{ DOWNLOAD AS PDF }
ABOUT AUHTORS
Deepak Chowrasia*, Nisha Sharma
University Institute of Pharmacy, Chhatrapati Shahu Ji Maharaj University, Kanpur (U.P.)-208024, India
*debpratim008@gmail.com
ABSTRACT
Cancer remains a global concern not only for clinicians, but also for chemist owing to its alarming incidence rate. Conventional cancer chemotherapeutics are inadequate requiring newer molecule for its cure and management. Heterocyclic chemistry emerges as a distinct branch of organic chemistry responsible for yielding uncountable pharmacologically active substances among nitrogen containing ring systems are thoroughly investigated owing to their unique characteristic. The present study to explore feasibility of nitrogen containing synthetic molecules as novel anticancer agents.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2487
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 5, Issue 5 Received On: 06/01/2017; Accepted On: 21/01/2017; Published On: 01/05/2017 How to cite this article: Chowrasia D, Sharma N; Heteroatom (Nitrogen) based Synthetic templates as Anticancer Agents; PharmaTutor; 2017; 5(5); 93-98 |
INTRODUCTION
Cancer “an extraordinary disease of human custom” characterized by uncontrolled cellular proliferation. The disease distinctly scores as second leading cause of death worldwide. Conventional treatment modalities for its management are inadequate, requiring novel intervention to conquer the increasing cancer incidence. As per global indices, approximately one in 5 of all individual children born will develop cancer in their lifetime. Despite extensive clinical investigation, specific root cause of cancer is still questionable. Although, conjunction of various physical, chemical, environmental, and genetic factors are thought to be responsible for its occurrence. Rapid progression and uncontrolled mortality rates alarmed scientists to search for better interventions to minimize human suffering however metastasis, multi-organ involvement, delayed detection, inadequate/costly treatment plan, resistance towards anticancer drugs and their pronounce side effects are some of the fingertips points rendering its cure & management. Furthermore, severe side effect of cancer regime promotes poor patient compliance leading to progression of the disease to advance stage causing death of victim. Over the past years numerous molecules from natural origins are screened to elucidate their anticancer activity however only few are clinically successful. Bulkier molecules, lengthy/costlier extraction process, requirement of huge quantity of solvents, slow extraction rate, laborious purification process, tedious analysis methodologies, and lower yield of desired constituent are some of the brusque in search & development of herbal anticancer agents. In contrast synthetic molecules are extraordinarily free from above mentioned limitations. Development in the field of heterocyclic chemistry tooled synthetic chemist to explore novel heteroatom based cyclic molecules to potentiate cancer regime armamentarium. Nitrogen being possessing specific characteristics such as unique electronic configuration, electronegativity, and basicity acting as a thrust molecule for design and development of novel anticancer agents. Furthermore combination of nitrogen with other heteroatoms especially sulphur yield heterocyclic compounds with better pharmacological action. The present paper explores some of the feasibility of same to design novel anticancer agents.
Anticancer prospective of heterocyclic system containing nitrogen
Hydrazide-hydrazone derivative synthesised by Mohareb et al.1 by reacting cyanoacetyl hydrazine with 3-acetypyridine. The yielded molecule undergoes series of heterocyclization to yield novel heterocyclic compounds. The antitumor evaluation of the newly synthesized compounds are screened against three different cancer cell lines namely MCF-7, NCI-H460 and SF-268; most of them showed good anticancer activity.
