 About Authors:
About Authors:
Dhiren Shah*1, Jatin Patel, Krunal Parikh
m.pharmacy
Seth G.L. Bihani S.D. College of Technical Education, R.U.H.S.
Sri Ganganagar, Rajasthan, INDIA
*dhiren.pharmacist@gmail.com
Abstract :
Involved Evaluation and Standardization techniques for crude drugs, mono or Polyherbal Frormulation. They involved the macroscopic techniques, microscopic techniques, physical evaluation and biological evaluation. They also involved the Quantitative analysis of Organophosphorus insecticides, Organochlorine and pyrethroid insecticides, microbial content determination.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1506
EVALUATION AND STANDARDIZATION: GENERAL INTRODUCTION
Evaluation of drug means confirmation of its identity and determination of its quality and purity and detection of nature of adulteration.
IDENTITY: refer to exact authentic biological source of the drug can be established by actual collection of the drug from a plant or animal which has been identified or comparing a representative unknown sample with a published description of the drug with authentic drug sample.
QUALITY: refer to the intrinsic value of drug i.e. the concentration or amount of medicinal principles or active constituents present. These constituents are classified as non-living cell inclusion and have been studied under the heading, the chemical classification. This includes fixed oils, carbohydrates, glycoside, alkaloids, resin, fats or waxes, volatile oil, tannins, vitamins, alergens, etc. A high grade of quality in a drug is of prime importance. An effort should be made to obtain mainly this high quality.
[adsense:468x15:2204050025]
The high grade quality of the drug be accomplished by:
1) Collection of the drug from the correct natural source at proper time and in the proper manner.
2) Preparation of the collected drug by proper cleaning, drying, garbling.
3) Proper preservation of the cleaned, dried pure drug against contamination through moisture, fungi, filth and insects.
PURITY: of the drug depends upon the absence of foreign matters whether organic or inorganic.
The crude drugs can be identified on the basis of their morphological, histological, chemical, physical, and biological studies.
The evaluation of crude drug is necessary because of three reason:
1. Biochemical variation in the drug
2. Deterioration due to treatment and storage
3. Substitution and adulteration, as a result of carelessness, ignorance or fraud
The different techniques involved in standardization of crude drugs are as follows:
· Organoleptic evaluation
· Microscopic evaluation
· Physical evaluation
· Biological evalution
Standardization of herbal drugs is not an easy task as numerous factors influence the bio efficacy and reproducible therapeutic effect. In order to obtain quality oriented herbal products, care should be taken right from the proper identification of plants, season and area of collection and their extraction and purification process and rationalizing the combination in case of polyherbal drugs.
Organoleptic evaluation
Organoleptic (Lit. “Impression on the organs”) evaluation of crude drugs refers to the evaluation of a drug by colour, odour, taste, size and shape, occasionally the sound or snap of fracture and special fetures including touch, texture, etc..
Organoleptic evaluation is also called MORHOLOGICAL or MACROSCOPICAL evaluation.
It is a technique of qualitative evaluation based on study of morphological and sensory profiles of whole drugs. Organoleptic evaluation means conclusions drawn from studies resulted due to impression on organ of senses.
PARAMETERS used for this type of evaluation can be explained as follows.
1) COLOUR
The colour is used in indicating the general origin of drug. e.g. material derived from the aerial part of the plant is usually green and the underground plat material is usually devoid of green colour.
2) SIZE
The length, width and thickess of the crude material are of great importance while evaluating a crude drug.
PROCEDURE: A graduated ruler in millimetres is adequate for the measurement of the length, width and thickness of crude maerials small seeds and fruits may be measured by aligning 10 of them on a sheet of calibrated paper, with 1mm spacing b/w lines and dividing the result by 10.
3) ODOUR AND TASTE
ODOUR:
To an expert, odour and taste of crude material are extremely sensitive criteria based on individuals perception. Therefore, he description of this feature may sometime cause some difficulties.
