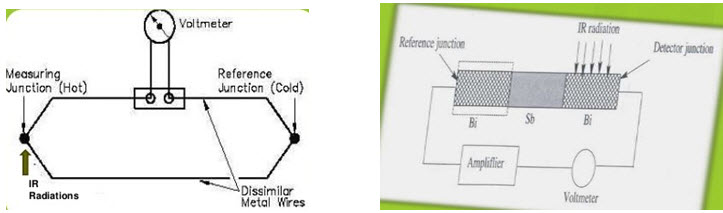
• Potential varies in the 2 junctions with the difference in temperature.
• One junction is cold junction which is reference junction kept at constant temperature not exposed to IR radiations.
• The other junction is hot junction exposed to IR radiations which increases temperature of junction.
• Temperature difference due to falling IR radiations generates the potential difference which amounts to IR radiations.
• Hot junction is generally blackened to improve its heat absorbing capacity.
• Advantage- Simple & used for short IR wavelength
• Disadvantage- slower response time & sensitivity
b. Bolometers-
• Bolometer is derived from a Greek word (bolometron)
Bolo- for something thrown
Metron- measure
• Construction-
• It consists of an absorptive element such as a thin layer of metal
• Most bolometers use semiconductor or superconductor absorptive elements rather than metals.
• Its resistance changes due to increase in temperature when IR radiation falls on it.
• It is electrical resistance thermometer which can detect & measure feeble thermal radiation.
• The electrical resistance increases approx. 0.4% for every Celsius degree increase of temperature.
• It is made of 2 platinum strips, covered with lamp black, one strip is shielded from radiation & one exposed to it.
• The strips form 2 branches of Wheatstone bridge.

• Working-
• The circuit thus effectively operate as resistance temperature detector.
• When IR radiations fall on the exposed strip, unbalance occur due to change in resistance , this causes current to flow through Galvanometer & the amount of current flowing is the measure intensity of IR radiation.
• The response time is 4 seconds.
c. Golay cell-
• It consists of a small metal detector closed by a rigid blackened metal plate (2mm) , flexible slivered diaphragm at the other end filled with xenon gas.
• Its response time is 20 milliseconds, hence faster than other thermal detectors
• It is suitable for wavelengths greater than 15μ.
• Also known as golay pneumatic detector.
• Gas filled chamber undergoes a pressure rise when heated by radiant energy.
• It is based on expansion of gas measuring device.
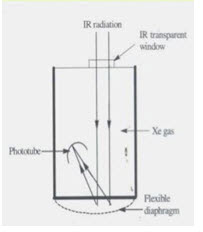
• Working-
• The radiation falls on the blackened metal plate, this heats the gas which leads to deformation of flexible silvered diaphragm.
• The light from a lamp inside the detector is made to fall on the diaphragm which reflects the light on to a phototube.
• The signal seen by the phototube/photocell is modulated in accordance with the power of the radiation beams incident on the gas cell.
• Advantage-
• Wavelength is very wide.
• Response time is much faster than bolometer or thermocouple.
d. Thermistor-
• Made of fused mixture of metal oxides or sintered oxides of Mn, Co, Ni.
• As the temperature of mixture increases, its electrical resistance decreases
• Response time is very slow i.e. 4 seconds.
• Material should be thermally sensitive.
e. Pyroelectric detectors-
• This is made from a single crystalline wafer of pyroelectric material (deuterium triglycine sulphate) sandwiched between two electrodes which are IR transparent.
• Pyroelectric materials are dielectric materials which have special thermal & electrical properties.
• Principle involved is electric polarization.
• When an electric field is applied and the field is removed, the polarization persists, it is temperature dependent and the semi-conductor acts as a capacitor.
• The incident infrared heating causes a change in the capacitance of the material.
• The pyroelectric effect depends on the rate of change of the detector temperature and not on the temperature itself, which allows for faster response time.