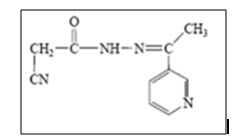
Figure 01: Cyanoacetylhydrazide derivative
Terzioglu2 et al. synthesized some novel 2,6-dimethyl-N-substituted phenylmethylene-imidazo[2,1-b][1,3,4]thiadiazole-5-carbohydrazides from 2,6-dimethyl imidazo-[2,1-b][1,3,4]- thiadiazole-5-carbohydrazide and screened them to elucidate their anticancer activity. Among synthesised compounds 2,6-Dimethyl-imidazo[2,1-b][1,3,4]thiadiazole-5-carboxylic acid (2-hydroxy-benzylidene)-hydrazide showed favorable anticancer activity among the tested compounds
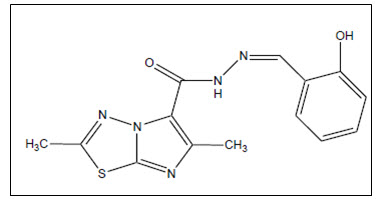
Figure 02 Phenylmethylene-imidazo [2,1-b][1,3,4]thiadiazole-5-carbohydrazid
Novel fused 1,2,4-triazine aryl derivatives containing the ethoxycarbonyl and carbohydrazide moiety was synthesised by Sztanke3 et al. The in-vitro anticancer evaluation of these compounds was done by using BrdU method for human LS180, SiHa and T47D carcinoma cells. It has been found that 8-(3-Chloro-phenyl)-4-oxo-4,6,7,8-tetrahydro-imidazo[2,1-c][1,2,4] triazine-3-carboxylic acid hydrazide exhibited potent avtivity against SiHa and LS180 tumour cells.

Figure 03 Fused 1,2,4-triazine aryl derivatives
Lembege4 et al. synthesized dihydroxy-xanthone carbaldehydes, compounds were further tested for their in vitro antiproliferative activity via. colorimetric method (MTT) against breast adenocarcinoma and squamous cell oral carcinoma cell lines. Among the series, 6-((2-(4,5-dihydro-1H-imidazol-2-yl)hydrazono)-methyl)-9-oxo-9H-xanthene-1,3-diyl diacetate was having highest antiproliferative activity.
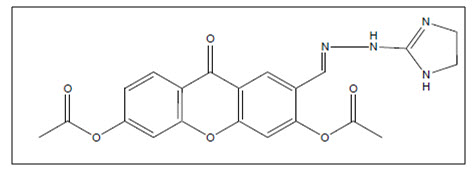
Figure 04 Dihydroxyxanthone carbalde
Eshkourfu5 et al. reported synthesis of some novel heterocyclic derivatives N′,N′2-bis[(1E)-1-(2-pyridyl)ethylidene]propanedihydrazide. The highest cytotoxic potential of the compound was observed on the MDA-361 or epithelial breast cancer. The investigated compounds are further studied for their mechanistic action
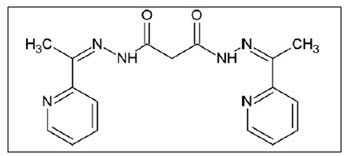
Figure 05 Ethylidene propanedihydrazide
Xia6 et al. synthesized series of novel 1-arylmethyl-3-aryl-1H-pyrazole-5-arbohydrazide hydrazone derivatives and the effects of all compounds were screened on A-549 cell growth were investigated. The results showed that all compounds had almost inhibitory effects on the growth of A-549 cells. The study on structure activity relationships and lipophilicities of compounds indicate, compounds with Log-P values in the range of inhibitory effects on the growth of A-549 cells, and among of them the hydrazone derived from salicylaldehyde had much more inhibitory effects.
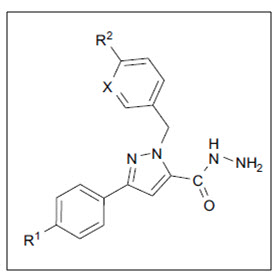
Figure 06 Pyrazole-5-Arbohydrazide Hydrazone
Rostom7 et al. synthesized novel series of 1-(4-chlorophenyl)-4-hydroxy-1H-pyrazole-3-carboxylic acid hydrazide derivatives including some 4-substituted-1,2,4-triazolin-3-thiones, 2-substituted-1,3,4-thiadiazole, and 2-substituted-1,3,4-oxadiazoles. Among synthesized compounds, ten were selected by the National Cancer Institute (NCI) for in-vitro antitumor screening. Seven compounds, exhibited potential and broad spectrum antitumor activity against most of the tested tumour cell lines.