· Indistinct
· Distinct
* Aromatic
* Balsamic
* Spicy
* Fruity
* Mouldy or musty
* Rancid
· Weak
· Strong
PROCEDURE: If the material is expected to be innocuous, place a small portion of the sample in the palm of the hand or a beaker of suitable size, and slowly and repeatedly inhale the air over the material. If no odour is perceptible, crush the sample between the thumb and index finger or between the palms of the hands using gentle pressure. If the material is known to be dangerous, crush by mechanical means and then pure a small quantity of boiling water onto the crushed sample in a beaker. First determine the strebgth of the odour (none, weak, distinct, strong) and then the odour sensation (aromatic, fruity, musty, mouldy, rancid, etc.) A direct comparision of the odour with a defined substance is advisable (e.g. peppermint should have an odour similar t menthol, cloes an odour similar to eugenol)
TASTE
Tastes are f tw types
· True taste
* Acid (S*ur)
* Saline (Salty)
* Saccharine (Sweet)
* Bitter
* Alkaline
* Metallic
· False taste (Sensati*ns t* the t*ngue)
* Mucilagin*us (s*ft slimy feeling)
* *il (Bland sm**th feeling)
* Astringent (C*ntracti*n *f m*uth tissue)
* Pungent (Warm biting sensati*n)
* Acrid (Unpleasant, Irritating)
* Nause*us (Induce v*miting)
SURFACE CHARACTERSTICS, TEXTURE AND FRACTURE CHARACTERSTICS:
Examine the untreated sample. If necessary, a magnifying lens (6x to 10x) may be used. Wetting with water or reagents, as required, may be necessary to observed the characteristics of a cut surface. The texture is best examined by taking a small quantity of material and rubbing it between the thumb and forefinger, it is usually described as ‘smooth’, ‘rough’,’guity’. Touch the material to determine if it is soft or hard, bend and rupture it to obtain information on brittleness and the appearance f the fracture plane whether it is fibrous, smooth, rough, granular, etc.
All this characteristics aare valuable in Indicating the general type of material and the presence of more then one component.
For convenience te description of macroscopic characters may be divided into four headings as-
a) Shape and size
b) Colour and external marking
c) Fracture and internal colour
d) Odour and taste
In some official crude drugs monograhs the entire macroscopic description consists of an organoleptic evaluation and is only means of evaluation given macroscopy refers to visual appearance to baked eyes. The macroscopic study depends on the part of the plant from which the drug is obtained.
According to the plant part used all organized drugs can be classified into following groups.
A) Underground plant parts
B) Barks
C) Woods
D) Leaves
E) Flower fruit
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
The Standardization of crude drug materials includes the following steps:-
1. Authentication: - Each and every step has to be authenticated.
a) Stage of collection.
b) Parts of the plant collected.
c) Regional status.
d) Botanical identity like phytomorphology, microscopical and histological analysis (characteristic of cell walls, cell contents, starch grains, calcium oxalate crystals, trichomes, fibers, vessels etc).Various histological parameter studies are:-
1. Leaf constant: - Palisade ratio, Vein islet number, Vein termination, Stomatal number, and
2. Stomatal index.
3. Trichomes
4. Stomata.
5. Quantitative microscopy.
6. Taxonomical identity.
7. Foreign matter.
8. Organoleptic evaluation.
9. Ash values and extractive values.
10. Moisture content determination.
11. Chrometographic and spectroscopic evaluation.
12. Heavy metal determination.
13. Pesticide residue.
14. Microbial contamination.
15. Radioactive contamination.
16. The stability parameters for the herbal formulations which include physical, chemical and microbiological parameters are as follow:
Physical parameters include color, odor, appearance, clarity, viscosity, moisture content, pH, disintegration time, friability, hardness, flow ability, flocculation, sedimentation, settling rate and ash values.
Chemical parameters include limit tests, chemical tests, chemical assays etc.
Chromatographic analysis of herbals can be done using TLC, HPLC, HPTLC, GC, UV, GC-MS, fluorimetry etc.
Microbiological parameters include total viable content, total mold count, total enterobacterial and their count. Limiters can be utilized as a quantitative or semi quantitative tool to ascertain and control the amount of impurities like the reagents used during abstraction of various herbs, impurities coming directly from the manufacturing vessels and from the solvents etc.
2. F.O.M. (Foreign organic matter)determination:
The parts of the organs of the crude drug other than those named in definition and description of drug are defined as foreign organic matter.
The maximum limit for the foreign organic matter is defined in monograph of crude drug. if it exceeds the limits, deterioration in quality of drug take place.
DETERMINATION OF FOREIGN MATTER
Weigh 100 –500 g of the drug sample to be examined or the minimum quantity prescribed in the monograph, and spread it out in a thin layer. The foreign matter should be detected by inspection with the unaided eye or by the use of a lens (6x). Separate and weigh it and calculate the percentage present.