• Pyroelectric detectors are used in mid-infrared region, works at room temperature and applied in most FT-IR instruments.
• It involves multiple scanning.
• For a high sensitivity a Hg cadmium telluride (MCT) detector is used cooled by liquid N2
4. Recorder:
• Recorders are used to record the IR spectrum.
• The radiant energy received by detector is converted into measurable electrical signal & is amplified by amplifier
• The amplified signals are recorded & plotted
• It records transmittance of sample as well as function of wave number.
VI. APPLICATIONS
1. Identification of functional group and structure elucidation
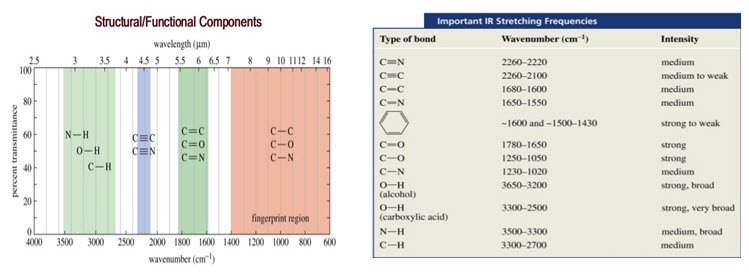
• Entire IR region is divided into group frequency region and fingerprint region. Range of group frequency is 4000-1500 cm-1 while that of finger print region is 1500-400 cm-1.
• In group frequency region, the peaks corresponding to different functional groups can be observed. According to corresponding peaks, functional group can be determined.
• Each atom of the molecule is connected by bond and each bond requires different IR region so characteristic peaks are observed. This region of IR spectrum is called as finger print region of the molecule. It can be determined by characteristic peaks.
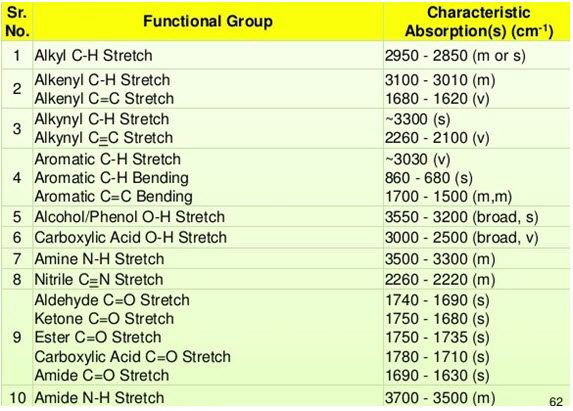
Table 1: IR Absorption
2. Identification of substances
• IR spectroscopy is used to establish whether a given sample of an organic substance is identical with another or not. This is because large number of absorption bands is observed in the IR spectra of organic molecules and the probability that any two compounds will produce identical spectra is almost zero. So if two compounds have identical IR spectra then both of them must be samples of the same substances.
• IR spectra of two enantiomeric compound are identical. So IR spectroscopy fails to distinguish between enantiomers.
• Criteria: sample & reference must be tested in identical conditions like physical state, temperature, solvent, etc.
• For example, an IR spectrum of benzaldehyde is observed as follows.
• C-H stretching of aromatic ring- 3080 cm-1
• C-H stretching of aldehyde- 2860 cm-1 and 2775 cm-1
• C=O stretching of an aromatic aldehyde- 1700 cm-1
• C=C stretching of an aromatic ring- 1595 cm-1
• C-H bending- 745 cm-1 and 685 cm-1
• No other compound then benzaldehyde produces same IR spectra as shown above.
• The Fingerprint Region (1200 to 600cm-1):
• Small differences in structure & constitution of molecule result in significant changes in the peaks in this region.
• Hence this region helps to identify an unknown compound.
• Computer Search System:
• Newer IR instruments offer computer search systems to identify compounds from stored infrared spectral data.
• The position & magnitudes of peaks in the spectrum is compared with profiles of pure compounds stored.