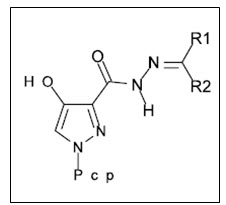
Figure 07 Pyrazole-3-carboxylic acid hydrazide
Liang8 et al. synthesized series of N-(2-oxoindolin-3-ylidene)hydrazides and identified as moderately potent inhibitors against c-Met kinase by virtual pharmacophore based screening and synthetic methods. The structure activity relationship (SAR) of molecule was studied and its binding mode with c-Met kinase was analyzed by virtual molecular modeling. The compound showed prominent activity against various cancer cell lines.
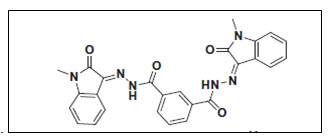
Figure 08 Oxoindolin-3-ylidene)hydrazide
Kamal et al.9 synthesized series of N-[2-anilino-3-pyridyl]carbonyl-1-benzenesulfonohydrazide derivatives) and five of them were selected by the National Cancer Institute (NCI) and evaluated for their in vitro anticancer activity. Three of the investigated compounds showed potent anticancer activity in the primary assay. Most of these compounds showed better inhibitory activity in comparison to the standard drugs.
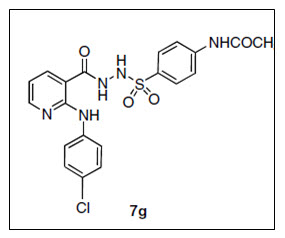
Figure 09 Benzenesulfonohydrazide derivatives
Sztanke et al.10 synthesised and screened anticancer activities of fused 1,2,4-triazine aryl derivatives containing ethoxycarbonyl and carbohydrazide substituent. In-vitro antitumour activities of heterobicyclic hydrazides were evaluated by BrdU method against human LS180, SiHa and T47D carcinoma cells. Amongst them, hydrazide exhibited remarkable inhibitory effect against SiHa, LS180 tumour cells, and simultaneously was found to be non-toxic towards the human normal cell line HSF cells.
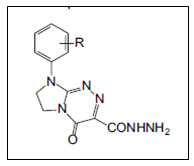
Figure 10- 1,2,4-triazine hydrazide
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Series of 2(4-alkoxyphenyl)cyclopropyl hydrazide and triazolo derivatives from 4-hydroxycinnamic acid were synthesised by Prithwiraj et al.11 in clean, mild, efficient, and straight forward synthetic protocol. These compounds consisting of different alkoxy substitutions, phenylcyclopropyl backbone and different heterocyclic groups were screened in vitro for anticancer activity against 4 different cell lines concluding some levels of resistance to pro-apoptotic stimuli
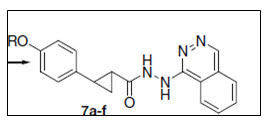
Figure 11 Alkoxyphenyl cyclopropyl hydrazide
Sandrine et al.12 shown potent anticancer activity of hydrazinoaza and N-azapeptoids on murine leukemia L1210 cells
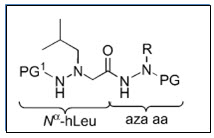
Figure 12 N-Azapeptoids derivative
Vogel et al.13 synthesized 2-phenylindole-3-carbaldehydes by from hydrazides of benzoic acid and pyridine carboxylic acids. The compounds inhibited growth of two cell lines viz. MDA-MB 231 and MCF-7 breast cancer cells with IC50 values of 20–30 nM for most active derivatives
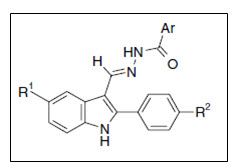
Figure 13 Phenylindole hydrazide
1-(substituted benzenesulphonyl)-5-oxopyrrolidine-carboxyl acid hydrazides derivatives are screened by Purkayastha et al.14 for anticancer activity in Swiss albino mice with Ehrlich ascites carcinoma cell line. The percentage inhibition of growth in ascitic cell count as well as fluid weight has been taken as activity parameters to rule out anticancer property. The comparative study indicates superior activity of phenylhydrazides over simple hydrazide derivatives.
