ABOUT AUTHORS
Mudasir Maqbool1*, Mohmad Amin Dar1, Shafiqa Rasool1, Rabiah Bashir1, Misba Khan 2
1Department of Pharmaceutical Sciences,
University of Kashmir, Hazratbal Srinagar, Jammu and Kashmir, India
2Mader-E-Meharban Institute of Nursing Sciences and Research,
Jammu And Kashmir, India
ABSTRACT
The maintenance of a healthy liver is vital to overall health of the human beings. Since the liver is involved in almost all biochemical processes and there are many different diseases that will affect it. The liver is often abused by environmental toxins, which are eating habits, alcohol and overdose of certain drugs which can damage and weaken the liver and eventually lead to many diseases. Medicinal herbs are significant source of hepatoprotective drugs. Mono and poly-herbal preparations have been used in various liver disorders. According to one estimate, more than 700 mono and poly-herbal preparations in the form of decoction, tincture, tablets and capsules from more than 100 plants are in clinical use. From the literature review near about 178 medicinal plants are reported to possess a hepatoprotective activity. A drug having beneficial effect on the liver is known as hepatoprotective drug. On the other hand, drugs having toxic effect on the liver are better known as hepatotoxic drugs. The most commonly used parameters to assess the hepatoprotective activity are morphological e.g. Liver weight and volume, biochemical estimations, such as measurement of transaminase activity, SGPT, SCOT, alkaline phosphatase, serum bilirubin, total serum proteins, albumin, globulin and prothrombin time, functional parameters, pentobarbitone and hexobarbitone sleeping time and finally histopathological study regarding presence of necrosis, fatty degeneration and cirrhosis. In this review, we will briefly discuss hepatotoxicity and hepatoprotective agents.
Reference Id: PHARMATUTOR-ART-2684
INTRODUCTION
Liver is the largest and most complex internal organ of living systems. It serves an important role in maintenance of internal environment through its multiple and diverse functions. It is involved in the intermediary metabolism of proteins, fats and carbohydrates. It acts as a storage depot for proteins, glycogen, various vitamins and metals. It also has a role in regulation of blood volume by transferring blood from portal to systemic circulation and its reticuloendothelial system participates in immune mechanism. It plays central role in detoxification and excretion of many endogenous and exogenous compounds. Liver diseases are fatal and leading cause of illness and deaths worldwide (Wang et al., 2014a). As per research, liver disorders cause about 18000 to 20000 deaths every year globally (Fatma and Uphadhyay, 2015; Akila and Prasanna, 2014). In United States, about 2-5 % of hospital admissions are due to liver injury out of which 10% results in acute liver failures (Pandit et al., 2012 ;Ostapowicz et al., 2002). The crude incidence of liver disorder is 14 per 100000 per annum globally, whereas the standard incidence is 8.1 per 100000 per annum (Bedi et al., 2016). Acute liver failure rate is up to 13% of the cases in developed nations like USA whereas it is less (5%) in tropical countries like India (MeMahon, 2005).
Hepatotoxicity is most commonly seen in the form of malfunction or damage to the liver due to excess amount of drugs or xenobiotics (Navarro, 2006; Singh et al., 2011; Bahar et al., 2013). Hepatotoxicants are exogenous agents of clinical relevance which may include an overdose of certain medicinal compounds (acetaminophen, nimesulide, antitubercular drugs like isoniazid, rifampicin etc.), industrial chemicals (alcohol, CCl4, beta galactosamine, thioacetamide) etc., which causes liver injury (Willett et al., 2004; Bigoniya et al., 2009; Papay et al., 2009; Singh et al., 2011; Pandit et al., 2012). The exact mechanism of drug induced liver injury remains largely unknown, but it appears to involve two pathways – direct hepatotoxicity (Type A or DILI1 (drug induced liver injury1), intrinsic or predictable drug reaction) and indirect hepatotoxicity (Type B or DILI2 (drug induced liver injury2), unpredictable or idiosyncratic drug reaction,) or adverse immune reaction (Bigoniya et al., 2009). Some most common direct hepatotoxins are carbon tetrachloride, thioacetamide, acetaminophen, galactosamine, fulvine, phalloidin, ethyl alcohol, aflatoxins etc. whereas examples of indirect hepatotoxins are methyl testosterone, chlorpropamide, tetracycline, halothane, phenytoin, methyldopa, sulphonamides, allopurinol, rifampicin etc (Bigoniya et al., 2009). Hepatotoxicity is manifested by different types of injuries, depending on the nature and dose of the chemical. Hepatotoxicity may result into cytotoxic effects (necrosis, apoptosis), cholestasis, steatosis, fibrosis, cirrhosis, hepatitis and liver tumors (Lee, 2003). Hepatotoxicity related symptoms may include jaundice or icterus appearance causing yellowing of the skin, eyes and severe abdominal pain, nausea or vomiting, weakness, severe fatigue, continuous bleeding, skin rashes, generalized itching, swelling of the feet and/or legs, abnormal and rapid weight gain in a short period of time, dark urine and light coloured stools (Chang and Schaino, 2007).