• Computer then compares profiles similar to that of the analyte & result is displayed.
3. Studying the progress of the reaction
• Progress of chemical reaction can be determined by examining the small portion of the reaction mixture withdrawn from time to time.
• The rate of disappearance of a characteristic absorption band of the reactant group and/or the rate of appearance of the characteristic absorption band of the product group due to formation of product is observed.
• E.g.: oxidation of secondary alcohol by chromium (VI) is accompanied by decrease in the absorption intensity of OH group.
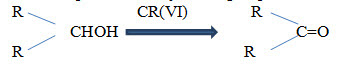
4. Detection of impurities
• When a compound is containing impurity, it reduces sharpness of individual bands, causing appearance of extra band or peak.
• IR spectrum of the test sample to be determined is compared with the standard compound.
• E.g.: small quantity of ketone in hydrocarbon can be detected as band near 1720cm 1, characteristic of ketone.
• Detection is favoured when impurity possess a strong band in IR region where the main substance do not possess a band.
• E.g.: impurity in bees wax (with petroleum wax)
5. Determination of molecular structure
• IR spectra mainly used to determine the molecular structure of unknown organic compound.
• It is done by correlation & interpretation of IR spectra.
• Used along with other spectroscopic techniques
• Identification is done based on position of absorption bands in the spectrum.
• E.g.: C=O at 1717cm 1
• Absence of band of a particular group indicates absence of that group in the compound.
• From the position of absorption bands in the spectrum it is possible to establish the nature of groups present in the molecule.
• E.g.: if the spectrum contain strong bond between 1690-1760cm-1, the compound must contain a carbonyl group.
6. Isomerism in organic chemistry
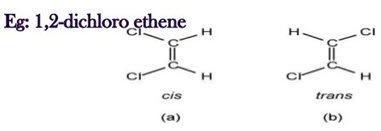
i. Geometrical isomerism:
• Vibration in IR is caused is caused by change in dipole moment.
• This technique clearly makes a distinction in cis & trans isomers.
• Trans isomers give a simpler spectrum than cis due to symmetry
• In trans isomers no (zero) dipole occur, so no peak is observed but in case of cis, dipole moment occur, so peak is formed at 1580cm-1.
• Generally, the configuration around a di-substituted double bond is easy to determine from the IR spectra.
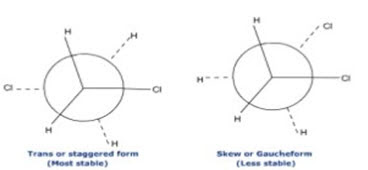
ii. Conformers (Rotational Isomers)
• Identified with the help of high resolution IR spectrometers.
• E.g.: ethanol normal band O-H at 3636cm-1 or weak band at O-H 3622cm-1.
• IR spectroscopy helps in detection of skew (gauche) & trans (staggered) conformation.
• Two bands at 1291 & 1235cm-1.
• Trans form predominates at low temperature while skew at higher temperature.
iii. Tautomerism
• Existence of 2 or more chemical compounds capable of interconversion usually by exchanging a hydrogen atom between the 2 atoms.
• E.g.: Thiocarboxylic acid. Both the molecule give different peaks in IR spectrum due to presence of C=S & C=O & in this way they can easily identified.
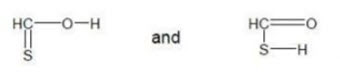
7. Functional group isomerism
• Isomerism shown by the compounds having same molecular formula but different functional group can also be determined using IR spectroscopy.
• E.g.: dimethyl ether (CH3-O-CH3) & ethanol (CH3-CH2-OH)
• In dimethyl ether, the functional group is ether (-O-), whereas in ethanol the functional group is alcohol (-OH-).
• The characteristic peak of –OH group appears at 3500-3100cm-1 which differentiate ethanol from dimethyl ether.