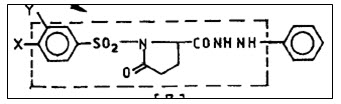
Figure 14 Benzenesulphonyl carboxyl acid hydrazides
Burja et al.15 had synthesized series of pyrazolone-fused combretastatins and evaluate their cytotoxic as well as antitubulin activity. The hydrazide and the pyrazolone-fused combretastatins were highly cytotoxic against various tumor cell lines as well as active against cisplatin resistant cells. The compounds were also concluded to be potent inhibitors of tubulin polymerization
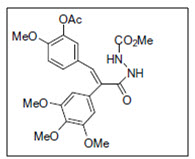
Figure 15 Pyrazolone hydrazide
Alvarez et al.16 synthesized and biologically evaluated series of diarylmethyloxime and diarylmethylhydrazone analogues containing indole ring and different modifications are done on the nitrogen present on the bridge. Several compounds showed potent inhibitory action on tubulin polymerization as well as cytotoxic activity against cancer cell lines. It is further concluded N-methyl-5-indolyl derivatives are active than ethyl substituted derivatives.
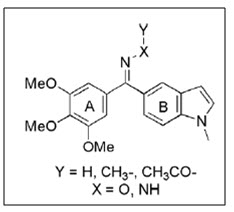
Figure 16 Diarylmethylhydrazone
Galal et al.17 synthesized benzimidazole-5-carboxylic acid hydrazides shown a potent anticancer activity. The compound shown cytotoxicity against both human lung cancer cell line A-549 and human breast cancer cell line MCF-7.
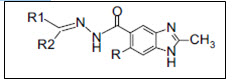
Figure 17 Benzimidazole-5-carboxylic acid hydrazides
CONCLUSION
Heterocyclic chemistry contributes about one-third of total pharmacologically active molecules among which nitrogen containing ring systems are much explored and studied building block for development of therapeutically active molecule especially anticancer agents due to unique characteristic of nitrogen. Summarily, hydrazide/hydrazone derivatives containing heterocyclic moiety may yield compound with potent anticancer activity.
REFERENCES
01.Mohareb, Rafat M.; Fleita, Daisy H.; Sakka, Ola K.; Novel synthesis of hydrazide-hydrazone derivatives and their utilization in the synthesis of Coumarin, Pyridine, Thiazole, and Thiophene derivatives with antitumor activity; Molecules 2011, 16, 16-27.
02.Terzioglu, Nalan; Gursoy, Aysel; “Synthesis and anticancer evaluation of some new hydrazone derivatives of 2,6-dimethylimidazo[2,1-b]-[1,3,4]thiadiazole-5-carbohydrazide”; Eur. J. Med. Chem. 2003, 38: 781-786.
03.Sztanke, K.; Pasterhak, K.; Rzymowska, J.; " Synthesis, structure elucidation and identification of antitumoural properties of novel fused 1,2,4-triazine aryl derivatives "; Eur. J. Med. Chem. 2008, 43: 1085-1094.
04.Varache-Lembege, M.; Moreau, S.; Larrouture, S.; Montaudon, D.; Robert, J.; Nuhrich, A.Varache, M.; Moreau, S.; Larrouture, S.; “Synthesis and antiproliferative activity of aryl and heteroaryl-hydrazones derived from xanthene carbaldehyde"; Eur. J. Med. Chem.2008, (43),1336-1343.
05.Rabia, Eshkourfu; Bozidar,Cobeljic; Miroslava, Vujcic; Iztok, Turel; Andrej, P.; Kristina Sepcic,; Manja Z.; Sinisa, R.; Tatjana S.; Dragana M.; Katarina, A.; Dusan, S.;"Synthesis, characterization, cytotoxic activity and DNA binding properties of the novel dinuclear cobalt(III) complex with the condensation product of 2-acetylpyridine and malonic acid dihydrazide"; J. of Inorg. Med. Chem, 2011, 1196-1203.
06.Yong X.; Chuan-Dong, F.; Bao-Xiang, Z.; Jing, Z.; Dong-Soo, S.; Jun-Ying, M.; "Synthesis and structure activity relationships of novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide hydrazone derivatives as potential agents against A549 lung cancer cells"; Eur. J. Med. Chem. 2008, (43), 2347-2353..
07.Rostom, S.; Shalaby, Manal A.; El-Demellawy, Maha A.; “Polysubstituted pyrazoles, Synthesis of new 1-(4-chlorophenyl)-4-hydroxy-1H-pyrazole-3-carboxylic acid hydrazide analogs and some derived ring systems. A novel class of potential antitumor and anti-HCV agents”; Eur. J. Med. Chem. 2003, 38: 959-974.
08.Zhongjie, L.; Dengyou, Z.; Jing, A.;, Limin, C.; Hengshuai, W.; Xiangqian, K.; Mingyue, Z.; Hong, Liu; Cheng, L. Meiyu, Geng; Hualiang, Jiang; Kaixian, Chen; “Identification and synthesis of N-(2-oxoindolin-3-ylidene)hydrazide derivatives against c-Met kinase”; Bioorg. Med. Chem. lett. 2011, (21), 3749-3754.
09.Kamal, A.; Naseer , M.; Reddy, K. S.; Rohini, K. ; “Synthesis of a new class of 2-anilino substituted nicotinyl arylsulfonylhydrazides as potential anticancer and antibacterial agents”; Bioorg. Med. Chem. 2007, (15), 1004-1013.
10.Sztanke, Krzysztof; Pasternak, K.; Rzymowska, J.Ma1gorzata, S. Martyna, K.; “Synthesis, structure elucidation and identification of antitumoural properties of novel fused 1,2,4-triazine aryl derivatives”; Eur. J. Med. Chem. 2008, (43): 1085-1094.
11.Prithwiraj, D.; Michel, B.; Lamoral-Theys, Delphine; Kiss, Bedos-Belval, R.Florence; Nathalie, S.; Synthesis and anticancer activity evaluation of 2(4-alkoxyphenyl)cyclopropyl hydrazides and triazolo phthalazines”; Bioorg. Med. Chem 2010, (18), 2537-2548.
12.Karine, Sandrine; Aubin, Jeanguy; Arlot-Bonnemainsc, Yannick; Miche, le Baudy-Flocha; “Hydrazino-Aza and N-Azapeptoids with Therapeutic Potential as Anticancer Agents”; Bioorg. Med. Chem. lett. 2003,(11), 4881-4889.
13.Vogel, Susanne; Doris, Kaufmann; Michaela, Pojarova; Christine M.; Tobias, P.; Sybille, K. Patrick, J. B.; Erwin, A.; “Aroyl hydrazones of 2-phenylindole-3-carbaldehydes as novel antimitotic agents”; Bioorg. Med. Chem., 2008, (16), 6436-6447.
14.Satyendra, P.; De, A.U.; “Synthesis Activity and Qsar Studies of Some I-(Substituted Benzenesulpkonylb5- Oxopyrrolidine-Z-Carboxylic Acid Hydrazides”; Bioorg. Med. Chem. lett., 1994, (4), 377-380.
15.Burja, Bojan; Cimbora-Zovko, Tamara; Sanja, Tomic; Jelusic, Tihana. Marijan,; Slovenko, Polanc ; Maja, Osmak; “Pyrazolone-fused combretastatins and their precursors”; Bioorg. Med. Chem., 2010, (18), 2375-2387.
16.Alvarez, Concepcion.; Alvarez, R.; Purificacion, C.; Perez-Melero, C.; Medardea, Manuel; “Diarylmethyloxime and hydrazone derivatives with 5-indolyl moieties as potent inhibitors of tubulin polymerization”; Bioorg. Med. Chem., 2008, (16), 5952-5961.
17. Galal, Shadia A.; Hegab, Khaled H.; Kassab, Ahmed S.; Rodriguez, Mireya L.; Kerwin, Sean M.; El-Khamry, A.; Eldiwani, Hoda I.; “New transition metal ion complexes with benzimidazole-5-carboxylic acid hydrazides with antitumor activity”; Eur. J. Med. Chem. 2009, (44), 1500-1508.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