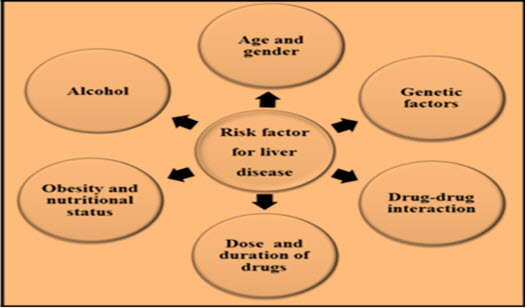
(Singh et al., 2016)
Hepatoprotective Agents
Hepatoprotective agents have been given attention due to their active roles in the additional treatment of liver disease (Flatland, 2003; Sartor and Trepanier, 2003; Twedt, 2004). In order for a compound to be used as a drug, it must be safe, harmless and effective for its intended use. The drug can be released in the market only after undergoing an extensive Food and Drugs Administration’s (FDA) drug approval process which is lengthy and costly. Apart from modern drugs, there are several hepatoprotective agents like L-carnitine (Yapar et al., 2007), Vitamin C (Adikwu and Deo, 2013), N-acetylcysteine (Maheswari et al., 2014), and Milk Thistle (Silymarin) . A review of available literature regarding medicinal plants with hepatoprotective activity revealed that different hepatotoxins were used by different workers to evaluate the activity in invitro and in vivo models. In some studies more than one hepatotoxin was used to screen the same plant. The most commonly used hepatotoxin was carbon tetrachloride (CC14 ). Near about 80% of studies CCl4 was used, irrespective of the route of administration. The total dose of CCI4 administered was in the range of 0.2-2ml / kg in acute liver damage with one day treatment and in the range of 1.5-5ml / kg in divided doses over a period of one week for chronic (reversible), 12-20ml / kg for 5-12 week (irreversible).The most commonly used parameters to assess the hepatoprotective activity were morphological e.g. Liver weight and volume, biochemical estimations, such as measurement of transaminase activity, SGPT, SCOT, alkaline phosphatase, serum bilirubin, total serum proteins, albumin, globulin and prothrombin time, functional parameters, pentobarbitone and hexobarbitone sleeping time and finally histopathological study regarding presence of necrosis, fatty degeneration and cirrhosis.Some of the studies were invitro methods for screening medicinal plants was used, where an increase in percentage of cells and enhancement of the rate of consumption of oxygen and reversal of the enzymatic values such as SGPT, SCOT, ALT in primary cultured hepatocytes was noted, these methods have been most commonly used by foreign investigators (especially Japanese investigators).
They have standardised invitro screening procedures employing primary cultured hepatocytes. In India this invitro method of screening medicinal plants for their hepatoprotective activity is not much more used extensively, probably due to technical difficulties and non-availability of the facilities to culture and maintain hepatocytes. Large-scale primary screening is possible with this method and further detailed study can be done afterwards.In vivo methods are rather time consuming and costly, since number of animals (rat or mice) required are more and study of various parameters, such as biochemical and histopathological studies add to its cost and with all these only one plant can screened at a time. (Vargas-Mendoza et al., 2014;Das et al., 2011).
Silymarin
Silymarin is a standardized extract from the seeds of a plant called milk thistle (Silybum marianum L.; Family: Asteraceae). In rural areas, it has been used as a natural remedy to treat liver diseases (Saller et al., 2001). Silymarin helps to protect and enhance the regeneration of liver cells in most of the liver diseases like cirrhosis, hepatitis and jaundice (Flora et al., 1998). Silymarin possess membrane stabilizing, anti-oxidative, anti-lipid peroxidative (Pascual et al., 1993), anti-fibrotic (Jia et al., 2001), and immune-modulatory properties and helps in liver regeneration (Pradhan and Girish, 2006). Studies on human beings demonstrated that about 20-40% silymarin is excreted as sulphates and glucuronide conjugates in bile (Saller et al., 2001). There are a few reports of low level of silymarin toxicity causing allergic skin rashes and gastrointestinal disturbances (Saller et al., 2001).
Herbal Formulations
Numerous medicinal plants and their formulations are used to treat liver disorders in ethno medicine practice as well as traditional system of medicine in India. There are about 600 commercial herbal formulations available in market all over the world, which are claimed to have hepatoprotective activity (Bedi et al., 2016). In India, about 40 anti-hepatotoxic, patented, polyherbal formulations representing a variety of combination of 93 medicinal plants from 44 families are available (Sharma et al., 1991). More than 700 mono and poly-herbal hepatoprotective preparations from more than 100 plants are in clinical use in the form of decoction, tincture, tablets and capsules. Recent global increase in the utilization of herbal drugs has also been reported in the literature (Girish et al., 2009).
Medicinal Plants
Eastern countries have been using herbal drugs to treat liver diseases since ancient time (Rajaratnam et al., 2014). The ancient Chinese and Egyptian written records are available which describe medicinal uses of plants (Rajaratnam et al., 2014). In ancient India (Vedic period) and China (Xia dynasty), records on use of herbal medicines track back to 2100 BC. The first written reports date back to 600 B.C. with the Charka Samhita of India and the early notes of the Eastern Zhou dynasty of China around 400 B.C (Onyije and Avwioro, 2012). Ayurveda, an indigenous system of medicine in India, has a long tradition of treating liver disorders with plant drugs. Minimizing side effects and increasing therapeutic efficacy of medicines is the basic need of today. Alternative system of medicine like Ayurveda, Unani etc. has been proved to be effective with minimum side effects. With rich diversity of plants, over 45,000 diverse plant species are found in India out of which about 15,000-20,000 plants have medicinal and therapeutic properties. Of these, only about 7,000-7,500 are being used by traditional practitioners (Bedi et al., 2016). As per WHO report, around three quarters of the world’s population uses herbs and other traditional medicines to cure various diseases, including liver disorders (Chaudhury and Refei, 2001). The medicinal plant such as Guduchi (Sharma and Pandey, 2010), Elephantopus scaber (Ho et al., 2012), Aquilegia vulgaris (Adamska et al., 2003), Strychnos potatorum (Sanmugapriya & Venkataraman, 2006), Tridax procumbens (Ravikumar et al., 2006), Picrorhiza kurroa (Mohd et al., 2012), Silybum marianum (Hermenean et al., 2015), Andrographis paniculata (Nasir et al., 2013), Azadirachta indica (Johnson et al., 2015) and Glycyrrhiza glabra (Sharma and Agrawal, 2014) has proven hepatoprotective properties and are used to treat liver disorders. Guduchi (Tinospora sp.) is one of the most versatile rejuvenating shrubs, also known as ’Giloya’ in Indian vernacular, and is reported to have many therapeutic applications (Pandey et al., 2012). Guduchi, as it is most commonly called, has been described as “one which protects the body” (Gawhare, 2013).
Some of the plant constituents possessing hepatoprotective activity (Handa et. al., 1986).

