About Authors:
Arpit Sharma
School of Pharmaceutical Sciences, Shoolini University
Solan, H.P. India
asarpitsharma1@gmail.com
Abstract
The gastro retentive drug delivery system is a novel approach for the drugs having narrow absorption window in the gastrointestinal tract and has poor absorption. Gastro retentive drug delivery system mainly prolongs the gastric emptying time, thereby targeting site-specific drug release. Several techniques such as floating drug delivery system, low density system, raft system, mucoadhesive system, high density system, super porous hydro gel and magnetic system, have been employed. The physiological problems like short gastric residence time and unpredictable gastric emptying time were overcome with the use of floating dosage forms which provide opportunity for both local and systemic effect. Floating drug delivery system enable prolonged and continuous input of the drug to the upper part of the gastro retentional tract and improve the bioavailability of medication that is characterized by a narrow absorption window. The present review addresses briefly about the floating drug delivery system.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1287
Introduction
Floating drug delivery systems are retained in the stomach and are useful for drugs that are poorly soluble or unstable in intestinal fluid. Oral administration is the most convenient and preferred means of any drug delivery to the systemic circulation. The reason that the oral route achieved such popularity may be in part attributes to its ease of administration [1]. Oral sustained drug delivery system is complicated by limited gastric residence time (GRTs). Fast GI transit prevents complete drug release in the absorption zone and reduce the efficacy of the administered dose since the majority of drugs are absorbed in stomach or the upper part of small intestine[2][3]. To overcome these limitations, various approaches have been proposed to increase gastric residence of drug delivery system in the upper part of the gastrointestinal tract includes floating drug dosage system (FDDS), swelling or expanding system and other delayed gastric emptying devices. Among these systems, FDDS have been most commonly used.
Floating drug delivery system
Floating drug delivery system have a bulk density less than that of gastric fluids and so remain buoyant in the stomach without affecting the gastric emptying rate for a prolong period of time. While the system is floating on the gastric contents, the drug is released slowly at the desired rate from the system. After release of drug, the residual system is emptied from the stomach. This result in an increased GRT and a better control of fluctuation in plasma drug concentration. However, beside a minimal gastric content needed to allow the proper achievement of the buoyancy retention principle, a minimal level of floating force (F) is also required to keep the dosage form reliably buoyant on the surface of meal. To measure the floating force equivalent to F (as a function of time) that is required to maintain the submerged object. The object floats better if RW is on the higher positive side. This apparatus helps in optimizing FDDS with respect to stability and durability of floating forces produced in order to prevent the drawbacks of unforeseeable intragastric buoyancy capability variation.
RW or F = F (buoyancy) – F (gravity)
= (Df – Ds ) gV
Where RW = total vertical force, Df = fluid density, Ds = object density, V = volume and g = acceleration due to gravity.
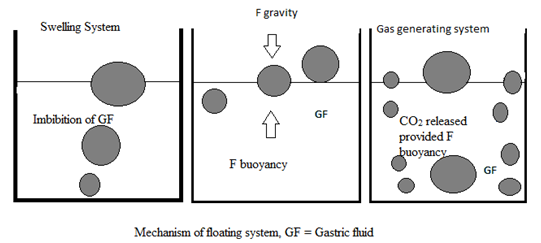
Classification of floating drug delivery system
Based on the mechanism of buoyancy FDDS can be classified into
A. Single unit floating dosage system
a) Effervescent system (gas-generating system)
b) Non-effervescent system
B. Multiple unit floating dosage system
a) Non-effervescent system
b) Effervescent system (gas-generating system)
c) Hollow microsphere
C. Raft forming system
A.Single unit floating dosage system
a) Effervescent system (gas-generating system)
These buoyant system utilized matrices prepared with swellable polymer like HPMC, polysaccharide like chitosan, effervescent component like sodium bicarbonate, citric acid and tartaric acid or chamber containing a liquid that gasifies at body temperature. The optimal stoichiometric ratio of citric acid and sodium bicarbonate, for gas generation is reported to be 0.76:1. The common approach for preparing these systems involves resin beads loaded with bicarbonate and coated with ethyl cellulose. The coating, which is insoluble but permeable, allows permeation of water. Thus, carbon dioxide is released, causing the beads to float in the stomach[4].
Excipients used most commonly in these systems include HPMC, polyacrylate polymer, polyvinyl acetate, carbopole, agar, sodium alginate, calcium chloride, polyethylene oxide and polycarbonates.
Ozdemir et al[5] prepared floating bilayer tablets with controlled release for furosemide. The low solubility of drug could be enhanced by using the kneading method, preparing a solid dispersion with β-cyclodextrin mixed in a 1:1 ratio. One layer contained the polymer HPMC 4000, HPMC 100, and CMC (for the control of drug delivery) and the drug. The second layer contained the effervescent mixture of sodium bicarbonate and citric acid. Radiographic studies on 6 healthy males volunteers showed that floating tablets were retained in stomach for 6 hours and further blood analysis studies showed that bioavailability of these tablets was 1.8 times that of the conventional tablets was decreased and prolonged in the case of floating dosage forms.
Penners et al[6] prepared an expandable tablet containing mixture of polyvinyl lactams and polyacrylates that swell rapidly in an aqueous environment and thus stays in stomach over an extended period of time. In addition to this, gas forming agents were also incorporated so as soon as the gas formed, the density of system was reduced and thus the system tended to float on the gastric environment.
Talwar et al[7] prepared a once-daily formulation for oral administration of ciprofloxacin. The formulation was composed of 69.9% ciprofloxacin base, 0.34% sodium alginate, 1.03% xanthum gum, 13.7% sodium bicarbonates, and 12.1% cross-linked poly vinyl pyrrolidine. The cross linked PVP initially and the gel forming polymer later formed a hydrated gel matrix that entrapped the gas, causing the tablet to float and be retained in the stomach. The hydrated gel matrix created a diffusion path for the drugs, resulting in sustained release of the drug.
[adsense:468x15:2204050025]
b) Non-effervescent system
This type of system, after swallowing, swells unrestrained via imbibitions of gastric fluid to an extend that prevent their exit from the stomach.
Shah S.H. et al system may be referred to as the ‘plug-type systems’ since they have a tendency to remain lodged near the pyloric sphincter. One of the formulation method of such dosage forms involves the mixing of drug with a gel, which swells in contact with gastric fluid after oral administration and maintains a relative integrity of shape and a bulk density of less than one within gelatinous barrier. The air trapped by the swollen polymer confers buoyancy to these dosage forms. Examples of this type of FDDS include colloidal gel barrier[8], micro porous compartment system, alginate beads, and hollow microsphere. Another type is a fluid-filled chamber which include incorporation of a gas filled floatation chamber into a micro porous component that houses a drug reservoir. Aperture or openings are present along the top and bottom walls through which the gastrointestinal tract fluid enter to dissolve the drug. The other two walls in contact with the fluid are sealed so that the undissolved drug remains therein. The fluid present could be air, under partial vacuum or any other suitable gas, liquid, or solid having an appropriate specific gravity and an inert behavior. The device is of swallowable size, remains afloat within the stomach for prolonged time, and after the complete release the shell disintegrates, passes off to the intestine, and is eliminated.
Gas filled floatation chamber
A newer self-correcting floatable asymmetric configuration drug delivery system[9] has a 3-layer matrix to control the drug release. This 3-layer principle has been improved by development of an asymmetric configuration drug delivery system in order to modulate the release extend and achieve zero-order release kinetics by initially maintaining a constant area at the diffusing front with subsequent dissolution/erosion towards the completion of the release process. The system was designed in such a manner that it floated to prolong gastric residence time in vivo, resulting in longer total residence time within the gastrointestinal tract environment with maximum absorptive capacity and consequently greater bioavailability. This particular characteristic would be applicable to drugs that have pH dependent solubility, a narrow window of absorption, and are absorbed by active transport from either proximal or distal portion of the small intestine.
Yang et al[10] developed a swellable asymmetric triple layer tablet with floating ability to prolong the gastric residence time of triple drug regimen (tetracycline, metronidazole, and clarithromycin) in Helicobacter pylori-associated peptic ulcer using HPMC and poly (ethylene oxide) (PEO) as the rate-controlling polymeric membrane excipients. Tetracycline and metronidazole were incorporated into the core layer of the triple layer matrix for controlled delivery, while bismuth salt was included in one of the outer layer for instant release. The floatation was accomplished by incorporating a gas-generating layer consisting of sodium bicarbonates and calcium carbonate with swellable chamber. Over 6 to 8 hours of sustained delivery of tetracycline and metronidazole was achieved with this dosage form which was still floating.
Streubel et al[11] prepared single-unit floating tablets based on polypropylene foam system and matrix foaming polymer. Highly porous foam powder in matrix tablets provided density much lower than the density of released medium. It was concluded that varying the ratio of matrix-foaming polymer and the foam powder could alter the drug release pattern effectively.
Wu et al[12] prepared floating sustained release tablets of nimodipine by using HPMC and PEG 6000. Prior to formulation of floating tablets, nimodipine was incorporated into poloxamer-188 solid dispersion after which it was directly compressed into floating tablets. It was observed that by increasing the HPMC and decreasing the PEG 6000 content a decline in in-vitro release of nimodipine was observed.
Nur and Zhang et al[13] prepared floating tablet of captopril using HPMC (4000 and 15000 cps) and carbopol 934P. It was concluded that the buoyancy of tablet is governed by both the swelling of hydrocolloid particle on the tablet surface when it contacts the gastric fluid and the presence of internal voids in the centre of tablet (porosity). A prolonged release from these floating tablets was observed as compared with the conventional tablets and a 24-hours controlled release from the dosage foam of captopril was achieved. Single-unit formulations are associated with problems such as sticking together or being obstructed in the gastrointestinal tract, which may have a potential danger of producing irritation. The main drawback of such system is “all or none” phenomenon. In such cases there is danger of passing of the dosage forms to intestinal part. To overcome this difficulty multiple unit dosage forms are designed.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
B. Multiple unit floating system
In spite of extensive research and development in the area of HBS and other floating tablets, these systems suffer from an important drawback of high variability of gastrointestinal transit time, when orally administered, because of their all or nothing gastric emptying nature. In order to overcome the above problem, multiple unit floating system were developed, which reduce the intersubject variability in absorption and lower the probability of dose dumping. Report have been found on the development of both effervescent and effervescent multiple unit system[]. Much research has been focused and the scientists are still exploring the field of hollow microsphere, capable of floating on the gastric fluid and having improved gastric retention properties.
a) Non-effervescent system
Not much report was found in the literature on non-effervescent multiple unit system, as compared to the effervescent system. However, few workers have reported the possibility of developing such system containing indomethacin, using chitosan as the polymeric excipients. A multiple unit HBS containing indomethacin as a model drug prepared by extrusion process is reported[14]. A mixture of drug, chitosan and acetic acid is extruded through a needle, and the extrudate is cut and dried. Chitosan hydrate and floats in the acidic media, and the required drug could be obtained by modifying the drug-polymer ratio.
b) Effervescent system (gas-generating system)
Ikura et al reported sustained release floating granules containing tetracycline hydrochloride. The granules are mixture of drug granules are a mixture of drug granulates of two stages A and B, of which A contains 60 parts of HPMC,40 parts of polyacrylate acid and 20 parts of drug and B part contain 70 part of sodium bicarbonate and 30 part of tartric acid. 60 parts by weight of granules of stage A and 30 parts by weight of granules B are mixed along with lubricant and filled in capsule. In dissolution media, the capsule shell dissolves and liberates the granules, which showed a floating time of more than 8 hours and sustained drug release of 80% drug in 6.5 hours. Floating mini capsule of pepstatin having a diameter of 0.1-0.2 mm has been reported by Umezawa[15]. These mini capsules contain a central core and a coating. The central core consists of a granule composed of sodium bicarbonate, lactose and a binder, which is coated with HPMC. Pepststin is coated on the top of the HPMC layer. The system floats because of the CO2 release in gastric fluid and the pepstatin reside in the stomach for prolonged period. Alginates have received much attention in the development of multiple unit system. Alginates are non toxic, biodegradable linear copolymer composed of L-glucuronic acid and L-mannuronic acid residue. A multiple unit system prepared by Iannuccelli et al comprises of calcium alginate core and calcium alginate/PVA membrane, both separated by an air compartment. In presence of water, the PVA leaches out and increase membrane permeability, maintaining the integrity of the air compartment. Increase in molecular weight and concentration of PVA, resulted in enhasment of floating properties of the system. Freeze drying technique is also reported for the preparation of floating calcium alginate beads[16]. Sodium alginate solution is added drop wise into the aqueous solution of calcium chloride, causing the instant of the droplet surface, due to formation of calcium alginate. The obtained beads are freeze dried resulting in a porous structure, which aid in floating. The author studied the behavior of radio labeled floating beads and compared with non floating beads in human volunteer using gamma scintigraphy. Prolonged gastric residence time of more than 5.5 h was observed for floating beads. The non floating beads had a shorter residence time with a mean onset emptying time of 1 h.
Ichikawa et al[17] developed a new multiple type of floating dosage system having a pill in a core, composed of effervescent layer and swellable membrane layer coated on sustain release pill. The inner layer of effervescent agent containing sodium bicarbonate and tartric acid was divided into 2 sub layer to avoid direct contact between the two agents. These sub layer were surrounded by swellable polymer membrane containing polyvinyl acetate and purified shellac. When this system was immersed in the buffer at 37ºC, it settled down the solution permeated into the effervescent layer through the outer swellable membrane. CO2 was generated by the neutralization reaction between the two effervescent agents, producing swollen pills (like balloons) with density less than 1.0 g/ml.
c) Hollow microsphere
Hollow microsphere are considered as one of the most promising buoyant system, as they possess the unique advantage of multiple unit system as well as better floating properties, because of central hollow space inside the microsphere. The general technique involved in the release and better floating properties mainly depend on the type of polymer, plasticizer and the solvent employed for the preparation. Polymer such as polycarbonate Eudragit® S and cellulose were used in the preparation of hollow microsphere, and the drug release can be modulated by optimizing the polymer quantity and the polymer-plasticizer ratio.
Sustained release floating microsphere using polycarbonates were developed by Thanoo et al[18], employing solvent evaporation technique. Aspirin, griseofulvin and p-nitroaniline were used as model drugs. Dispersed phase containing polycarbonate solution in dichloromethane, and micronized drug, was added to the dispersed medium containing sodium chloride, polyvinyl alcohol and methanol. The dispersion was stirred for 3-4 h to assure the complete solvent evaporation, and the microsphere obtained were filtered, washed with cold water and dried. The spherical and hollow nature of the microsphere was confirmed by scanning electron microscopic studies. The microsphere showed a drug payload of more than 50%, and the amount of drug incorporated is found to influence the particle size distribution and drug release. The larger proportion of bigger particle was seen at high drug loading, which can be attributed to the increased viscosity of the dispersed phase.
Kawashima et al[19] described hollow microsphere (micro balloons) with drug in their outer polymer shell, prepared by a novel emulsion solvent diffusion method. A solution of drug and enteric acrylic polymer (Eudragit® S) in a mixture of ethanol and dichloromethane is added to the aqueous phase containing polyvinyl alcohol (0.75% w/v) and stirred continuously to obtained o/w emulsion. The microsphere obtained are filtered, water washed, and dried. The diffusion and evaporation profile of ethanol and dichloromethane, suggested a rapid diffusion of ethanol from the droplets into the aqueous phase, which might reduce the polymer solubility in the droplet because of insoluble property of Eudragit® S in dichloromethane. Hence, the polymer precipitation occurs instantly at the droplet surface, forming a film-like shell enclosing dichloromethane and drug. The microsphere showed good flow and packing properties, and a floating time of more than 12 h on acidic medium containing surfactant.
Joseph et al[20] developed a floating dosage form of piroxicam based on hollow polycarbonate microsphere. The microsphere by solvent evaporation technique. Encapsulation efficiency of 95% was achieved. In-vivo studies were performed in healthy male albino rabbits. Pharmacokinetic analysis was derived from plasma concentration vs. time plot and revealed that the bioavailability from the piroxicam microsphere alone was 1.4 that of the free drug and 4.8 times that of dosage form consisting of microsphere plus the loading dose and was capable of sustained delivery of the drug over a prolonged period.
C. Raft forming system
Raft forming systems have received much attention for the delivery of antacid and drug delivery for gastrointestinal infection and disorder. The mechanism involve in the raft formation include the formation of viscous cohesive gel in contact with gastric fluid, wherein each portion of the liquid swells forming a continuous layer called raft. The raft floats on gastric fluid because of low bulk density created by formation of CO2. Usually, the system contain a gel forming agent and alkaline bicarbonates and carbonates responsible for the formation of CO2 to make the system less dense and float on the gastric fluid[21]. Jorgen et al[22] described an antacid raft forming floating system. The system contain the gel forming agent (e.g. alginic acid), sodium bicarbonates and acid neutralizer, which form a foaming sodium alginate gel (raft) when in contact in gastric fluid. The raft thus formed floats on the gastric fluid and prevents the reflux of gastric content (i.e. gastric acid) into the esophagus by acting a barrier between the stomach and esophagus. A patent assigned to Reckitt and Colman Products Ltd., describes a raft forming formulation for the treatment of helicobacter pylori (H. Pylori) infection in the GIT. The composition contained drug, alginic acid, sodium bicarbonates, calcium carbonates, mannitol and a sweetener. These ingredient were granulated, the citric acid was added to the granules. The formulation produces effervescence and aerates the raft formed, making it float.
Advantages of floating drug delivery system[23][24]
1. The gastroretensive systems are advantageous for drugs absorbed through the stomach. E.g. Ferrous salt, antacids.
2. Acidic substance like aspirin causes irritation on the stomach wall when come in contact with it. Hence HBS formulation may be useful for the administration of aspirin and other similar drugs.
3. Administration of prolong floating dosage forms, tablets, and capsule, will result in dissolution of the drug in the gastric fluid. They dissolve in the gastric fluid would be available for absorption in small intestine after emptying of the stomach contents. It is therefore expected that a drug will be fully absorbed from floating dosage forms if it remains in the solution form even at alkaline pH of the intestine.
4. The gastroretensive system are advantageous for drug meant for local action in the stomach. E.g. antacids.
5. When there is a vigorous intestinal movement and a short transit time as might occur in certain type of diarrhea, poor absorption is expected. Under such circumstances it may advantageous to keep the drug in floating condition in stomach to get a relative better response.
Disadvantages of floating drug delivery system
There are certain situation where gastroretention of the drug is not desirable.
1. Some drugs present in the floating system cause irritation to gastric mucosa. Eg. Aspirin and NSAID.
2. The drugs which are having multiple absorption site in the gastrointestinal tract.
3. These systems require a high level of fluid in the stomach for drug delivery to float and work efficiently.
4. The drugs which get degraded due to gastric enzyme.
5. The drugs which are not stable in the gastric pH.
Application of floating drug delivery system
Floating drug delivery system offers several applications for drug having poor bioavailability because of the narrow absorption window in upper part of gastrointestinal tract. It retains the dosage forms at the site of absorption and thus enhances the bioavailability. These are summarizes as follows.
1. Sustained drug delivery
HBS systems can remain in the stomach for long period and hence can release the drug over a prolong period of time. The problem of short gastric residence time encountered with an oral CR formulation hence can be overcome with these systems. These system have a bulk density of <1 as a result of which they can float on the gastric content. These systems are relatively large in size and passing from pyloric opening is prohibited. E.g. Sustained release floating capsules of nicardipine hydrochloride were developed and were evaluated in-vivo. The formulation compared with commercially available MICARD capsule using rabbits. Plasma concentration time curves showed a longer duration for administration (16 hours) in the sustained release floating capsule compared with conventional MICARD capsule (8 hours) [25].
2. Site-specific drug delivery
These systems are particularly advantageous for drugs that are specifically absorbed from stomach or the proximal part of small intestine, e.g. riboflavin and furosemide. E.g. furosemide is primarily absorbed from the stomach followed by the duodenum. It has been reported that a monolithic floating dosage form with prolonged gastric residence time was developed and the bioavailability was increased. AUC obtained with the floating tablets was approximately 1.8 times those of conventional furosemide tablets.
3. Absorption enhancement
Drugs that have poor bioavailability because of site-specific absorption from the upper part of gastrointestinal tract are potential candidates to be formulated as floating drug delivery system, thereby maximizing their absorption. E.g. a significantly increase in the bioavailability of floating dosage forms (42.9%) could be achieved as compared with commercially available LASIX tablets (33.4%) and enteric coated LASIX-long product (29.5%) [26].
Conclusion
Gastro-retentive floating drug delivery system have emerged as an efficient means of enhancing the bioavailability and controlled delivery of many drugs. The increasing sophistication of delivery technology will ensure the development of increase number of gastro retentive drug delivery to optimize the delivery of molecules that exhibit absorption window, low bioavailability and extensive first pass metabolism.
References
1. Chien Y.W., Novel Drug Delivery System, 2nd edition, Revised and expanded, Marcel Dekker, New York, 1992, 139-140.
2. Choi B.Y., Park H.J., Hwang S.J., and J.B., Preparation of alginate beads for floating drug delivery system: effect of CO2 gas-forming agent, Int. J. Pharm. 239, 2002, 81-91.
3. Rouge N., Buri P., Doelker E., Drug absorption sites in the gastrointestinal tract and dosage forms for site-specific delivery, Int. J. Pharm. 136, 1996, 117-139.
4. Rubinsten A., Friend D.R, Specific delivery to the gastrointestinal tract, in: Domb A.J (Ed.), Polymeric Site-Specific Pharmacotherapy, Wiley, Chichester, 1994, 282-283.
5. Ozdemir N., Ordu S, Ozkan Y. Studies of floating dosage form of furosemide: in vitro and in vivo evaluation of bilayer tablet formulation. Drug Dev. Ind. Pharm. 2000,26,857-866.
6. Penner G.,Lustig K., Jorg P.V.G. Expandable pharmaceutical forms. US patent 5,651,985, 1997.
7. Talwar N., Sen H., Staniforth J.N, orally administered controlled drug delivery system providing temporal and spatial control. US patent 6261601, 2001.
8. Sheth P.R. and tossounian J.L. U.S. Patent no. 4140755, 1979.
9. Yang L., Fassihi R. Zero order release kinetics from self correcting floatable configuration drug delivery system. J. Pharm Sci. 1996; 85:170-173.
10. Yang L., Esharghi J., Fassihi R A new intra gastric delivery system for the treatment of helicobacter pylori associated gastric ulcer: in vitro evaluation. J. Cont. Rel. 1999; 57:215-222.
11. Streubel A., Siepmann J., Bodmeier R. Floating matrix tablets based on low density foam powder: effect of formulation and processing parameter on drug release. Eur. J. Pharm. Sci. 2003;18:37-45.
12. Wu W, Zhou Q, Zhang H.B, Ma G.D, Fu C.D. Studies on Nimodipine sustained released tablet capable of floating on gastric fluid with prolonged gastric resident time. Yao Xue Xue Bao. 1997;32:786-790.
13. Nur A.O, Zhang J.S. Captopril floating and/or bioadhesive tablets: design and release kinetics. Drug Dev. Ind. Pharm. 2000; 26:965-969.
14. Tardi P., Troy H., European patent no. EP 1432402. 2002
15. Umezaba, Hamao United States Patent 4101650. 1978
16. Stops F., Fell J.T., Collett J.H., Martini L.G. Floating dosage form to prolong gastro retention-the characterization of calcium alginate beads, Int. J. Pharm. 2008, 350,301-311.
17. Chikawa M., Watanabe S, Miyake Y. A new multiple unit oral floating dosage system. 1: Preparation and in vitro evaluation of floating and sustained-release kinetics. J. Pharm. Sci. 1991;80:1062-1066
18. Thanoo B.C, Sunny M.C and Jaikrishnan A., Oral-sustained release drug delivery system using polycarbonate microsphere capable of floating on gastric fluid, J. Pharm. Pharmacol 1993,45,21-24.
19. Sato Y., Kawashima Y., Takeuchi H. and Yamamoto H., in vivo evaluation of riboflavin containing microballoons for floating controlled drug delivery system in healthy human volunteers, J. Cont. Rel. 2003, 93, 39-47.
20. Joseph N.H. Laxmi S., Jaikrishnan A. A floating type oral dosage form for piroxicam based on hollow microsphere: in vitro and in vivo evaluation in rabbits. J. Cont. Rel. 2002;79:71-79.
21. Washington N., Investigation into the barrier action of an alginate gastric reflux suppressant, Liquid Gabiscon, Drug Investig. 1987, 2,23-30.
22. Foldager J., Toftkjor H., Antacid composition. US Patent 5068109, 1991.
23. Babu VBM, Khar RK. In vitro and in vivo studies of sustain release floating dosage form containing salbutamol sulphate. Pharmazie. 1990; 45:268-270.
24. Hetal N Kikani, A thesis on, Floating drug delivery system, The North Gujarat University, Patan, 2000-2001; 11-12.
25. Fell J T, Whitehead L, Collet H, Prolonged gastric retention using floating dosage form, Pharm Technol. 2000; 24(3):82-90.
26. Moursy NM, Afifi NH, Ghorab DM, El-saharty Y. Formulation and evaluation of sustained release floating capsule of Nicardipine hydrochloride . Pharmazie. 2003;58:38-43.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