8. Determination of orientation in aromatic compounds
• The IR absorption bands in the region 675-900cm-1 due to out-of-plane bending vibrations gives information about the relative positions of substituents on the benzene ring
• The position of these absorption bands in this region depends on the number of adjacent hydrogen atoms present in the ring.
• The o-substituted benzenes show a strong band in the region 735-770cm-1 but no band in the region 690-710cm-1
• The m-di-substituted benzenes show 2 strong bands in the regions 680cm-1 & 750-810cm
• The p-di-substituted benzenes exhibit only one band in the region 800-860cm-1
9. Detection of hydrogen bonding & distinguish inter & intramolecular
• IR spectroscopy is a powerful & widely used method for studying hydrogen bonding.
• Hydrogen bonding alters the vibration frequencies of OH & NH group.
• An inert simple alcohol exhibits a sharp absorption at 3620-3640cm 1 & broad at 3500-3200cm-1.
• As solution dilution increases, sharp peak is obtained.
• The sharp absorption at higher wavelength is due to OH stretching.
• It becomes more intense at higher dilution because association of solute molecules is less probable.
• At higher concentration, they form dimers, trimers & polymers & so it gives broad peaks.
• Intermolecular hydrogen bonding decreases with dilution, whereas interamolecular hydrogen bonding shows no effect.
10. Quantitative Analysis
• The quantity of the substance can be determined either in pure form or as a mixture of two or more compounds.
• In this, characteristic peak corresponding to the drug substance is chosen and log I0/It of peaks for standard and test sample is compared. This is called base line technique to determine the quantity of the substance.
• All quantitative spectrophotometric measurement are governed by Beer`s law.
• In IR spectrophotometric , deviation to beer`s law are more due to:
• Week intensity of light source
• Week detection by detectors
• By employing wider slit width .
• Disadvantages of IR in Quantitative analysis:
• Non adherence of Beer’s law
• Complexity of spectra
11. Limit test of polymorphs
• Polymorphs are same molecules differing only by crystalline forms.
• E.g. . chloramphenicol palmitate suspension IP, BP, USP
• Chloramphenicol palmitate - 3 polymorphic forms
• Form A – least bioavailable & least activity.
• Form B
• Form C
• Limit of Chloramphenicol palmitate in Pharmacopoeia – NMT 10 % of form A which is found out by IR spectroscopy.
• In IP and USP - 1 parts of polymorph A and 9 parts of polymorph B are mixed and spectrum is recorded.
• Test must not have peak greater than standard.
12. Identification of drugs
• Ibuprofen in IP, BP and USP identified by IR- because it contains COOH group so, chemical tests are not useful for identification.
• Steroids are difficult to be distinguished from ketones by any chemical test. U . V. spectrum is same as the chromophoric part is same in both. But IR spectrum can give complete difference between steroids and ketones.
• Steroid identified are Cortisone acetate, Dehydro cortisone acetate, Ethylsterone , Prednisolone , Prednisone, Progesterone All the sulpha drugs in BP’93 are identified
• Other compounds identified are Ethambutol, Ibuprofen Cholecalciferol , and Disodium EDTA.
• In IP and USP spectrum of the test and standard are compared.
13. Other Applications:
i) Determination of unknown contaminants in industry using FTIR
ii) Determination of cell walls of mutant & wild type plant varieties using FTIR
iii) Biomedical studies of human hair to identify disease states
iv) Identify odour & taste components of food
v) Determine atmospheric pollutants from atmosphere itself.
vi) Examination of old paintings & artifacts
vii) To determination of raw materials.
viii) Quality control of pharmaceutical formulation
ix) Determination of particle size
x) Determination of blend uniformity
xi) Determination or identification of polymorphic drugs
xii) Can be used to characterize samples in solid states’ water content measurement
xiii) Analysis of urine & other bio fluids ( urea, creatinine, protein)
xiv) Used in non-invasive measurement of glucose
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT admin@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE