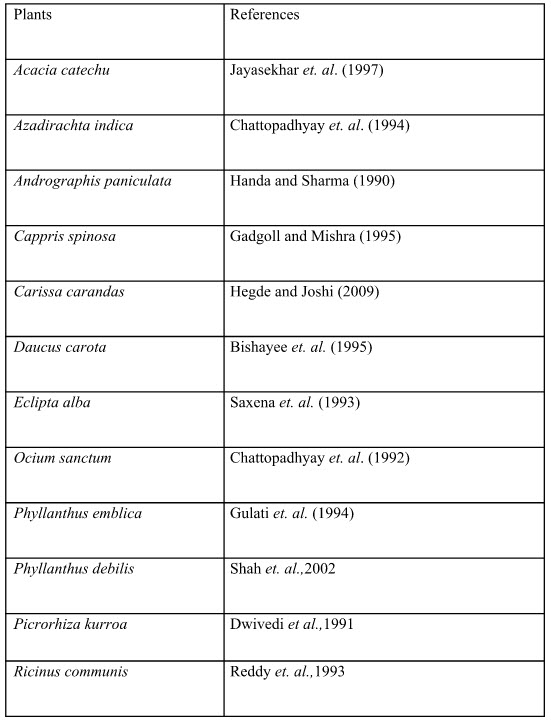
List of few plants with hepatoprotective property against toxic chemical induced liver damage in experimental animals.
CONCLUSION
The liver is of vital importance in intermediary metabolism, in the detoxification and in the elimination of toxic substances. Since the liver has considerable functional reserve, damage to the organ may not affect its activity. The maintenance of a healthy liver is vital to overall health of the human beings. Since the liver is involved in almost all biochemical processes and there are many different diseases that will affect it. The liver is often abused by environmental toxins, which are eating habits, alcohol and overdose of certain drugs which can damage and weaken the liver and eventually lead to many diseases. Therapies developed along the principles of Western medicine are often limited in efficacy, carry the risk of adverse effects, and are often too costly, especially for the developing world. Therefore, treating liver diseases with plant-derived compounds, which are accessible and do not require laborious pharmaceutical synthesis seems highly attractive.
REFERENCES
1. Adikwu E. and Deo O. (2013) “Hepatoprotective Effect of Vitamin C (Ascorbic Acid)” Journal of Pharmacology & Pharmacy 4(1): 84-92.
2. Akila M. and Prasanna G. (2014) “Hepatoprotective Effect of Indigofera Linnael Ali. On Carbon Tetrachloride Induced Wistar Albino Rats” International Research Journal of Pharmacy 5(5): 392-395.
3. Adamska T., Młynarczyk W., Jodynis-Liebert J., Bylka W. Matławska I. (2003) “Hepatoprotective Effect of the Extract and Isocytisoside from Aquilegia vulgaris Phytotherapy Research 17(6): 691-696.
4. Bishayee, A., Sarkar, A. and Chatterjee (1995), Hepatoprotective activity of carrot (Daucus Carota) against carbon tetrachloride intoxication in mouse liver, J Ethnopharmacol, 47: 69-74.
5. Bedi O., Bijjem K.R.V., Kumar P., Gauttam V. (2016) “Herbal Induced Hepatoprotection and Hepatotoxicity: A Critical Review” Indian Journal of Physiological Pharmacology 60(1): 6-21.
6. Bahar E., Ara J., Hossain M., Nath B., Runi N. (2013) “Cytotoxic (In-Vitro) Effect of Methanol and Petroleum Ether Extracts of the Aerva lanata” Journal of Pharmacognosy and Phytochemistry 2(1): 92-100.
7. Bigoniya P., Singh C. S., Shukla A., (2009) “A Comprehensive Review of Different Liver Toxicants Used in Experimental Pharmacology” International Journal of Pharmaceutical Sciences and Drug Research 1(3): 124-135.
8. Chaudhury R.R. and Refei U.M. (2001) “Traditional Medicine in Asia” New Delhi, WHO Regional Office for South-East Asia, World Health Organization Regional Publication No.39, ISBN 92 9022 2247.
9. Chattopadhyay, R. R., Sarkar, S. K., Ganguly, S., Benerjee, R. N., Basu, T. K. and Mukherjee, A. (1994), Hepatoprotective activity of Azadirachta indica leaves on paracetamol induced hepatic damage in rats, Indian J Pharmacol, 26: 35-40.