Examples:
Table 1: Determination of Foreign matter
|
plant parts |
Drugs |
foreign matter limit |
|
leaves and herbs |
bearberry leaf |
not more than 8%foreign matter of which NMT |
|
birch leaf |
NMT 3%fragments of female catkins, nmt |
|
|
wornwood |
NMT5%stem with diameter>4mm, nmt |
|
|
fruits and seeds |
hawthrown seeds |
NMT2% foreign matter, nmt 5% deteriorated |
|
|
psyllium seeds |
NMT 1% foreign matter including |
|
Barks |
quillaia |
NMT 2 5 foreign matter |
|
|
cascara |
NMT 1% foreign matter |
Loss on drying
The test determine both water and volatile matter in the crude drug. loss on drying is the loss of mass expresed as w/w and can be determined by following procedure :
Method: Procedure set forth here determines the amount of volatile matter (i.e., water drying off from the drug). For substances appearing to contain water as the only volatile constituent, the procedure given below, is appropriately used. Place about 10 g of drug (without preliminary drying) after accurately weighing (accurately weighed to within 0.01 g) it in a tared evaporating dish. For example, for underground or unpowderdd drug, prepare about 10 g of the sample by cutting shredding so that the parts are about 3 mm in thickness. Seeds and fruits, smaller than 3 mm should be cracked. Avoid the use of high speed mills in preparing the samples, and exercise care that no appreciable amount of moisture is lost during preparation and that the portion taken is representative of the official sample. After placing the above said amount of the drug in the tared evaporating dish dry at 105º for 5 hours, and weigh. Continue the drying and weighing at one hour interval until difference between two successive weighings corresponds to not more than 0.25 per cent. Constant weight is reached when two consecutive weighings after drying for 30 minutes and cooling for 30 minutes in a desiccator, show not more than 0.01 g difference.
Table 2: Crude drug with limit for moisture content
|
Drug |
Moisture content(%w/w) |
|
Aloe |
Not more than10% |
|
Digitalis |
Not more than5% |
|
Ergot |
Not more than8% |
|
Acacia |
Not more than15% |
|
Starch |
Not more than15% |
Ash value:
The residue remaining after incineration is the ash content of the drug,which simply represents inorganic salts,naturally occuring in drug or adhering to it or deliberately added to it as form of adulteration.ash value is criteria to judge the identity or purity of drug.
The ash remaining following ignition of medicinal plant material is determined by three different method which measure total ash, acid insoluble ash, water soluble ash.
Determination of Total Ash
Incinerate about 2 to 3 g accurately weighed, of the ground drug in a tared platinum or silica dish at a temperature not exceeding 450º until free from carbon, cool and weigh. If a carbon free ash cannot be obtained in this way, exhaust the charred mass with hot water, collect the residue on an ashless filter paper, incinerate the residue and filter paper, add the filtrate, evaporate to dryness, and ignite at a temperature not exceeding 450º. Calculate the percentage of ash with reference to the air-dried drug.
Examples
Table 3: Determination of Total Ash
|
Drugs |
Total ash (% w/w) |
|
Aloes |
Not more than 5 % |
|
Ashoka |
Not more than 11% |
|
Amla |
Not more than 7 % |
|
Nutmeg |
Not more than 3 % |
Acid insouble ash:
Test measured the amount of silica present, especially as sand siliceous earth
Procedure:
Boil the ash obtained for 5 minutes with 25 ml of dilute hydrochloric acid; collect the insoluble matter in a Gooch crucible, or on an ashless filter paper, wash with hot water and ignite to constant weight. Calculate the percentage of acid-insoluble ash with reference to the air dried drug.
Example:
Table 4: Acid insouble ash
|
Drugs |
Acid insoluble ash (%w/w) |
|
Agar |
Not more than 1.0 |
|
Amla |
Not more than 2.0 |
|
Bael |
Not more than 1.0 |
Water soluble ash:
Determination of Water Soluble Ash:
Boil the ash for 5 minutes with 25 ml of water; collect insoluble matter in a Gooch crucible, or on an ashless filter paper, wash with hot water, and ignite for 15 minutes at a temprature not exceeding 450º. Substract the weight of the insoluble matter from the weight of the ash; the difference in weight represents the watersoluble ash. Calculate the percentage of water-soluble ash with reference to the airdried drug.
Examples:
Table 5: Water soluble ash
|
Drugs |
Water soluble ash (%w/w) |
|
Ginger |
Not more than 1.7 |
5. Extractive value:
The extracts obtained by exhausting crude drugs are indicative of their chemical constituent(app.). taking into consideration the diversity of chemical nature and property of contents of drugs, various solvents are used for determination of extractive.
Determination of Alcohol Soluble Extractive
Macerate 5 g of the air dried drug, coarsely powdered, with 100 ml of Alcohol of the specified strength in a closed flask for twenty-four hours, shaking frequently during six hours and allowing to stand for eighteen hours. Filter rapidly, taking precautions against loss of solvent, evaporate 25 ml of the filtrate to dryness in a tared flat bottomed shallow dish, and dry at 105º, to constant weight and weigh. Calculate the percentage of alcohol-soluble extractive with reference to the air-dried drug.
Examples:
Table 6: Determination of Alcohol Soluble Extractive
Alcohol soluble extractive value of some crude drug
|
Drugs |
Alcohol soluble extractive (%w/w) |
|
Aloe |
Not less than 10.0 |
|
Amla |
Not less than 40.0 |
|
Ashoka |
Not less than 15.0 |
|
Clove |
Not less than 3.0 |
|
Turmeric |
Not less than 8.0 |
Determination of Ether Soluble Extractive (Fixed Oil Content)
Transfer a suitably weighed quantity (depending on the fixed oil content) of the air dried, crushed drug to an extraction thimble, extract with Solvent ether (or petroleum ether, b.p. 40º to 60º) in a continuous extraction apparatus (Soxhlet extractor) for 6 hours. Filter the extract quantitatively into a tared evaporating dish and evaporate off the solvent on a water bath. Dry the residue at 105º to constant weight. Calculate the percentage of ether-soluble extractive with reference to the air-dried drug.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Example: non volatile ether soluble extractive value of crude drugs
Table 7: Determination of Ether Soluble Extractive (Fixed Oil Content
|
Drugs |
non volatile ether soluble extractive |
|
male fern |
Not less than 1.5 |
|
Linseed |
Not less than 25.0 |
Microscopical method :
This method allows more detailed examination of drug , and it can be used to identify the organized drug by their known histological character, it is mostly used for qualitative evaluation of organized crude drug in entire and powder form.
Leaf constants:
a. Palisade ratio:
Determination of Palisade Ratio
Palisade ratio is the average number of palisade cells under one epidermal cell. Place leaf fragments of about 5 × 5 mm in size in a test-tube containing about 5 ml of chloral hydrate solution and heat in a boiling water-bath for about 15 minutes or until the fragments become transparent. Transfer a fragment to a microscopical slide and prepare the mount of the upper epidermis in chloral hydrate solution and put a small drop of glycerol solution on one side of the cover-glass to prevent the preparation from drying. Examine with a 40x objective and a 6x eye piece, to which a microscopical drawing apparatus is attached. Trace four adjacent epidermal cells on paper; focus gently downward to bring the palisade into view and trace sufficient palisade cells to cover the area of the outlines of the four epidermal cells. Count the palisade cells under the four epidermal cells. Where a cell is intersected, include it in the count only when more than half of it is within the area of the epidermal cells. Calculate the average number of palisade cells beneath one epidermal cell, dividing the count by 4; this is the “Palisade ratio” (See Fig 1).
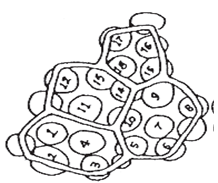
Figure 1: Palisade ratio
For each sample of leaf make not fewer than ten determinations and calculate the average number.
Palisade ratio =18.4/4=4.5
examples
|
Species |
Palisade ratio |
|
Atropa belladonna |
6 to 10 |
|
Cassia angustifolia |
5.1 to 7.5 |
|
Datura stramonium |
4 to 7 |
|
Datura tatula |
4 to 7 |
b. Stomatal no.:
It is average no. of stomata per sq. mm of the epidermis of the leaf ,it is effected by various factor like age of plant, size of leaf, enviourmental condition etc
Determination of Stomatal Number
Place leaf fragments of about 5x5 mm in size in a test tube containing about 5 ml of chloral hydrate solution and heat in a boiling water-bath for about 15 minutes or until the fragments become transparent. Transfer a fragments to a microscopic slide andprepare the mount the lower epidermis uppermost, in chloral hydrate solution and put a small drop of glycerol-ethanol solution on one side of the cover glass to prevent the preparation from drying. Examine with a 40 x objective and a 6x eye piece, to which a microscopical drawing apparatus is attached. Mark on the drawing paper a cross (x) for each stomata and calculate the average number of stomata per square millimeter for each surface of the leaf.
Table 8: Determination of Stomatal Number
|
Species |
number of stomata per sq.mm |
|
|
|
upper surface |
lower surface |
|
atropa belldona |
7.5 to 10 to 17.5 |
77.5 to 113 to176 |
|
cassia angustifolia |
180 to 200 to 223 |
195 to 220 to 257 |
c. Stomatal index:
Determination of Stomatal Index
The stomatal index is the percentage of the number of stomata formed by the total number of epidermal cells, including the stomata, each stoma being counted as one cell. Place leaf fragments of about 5 × 5 mm in size in a test tube containing about 5 ml of chloral hydrate solution and heat in a boiling water-bath for about 15 minutes or until the fragments become transparent. Transfer a fragment to a microscopic slide and prepare the mount, the lower epidermis uppermost, in chloral hydrate solution and put a small drop of glycerol-ethanol solution on one side of the cover-glass to prevents the preparation from drying. Examine with a 40x objective and a 6x eye piece, to which a microscopical drawing apparatus is attached. Mark on the drawing paper a cross (x)for each epidermal cell and a circle (o) for each stoma. Calculate the result as follows:
Stomatal index = s*100/E+S
Where S = the number of stomata in a given area of leaf ; and E = the number of epidermal cells (including trichomes) in the same area of leaf.
For each sample of leaf make not fewer than ten determinations and calculate the average index.
Examples:
Table 9: Stomatal Index
|
Species |
Stomatal index |
|
|
|
upper surface |
lower surface |
|
atropa belldona |
2.3 to 3.9 to 10.5 |
20.2 to 21.7 to 23.0 |
|
cassia angustifolia |
17.1 to 19.0 to 20.7 |
17.0 to 18.3 to 19.3 |
d. Vein islet number:
Determination of Vein-Islet Number
The mesophyll of a leaf is divided into small portions of photosynthetic tissue by anastomosis of the veins and veinlets; such small portions or areas are termed “Vein- Islets”. The number of vein-islets per square millimeter is termed the “Vein-Islet number”. This value has been shown to be constant for any given species and, for full-grown leaves, to be unaffected by the age of the plant or the size of the leaves. The vein-islet number has proved useful for the critical distinction of certain nearly related species.
The determination is carried out as follows :
For Whole or Cut leaves–- Take pieces of leaf lamina with an area of not less than 4 square millimeters from the central portion of the lamina and excluding the midrib and the margin of the leaf. Clear the pieces of lamina by heating in a test tube containing chloral hydrate solution on a boiling water-bath for 30 to 60 minutes or until clear and prepare a mount in glycerol-solution or, if desired, stain with safranin solution and prepare the mount in Canada Balsam. Place the stage micrometer on the microscope stage and examine with 4x objective and a 6x eye piece. Draw a line representing 2 mm on a sheet of paper by means of a microscopical drawing apparatus and construct a square on the line representing an area of 4 square millimeters. Move the paper so that the square is seen in the centre of the field of the eyepiece. Place the slide with the cleared leaf piece on the microscope stage and draw in the veins and veinlets included within the square, completing the outlines of those vein-islets which overlap two adjacent sides of the square. Count the number of vein-islets within the square including those overlapping on two adjacent sides and excluding those intersected by the other two sides. The result obtained is the number of vein-islets in 4 square millimeters. For each sample of leaf make not fewer than three determinations and calculate the average number of vein-islets per square millimeter.
For Leaf Fragments having an area less than 4 square millimeters– Take fragments of leaf lamina each with an area of not less than 1 square millimeter, excluding the midrib and the margin of the leaf. Clear and prepare a mount as stated above. Use a 10x objective and a 6x eyepiece and draw a line representing 1 mm on a sheet of paper by means of a microscopial drawing apparatus and construct a square on this line representing an area of 1 square millimetre. Carry out the rest of the procedure as stated above. The result obtained is the number of vein-islets in 1 square millimetre. For each sample of leaf make no less than 12 determinations and calculate the average number.
Quantitative microscope:
Lycopodium spore method:
It is an important technique for powdered drug ,especially when chemical &other method fail as accurate measure of quality.lycopodium is composed of spores of lycopodium elavatum.I.each spore is tetrahedral in shape,the base is rounded and the three side wall makes the three well marked covering ridge, which join one other at filled with fixed oil. The spore are excepetional uniform in size(25µm) and the shape tetrahedral so that one can always know that a definite no. of spore present in particular weight of lycopodium.on an average 94000 spores per mg of powdered lycopodium are present.using this figure one can calculate theweight of any number of spores under any condition under the microscope.
A powdered drug is evaluated by this technique ,if its contains:
· Well defined particles may be count ed e.g. starch grain or pollen grain.
· Single layered cells or tissue,the area of which may be tracedunder suitable magnification.
· The object of uniform thickness ,the length of which can be measured under suitable magnification and actual area can be calculated
Procedure:
Determine the loss on drying of powdered sample material of both sample &lycopodium powder at 105ºc.
Mix about 100 mg powdered sample drug and 50 mg of lycopodium powder using a small flexible spatula on a glass plate ,with a little suspending fluid.
In this mixture incoroporate a sufficient quantity of suspending fluid (glycerin:mucilage of tragacanth:water::2:1:2 or an oil) until a smooth line paste results .transfer it to stoppered tube by washing with excess of suspending fluid .adjust the final volume so that about 15 to 20 spore are observed in a field using a 4 mm of objective in microscope.
Oscillate the stopered container gently in order to obtain uniformity of the suspension.place one drop of suspension on each of two slide ,spread with a thin glass rod or needle ,apply the cover slip and leave aside for few minutes on the table in order to allow the fluid mixture to settle eventely.
Count the starch chacterstics structureof sample and lycopodium spores under microscope in each of 25 different field selected for observation.
Calculate the %purity of powdered drug by using the following equation:
% purity of crude drug=n*w*94000*100/s*m*p
N=number of charactersic structure of sample in 25 field
W=weight in mg of lycopodium
94000=number of lycopodium spore mer mg
S= number of lycopodium spore in same 25 field
M=weight in mg of sample ,calculated on the basis of sample dried at 105ºc
P=number of charcterstic sturucture of sample in 1 mg
(p is 2,86000 in case of ginger starch grain powder)
8. Qualitative tests:
a. detection of alkaloids :
The small portion of solvent free chloroform ,alcoholic and water extract are stirred seperately eith few drop of dilute hcl and filtered .the filterate may be tested carefully with various alkaloidal reagents such as:
Table 10: Detection ofAlkoids
|
Reagents |
Observation |
|
Mayer reagents (pottasium mercuric iodide solution ) |
Cream precipitate |
|
Dragondroff’ reagent(pottasiun bismuth iodide solution) |
Redissh brown ppt. |
|
Wagner’s reagent (iodine pottasium iodide solution) |
Redissh brown ppt. |
|
Hager’s reagents |
Yellow ppt. |
b. Detection of carbohydrate and glycosides:
· Small quantity of alcoholic and aq. Extracts are dissolved seperately in 5 ml of distilled water and filtered. The filterate may be subjected to molish test to detect presense of carbohydrate.
· Another small portion of extract is hydrolysed with dilute sulphuric acid for few hours in water bath and subjected to liebermann- burchard,legals,and brontrager test to detect presense of different glycosides.
c. Detection of saponins :
About 1 ml of alcoholic and aq extract is diluted seperately with disstilled water to 20 ml and shaken in graduated cylinder for 15 minutes.one cm layer of foam indicates presence of saponins.the test solution may be subjected to test for haemolysis.
d. Detection of protein and free mino acids:
Small quantity of alcoholic and aqueous extract are dissolved in few ml of water and subjected to million’s,biuret’s, and ninhydrin test.
e. Detection of volatile oil:
About 50 mg of powdered material is taken in a volatile oil estimation appartus and subjected to hydrodistilation.the disttilte is collected in graduated tube of assembely in which aq. Portion is automatically seperated from the volatile oil, if it present in the drug and returned back to disstilation flask
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Pesticide analysis:
For the purposes of the Pharmacopoeia, a pesticide is any substance or mixture of substances intended for preventing, destroying or controlling any pest, unwanted species of plants or animals causing harm during or otherwise interfering with the production, processing storage, transport or marketing of vegetable drugs. The item includes substances intended for use as growth-regulators, defoliants or desiccants and any substance applied to crops either before or after harvest to protect the commodity from deterioration during storage and transport.
Reagents. All reagents and solvents are free from any contaminants, especially pesticides, that might interfere with the analysis. It is often necessary to use special quality solvents or, if this is not possible, solvents that have recently been re-distilled in an apparatus made entirely of glass. In any case, suitable blank tests must be carried out.
Apparatus. Clean the apparatus and especially glassware to ensure that they are free from pesticides, for example, soak for at least 16 h in a solution of phosphate-free detergent, rinse with large quantities of distilled water R and wash with acetone and hexane or heptane.
Quanitative analysis of Organophosphorus insecticides.
Examine by gas chromatography, using carbophenothion R as internal standard. It may be necessary to use a second internal standard to identify possible interference with the peak corresponding to carbophenothion.
Test solution. Concentrate solution B in a current of helium for chromatography R almost to dryness and dilute to 100 μl with toluene R.
Reference solution. Prepare at least three solutions in toluene R containing the insecticides to be determined and carbophenothion at concentrations suitable for plotting a calibration curve.
The chromatographic procedure may be carried out using :
– a fused-silica column 30 m long and 0.32 mm in internal diameter the internal wall of which is covered with a layer 0.25 μm thick of poly (dimethyl) siloxane R.
– hydrogen for chromatography R as the carrier gas. Other gases such as helium for chromatography R or nitrogen for chromatography R may also be used provided the chromatography is suitably validated.
– a phosphorus-nitrogen flame-ionisation detector or a atomic emission spectrometry detector. Maintaining the temperature of the column at 80ºC for 1 min, then raising it at a rate of 30ºC/min to 150ºC, maintaining at 150ºC for 3 min, then raising the temperature at a rate of 4ºC/min to 280ºC and maintaining at this temperature for 1 min and maintaining the temperature of the injector port at 250ºC and that of the detector at 275ºC. Inject the chosen volume of each solution. When the chromatograms are recorded.
Quanitative analysis of Organochlorine and pyrethroid insecticides:
Examine by gas chromatography, using carbophenothion as the internal standard.It may be necessary to use a second internal standard to identify possible interferencewith the peak corresponding to carbophenothion.
Microbial content determination:
TESTS FOR STERILITY
The tests for sterility are intended for detecting the presence of viable forms of micro-organisms in or on pharmacopoeial preparations. The tests must be carried out under conditions designed to avoid accidental contamination of the product during the test.
The working conditions in which the tests are performed should be monitored regularly by sampling the air and surfaces of the working area and by carrying out control tests, The tests are based upon the principle that if micro-organisms are placed in a medium which provides nutritive material and water, and kept at a favourable temperature, the organisms will grow and their presence can be indicated by a turbidity in the originally clear medium. The tests for sterility are designed to reveal the presence of micro-organisms in the samples used in the tests; interpretation of results is based on the assumption that the contents of every container in the batch, had they been tested, would also have complied with the the tests. Since every container cannot be tested, a sufficient number of containers should be examined to give a suitable degree of confidence in the results of the tests.
Culture Media:
Fluid thioglycollate medium – For use with clear fluid products
Alternative thioglycollate medium – For use with turbnid and viscid products and for devices having tubes with small lumina.
Test Procedures:
The tests can be carried out using Method A, Membrane Filtration or Method B, Direct Inoculation. Method A is to be preferred where the substance being examined is (a) an oil, (b) an ointment that can be put into solution, (c) a non-bacteriostatic solid not readily soluble in the culture medium and (d) a soluble powder or a liquid that possesses inherent bacteriostatic and fungistatic properties.
Method A, Memberane filteration test
Prepare each membrane by aseptically transferring a small quantity (sufficient to moisten the membrane) of fluid A on to the membrane and filtering it. For each medium to be used, transfer aseptically into two separate membrane. Alternatively, transfer aseptically the combined quantities of the preparation being examined prescribed in the two media onto one membrane. Draw the liquid rapidly through the filter with the aid of vacuum. If the solution being examined has antimicrobial properties, wash the membrane (s) by filtering through it (them) not less than three successive quantities, each of approximately 100 ml, of sterile fluid A. The quantities of fluid used should be sufficient to allow growth of a small inoculum of organisms (approximately 50) sensitive to the antimicrobial substance in the presence of the residual inhibitory material on the membrane. After filtration, aseptically remove the membrane (s) from the holder, cut the membrane in half, if only one is used, immerse the membrane, or one-half of the membrane, in 100 ml of soyabean-casein digest medium and incubate at 20º to 25º for not less than 7 days. Similarly, immerse the other membrane, or other half of the membrane, in 100 ml of fluid thioglycollate medium and incubate at 30º to 35º for not less than 7 days.
Diluting fluids
a. Fluid A: Dissolve 1 g of peptic digest of animal tissue (such as bacteriological peptone) or its equivalent in water to make 1 litre, filter or centrifuge to clarify, adjust to pH 7.1±0.2, dispense into flasks in 100-ml quantities and sterilize at 121º for 20 minutes.
b. Fluid B: If the test sample contains lecithin or oil, use fluid A to each litre of which has been added 1 ml of polysorbate 80, adjust to pH 7.1±0.2, dispense into flasks and sterilize at 121º for 20 minutes.
Note – A sterile fluid shall not have antibacterial or antifungal properties if it is to be considered suitable for dissolving, diluting or rinsing a preparation being examined for sterility.
Method B: Direct Inoculation:
Method of test:
Remove the liquid from the test containers with a sterile pipette or with a sterile syringe or a needle. Aseptically transfer the specified volume of the material from each container to a vessel of the culture medium. Mix the liquid with the medium but do not aerate excessively. Incubate the inoculated media for not less than 14 days, unless otherwise specified in the monograph, at 30º to 35º in the case of fluid thioglycollate medium and at 20º to 25º in the case of soyabean-casein digest medium. When the material being examined renders the medium turbid so that the presence or absence of microbial growth cannot be determined readily by visual examination, transfer suitable portions of the medium to fresh vessels of the same medium between the third and seventh days after the test is started. Continue incubation of the transfer vessels for not less than 7 additional days after the transfer and for a total of not less than 14 days.
Observation and Interpretation of Results:
At intervals during the incubation period, and at its conclusion, examine the media for macroscopic evidence of microbial growth. If no evidence of growth is found, the preparation being examined passes the tests for sterility. If evidence of microbial growth is found, reserve the containers showing this and, unless it is demonstrated by any other means that their presence is due to causes unrelated to the preparation being examined and hence that the tests for sterility are invalid and may therefore be recommenced, perform a retest using the same number of samples, volumes to be tested and the media as in the original test. If no evidence of microbial growth is then found, the preparation being examined passes the tests for sterility. If evidence of microbial growth is found, isolate and identify the organisms. If they are not readily distinguishable from those growing in the containers reserved in the first test, the preparation being examined fails the tests for sterility. If they are readily distinguishable from those growing in the containers reserved in the first test, perform a second retest using twice the number of samples. If no evidence of microbial growth is found in the second retest, the preparation being examined passes the tests for sterility. If evidence of growth of any micro-organisms is found in the second retest the preparation being examined fails the tests for sterility.
Result and Conclusion:
Involved Evaluation and Standardization techniques for crude drugs, mono or Polyherbal Frormulation. They involved the macroscopic techniques, microscopic techniques, physical evaluation and biological evaluation. They also involved the Quantitative analysis of Organophosphorus insecticides, Organochlorine and pyrethroid insecticides, microbial content determination.
Summary report :
The given test are applied for quality, safety and efficacy built up in the product. And these tests are most important for pharmaceutical industry who manufacture from the crude drug.
BIBILOGRAPHY
-
Rasheeduz zafar et al, practical pharmacognosy, 1st edition reprint 2000, published by CBS publisher &distributor, new delhi p.1
-
Mohhamad Ali , textbook of pharmacognosy, second edition1998, published by CBS publisher p.n.52
-
Khandelwal K.R, Practical Pharmacognosy Technique &Experiment, published by Nirali Parkashan ,p.no.146
-
T.E.Walis, Textbook of pharmacognosy, 5th edition, published by CBS Publisher & Distributor,p.no.561
-
Dr.C.K.Kokate et al, 39th edition, Pharmacognosy, published by Nirali Parkashan, 20th edition 2004, p.no.
-
S.S Handa, Textbook Of Pharmacognosy, 2nd edition reprint 2005, Vallabh Prakashan delhi p.no.63
-
DR.C.K.Kokate, Practicle Pharmacognosy, 4thedition, reprint 2006, published by Vallabh Parkashan delhi,p.no. 122
-
Protocol for testing ayurvedic, siddha & unani medicines,Pharmacopoeial Laboratory For Indian Medicine Ghaziabad ,p.no.94,135-146
-
WHO Guideline for Herbal Drug Stanardization 7/8/2011 pharmainfo.net/reviews/who-guidelines-herbal-drug-standardization
-
Method Of Drug Evaluation, Bukisa bukisa.com/articles/332829_methods-of-drug-evaluation
-
Method Of Evaluation Of Crude Drug, Formulation Manual pharmaformulas.vinvarun.biz/2009/07/method-of-evaluation-of-crude-drugs.html
-
M.S.Wani Herbal Medicines and Its Standardization on- 7/8/2011 pharmainfo.net/reviews/herbal-medicine-and-its-standardization
-
Sunita panchawat ,et al, Standardization and Evaluation of Herbal Drug Formulation , Posted: Oct 08, 2009,ArticlesBase free online article directory articlesbase.com/alternative-medicine-articles/standardisation-and-evaluation-of-herbal-drug-formulations-1317004.html
-
WHO Guidelines for Quality Standardized Ayurvedic FormulationsWHO Guidelines for Quality Standardized Ayurvedic Formulations ,Integerative Health Care Institute integrativehealthcareinstitute.net/journal/articles/who-guidelines-for-quality-standardized-ayurvedic-formulations-2.html
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