10. Chattopadhyay, R. R., Sarkar, S. K., Ganguly, S., Medda, C. and Basu, T. K. (1992), Hepatoprotective activity of Ocimum sanctum leaf extract against paracetamol induced hepatic damage in rats. Indian J Pharmacol, 24: 163-165.
11. Chang C.Y and Schiano T.D. (2007) “Review Article: Drug Hepatotoxicity” Journal of Alimentary Pharmacology & Therapeutics 25(10): 1135-1151.
12. Dwivedi, Y., Rastogi, R., Garg, N. K. and Dhawan, B. N. (1991), Prevention of paracetamol induced hepatic damage in rats by picroliv, the standard active fraction from Picrorhiza kurroa, Phytother Res, 5:115-119.
13. Das A., Biswas P., Chakrabarty P. (2011) “Hepatotoxicity and Hepatoprotective Herbs: Herbal Remidies” International Journal of Research in Ayurveda and Pharmacy 2(4): 1073-1078.
14. Fatma N. and Uphadhyay R.P. (2015) “Euphorbia Nivulia Buch. Ham.: A Boon for Jaundice (A Case Study)” Annals of Plant Sciences 4(6): 1137-1139.
15. Flatland B. (2003) “Botanicals, Vitamins, and Minerals and the Liver: Therapeutic Applications and Potential Toxicities” Compendium on Continuing Education for the Practising Veterinarian Practitioners 25(7): 514-524.
16. Flora K., Hahn M., Rosen H., Benner K. (1998) “Milk Thistle (Silybum marianum) for the Therapy of Liver Disease” The American Journal of Gastroenterology 93(2): 139-143.
17. Gulati, R. K., Agrawal, S. and Agrawal, S. S. (1994), Hepatoprotective studies on Phyllanthus emblica Linn and quercetin, Ind J Expt Biol, 33: 261- 268.
18. Gawhare V.S. (2013) “A Review on Guduchi through Ayurvedic Texts” International Ayurvedic Medical Journal 1(3): 1-7.
19. Gadgoll, C. and Mishra, S.H. (1995), Preliminary screening of Achillea millefolium, Cichorium intybus and Capparis spinosa for anti- hepatotoxic activity, Fitother, 66: 319-323.
20. Girish C., Koner B.C., Jayanthi S., Rao K.R., Rajesh B., Pradhan S.C. (2009) “Hepatoprotective Activity of Six Polyherbal Formulations in Paracetamol Induced Liver Toxicity in Mice” Indian Journal of Medical Research 129(5): 569-578.
21. Ho W. Y., Yeap S.K., Ho C.L., Rahim R.A., Alitheen N.B. (2012) “Hepatoprotective Activity of Elephantopus scaber on Alcohol-Induced Liver Damage in Mice” Evidence-Based Complementary and Alternative Medicine 417953: 8.
22. Hermenean A., Stan M., Ardelean A., Pilat L., Mihali C.V., Popescu C., Nagy L., Deak G., Zsuga M., Keki S., Bacskay I., Fenyvesi F., Costache M., Dinischiotu A., Miklos V. (2015) “Antioxidant and Hepatoprotective Activity of Milk thistle (Silybum marianum L. Gaertn.) Seed Oil” Life Sciences 10(1):225-236.
23. Handa S. S., Sharma. A. and Chakraborty, K. K. (1986), Natural products and plants as liver protecting drugs, Fitother, 57: 307-351.
24. Handa S. S. and Sharma. A. (1990), Hepatoprotective activity of andrograpolide against galactosamine and paracetamol intoxication in rats, Indian J Med Plant, 92: 284-292.
25. Hegde, K. and Joshi, A. B. (2009), Hepatoprotective effect of Carissa carandus Linn root extract against CCl4 and paracetamol induced hepatic oxidative stress, Indian j Expt Biol, 47: 660- 666.
26. Jayasekhar, P., Mohanan, P.V. and Rathinum, K. (1997), Hepatoprotective property of ethyle acetate extract of Acacia catechu, Indian J Pharmacol, 29: 426-428.
27. Johnson M., Olufunmilayo L.A., Anthony D.O., Olusoji E.O. (2015) “Hepatoprotective Effect of Ethanolic Leaf Extract of Vernonia amygdalina and Azadirachta indica against Acetaminophen-Induced Hepatotoxicity in Sprague-Dawley Male Albino Rats” American Journal of Pharmacological Sciences 3(3): 79-86.
28. Jia J.D., Bauer M., Cho J.J., Ruehl M., Milani S. Boigk G., Riecken E.O., Schuppan D. (2001) “Antifibrotic Effect of Silymarin in Rat Secondary Biliary Fibrosis is Mediated by Downregulation of Procollagenα1 (I) and TIMP-1” Journal of Hepatology 35(3): 392-398.
29. Lee W.M. (2003) “Drug-Induced Hepatotoxicity” The New England Journal of Medicine 349(5): 474-485.
30. Maheswari E., Saraswathy G.R., Thakur Santhranii T. (2014) “Hepatoprotective and Antioxidant Activity of N-acetyl Cysteine in Carbamazepine-Administered Rats” Indian Journal of Pharmacology 46(2): 211-215.
31. MeMahon B.J. (2005) “Epidemiology and Natural History of Hepatitis B” Seminars in Liver Disease 25(1): 3-8.
32. Mohd J., Akhtar A.J. Abuzer A., Tajuddin T.E., Sayeed S. (2012) “Hepatoprotective Evidence of Higher Altitude Medicinal Plant Picrorhiza kurroa Royle Ex Benth: Threatened with Extinction” Journal of Herbal Medicine and Toxicology 6 (2): 1-5.
33. Nasir A., Abubakar M.G., Shehu R.A., Aliyu U., Toge B.K. (2013) “Hepatoprotective Effect of the Aqueous Leaf Extract of Andrographis paniculata Nees against Carbon Tetrachloride Induced Hepatotoxicity in Rats” Nigerian Journal of Basic and Applied Science 21(1): 45-54.
34. Navarro V.J. and Senior J.R. (2006) “Drug-Related Hepatotoxicity” The New England Journal of Medicine 354(7): 731-739.
35. Onyije F. M. And Avwioro O. G. (2012) “Effect of Ethanolic Extract of Bauhinia Monandra Leaf on the Liver of Alloxan Induced Diabetic Rats” Journal of Physiology and Pharmacology Advances 2(1): 59-63.
36. Ostapowicz G., Fontana R.J., Schiodt F.V., Larson A., Davern T.J., Han S.H., McCashland T.M., Shakil A.O., Hay J.E., Hynan L. (2002) “Results of a Prospective Study of Acute Liver Failure at 17 Tertiary Care Centers in the United States” Annals of Internal Medicine 137(12): 947-954.
37. Pandit A., Sachdeva T., Bafna P. (2012) “Drug-Induced Hepatotoxicity: A Review” Journal of Applied Pharmaceutical Science 2 (5): 233-243.
38. Papay J.I., Clines D., Rafi R., Yuen N., Britt S.D., Walsh J.S., Hunt C.M. (2009) Drug-Induced Liver Injury Following Positive Drug Rechallenge” Regulatory Toxicology and Pharmacology 54(1): 84-90.
39. Pascual C., Gonz R., Armesto J., Muriel P. (1993) “Effect of Silymarin and Silybinin on Oxygen Radicals” Drug Development Research 29(1): 73-77.
40. Pradhan S.C. and Girish C. (2006) “Hepatoprotective Herbal Drug, Silymarin from Experimentalpharmacology to Clinical Medicines” Indian Journal of Medical Research 124(5): 491-504.
41. Pandey S., Gujrati V.R., Shanker K., Singh N., Dhawan K.N. (1994) “Hepatoprotective Effect of Liv.52 Against CVl4-Induced Lipid Peroxidation in Liver of Rats” Indian Journal of Experimental Biology 32(9): 674-675.
42. Reddy, B. P., Murthy, V. N., Venkateshwarlu, V., Kokate, C. K. and Rambhau, D. (1993), Antihepatotoxic activity of Phyllanthus niruri, Tinospora cordifolia and Ricinus communis, Indian Drugs, 87: 401-404.
43. Ravikumar V., Shivashangari K.S., Devaki T. (2006) “Effect of Tridax procumbens on Liver Antioxidant Defense System during Lipopolsaccharid- Induced in Dgalactosomine Sensitized Rats” Molecular and Cellular Biochemistry 269(1-2): 131-136.
44. Rajaratnam M., Prystupa A., Lachowska-Kotowska P., Załuska W., Filip R. (2014) “Herbal Medicine for Treatment and Prevention of Liver Diseases” Journal of Pre-Clinical and Clinical Research 8(2): 55-60.
45. Sanmugapriya E. and Venkataraman S. (2006) “Studies on Hepatoprotective and Antioxidant Actions Ofstrychnos potatorum Linn. Seeds on CCl4-Induced Acute Hepatic Injury in Experimental Rats” 105(1-2): 154-160.
46. Saxena, A. K., Singh, B. and Anand, K. K. (1993), Hepatoprotective effects of Eclipta alba on sub cellular levels in rats, J Ethnopharmacol, 40: 155-161.
47. Shah, M., Patel, P., Phadke, M., Menon, S., Mary, F. and Sane, R. T. (2002), Evaluation of the effect of aqueous extract from powders of root , stem, leaves and whole plant of Phyllanthus debilis against CCl4 induced rat liver dysfunction, Ind Drugs, 39: 333- 337.
48. Sharma V. and Pandy D. (2010) “Protective Role Tinospora cordifolia against Lead-Induced Heptotoxicity” Toxicology 17(1): 12-17.
49. Sharma V. and Agrawal R.C. (2014) “In Vivo Antioxidant and Hepatoprotective Potential of Glycyrrhiza Glabra Extract on Carbon Tetrachloride (CCl4) Induced Oxidative-Stress Mediated Hepatotoxicity” International Journal of Research in Medical Sciences 2(1): 314-320.
50. Saller R., Meier R., Brignoli R. (2001) “The Use of Silymarin in the Treatment of Liver Diseases” Drugs 61(14): 2035-2063.
51. Sharma A., Singh R.T., Sehgal V., Handa S.S. (1991) “Antihepatotoxicity Activity of Some Plants Used in Herbal Formulations” Fitoterapia 62: 131-138.
52. Singh D., Cho W.C. and Upadhyay G. (2016) “Drug-Induced Liver Toxicity and Prevention by Herbal Antioxidants: An Overview” Frontiers in Physiology 6: 363-381.
53. Sartor L.L. and Trepanier L.A. (2003) “Rational Pharmacologic Therapy of Hepatobiliary” Compendium on Continuing Education for the Practising Veterinarian 25(6): 432-445.
54. Singh A., Bhat T.K., Sharma O.P. (2011) “Clinical Biochemistry of Hepatotoxicity” Journal of Clinical Toxicology 4(1): 1-19.
55. Twedt D.C. (2004) “The Use of Nutraceutical in Liver Disease” Proceedings of the 28th Annual Royal Canin/OSU Symposium. Columbus, Oct 16-17: 63-66.
56. Vargas-Mendoza N., Madrigal-Santillan E, Morales-González A, Esquivel-Soto H, Esquivel-Chirino C, González-Rubio G.M., Gayosso-de-Lucio J.A. (2014) “Hepatoprotective effect of silymarin” World Journal of Hepatology 6(3): 144-149.
57. Wang F.S., Fan J.G., Zhang Z., Gao B., Wang H.Y. (2014a) “The Global Burden of Liver Disease: The Major Impact of China” Hepatology 60(6): 2099-2108.
58. Willett K.L., Roth R.A., Larry Walker L. (2004) “Workshop Overview: Hepatotoxicity Assessment for Botanical Dietary Supplements” Toxicological Sciences 79 (1): 4-9.
59. Yapar K., Kart A., Karapehlivan M., Atakisi O., Tunca R., Erginsoy S., Citil M. (2007) “Hepatoprotective Effect of L-Carnitine against Acute Acetaminophen Toxicity in Mice” Experimental and Toxicologic Pathology 59(2): 121-128.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









