{ DOWNLOAD AS PDF }
 ABOUT AUTHORS:
ABOUT AUTHORS:
Debpratim Chakraborty
Persuing M.Pharm (Pharmacognosy),
Jadavpur University, West bengal.
debpratim008@gmail.com
ABSTRACT
Polycystic Ovarian Syndrome is one of the most common female endocrine. Many papers reported the correlation between infertility that most eminently predisposes towards cancer in PCOS women from many years. From both clinical and experimental research one can conclude that correlation between PCOS and endometrial cancer is the strongest. Most studies on correlation between PCOS and ovarian cancer as well as breast cancer failed to show a link though Pulsatilla have a strong effect on it. Various synthetic drugs which mainly used in the treatment of PCOS and endometrial cancer but here the problem is those drugs has lots of side effect/Adverse effect so we should go with the natural product Pulsatilla Sp. Which is used on PCOSand various cancer. Pulsatilla is a well establish drug for PCOS with unknown mechanism of action. Deoxypodophyllotoxin, which was isolated from the roots of P. koreana, was found to inhibit the tube-like formation of HUVECs (human umbilical venous endothelial cells) and have a potent antitumor effect. The chemical constituent saponin D present in Pulsatilla root is responsible for the anti tumor activity. The results, which are expressed as Pulsatilla saponin D has such a potent antitumor effect (IR, 82 %) on a solid tumor, which is higher than Adriamycin (IR, 64%). 23-Hydroxybetulinic acid synthesized from Pulsatilla chinensis also shows anti tumoral activity. Though the potency of pulsatilla sp. Are less than synthetic marketed drugs but by structural modification we may get potent anti canceral agent with least toxicity by Pulsatilla.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2389
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 4, Issue 2 Received On: 07/09/2015; Accepted On: 14/09/2015; Published On: 01/02/2016How to cite this article: Chakraborty D; Effect of pulsatilla on polycystic ovarian syndrome and its associated cancer; PharmaTutor; 2016; 4(2); 34-40 |
INTRODUCTION
Polycystic Ovarian Syndrome is one of the most common female endocrine (or hormonal) disorders, characterized by multiple abnormal ovarian cysts. PCOS is accompanied by such conditions as anovulation, hipertestosteronism, lower cell sensitivity to insulin, type II diabetes, hyperlipidemia and obesity. Each of the above-mentioned conditions is an approved risk factor proved to predispose towards cancer. Women diagnosed with PCOS develop higher level of testosterone, insulin, growth factors such as IGF-1 (Insulin Growth Factor-1), which were experimentally proved to have potential for direct stimulation of cancer cells proliferation. Now a day various anti-cancer drug available in the market with highly toxicity, that’s why we should keep our attention towards natural remedies. The root of various species of Pulsatilla plant (pulsatilla koreana, pulsatilla chinensis) contain saponin which shows anti-cancer properties and different species of pulsatilla also helps to reduce PCOS.
Poly cystic ovary syndrome:
Polycystic ovary syndrome (PCOS), also called hyperandrogenic anovulation (HA), or Stein–Leventhal syndrome, is a set of symptoms due to a hormone imbalance in women. Symptoms include: irregular or no menstrual periods, heavy periods, excess body and facial hair, acne, pelvic pain, trouble getting pregnant, and patches of thick, darker, velvety skin. Associated conditions include: type 2 diabetes, obesity, obstructive sleep apnea, heart disease, mood disorders, and endometrial cancer.
PCOS is due to a combination of genetic and environmental factors.Risk factors include obesity, not enough physical exercise, and a family history of someone with the condition. Diagnosis is based on two of the following three findings: no ovulation, high androgen levels, and ovarian cysts. Cysts may be detectable by ultrasound.
Prevalence:
PCOS is the most common endocrine disorder among women between the ages of 18 and 44. It affects approximately 5% to 10% of this age group. It is one of the leading causes of poor fertility. The earliest known description of what is now recognized as PCOS dates from 1721 in Italy.
Few studies have attempted to define its prevalence in women with these symptoms. In a study of 175 anovulatory women presenting consecutively to a reproductive endocrine clinic, 30 percent of those with amenorrhea and 75 percent of those with oligomenorrhea had ultrasonographic evidence of polycystic ovaries. More than 60 percent of these women were hirsute, and 90 percent had elevated serum concentrations of luteinizing hormone or androgens (or both). [1] These findings are supported by a study in which clinical and biochemical, rather than ultrasonographic, criteria were used to make the diagnosis of polycystic ovary syndrome. In a series of women being treated at a regional infertility center in southwest England, 37 percent of those with amenorrhea and 90 percent of those with oligomenorrhea (overall, 73 percent of the cases of anovulatory infertility) were found to have the polycystic ovary syndrome. Subsequently, clinical and biochemical markers of the syndrome were correlated with ultrasonographic results, and a high degree of concordance was observed between the findings. Surprisingly, polycystic ovaries were detected by ultrasonography in 40 of 46 women (87 percent) presenting with hirsutism but with regular menses (i.e.,“idiopathic hirsutism”).
Clinical representation:
Typical clinical features of the polycystic ovary syndrome are summarized in Table
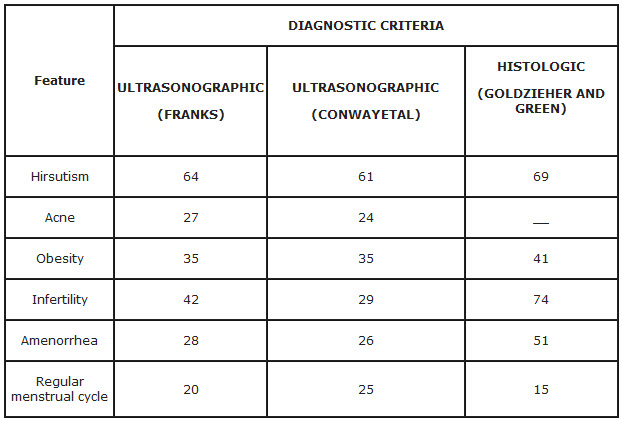
*Data were compiled from two recent studies in which ultrasonography was used as the primary method of diagnosis (Franks and Conway ) and from the classic review by Goldzieher and Green in which the diagnosis was based on proved histologic features of ovaries after wedge resection. Note the similarity in the distribution of symptoms in the three studies.
PCOS and its link with cancer:
Many papers reported the correlation between infertility that most eminently predisposes towards cancer in PCOS women from many years. In a study of 1983 it’s reported that 3-times higher risk of endometrial cancer in patients suffering from chronic anovulation. Women who were infertility-patient displayed 7.6-fold risk of endometrial cancer. From the above mentioned statement, one can conclude that infertility increases risk of endometrium cancer, especially if accompanied by chronic anovulation. Many papers aimed at linking breast cancer to hormonal factors which is also the case in PCOS. Brinton et al.[2] did not show correlation between infertility and breast cancer. These conclusions were confirmed in other research. . However, one research showed 1.8-fold risk in infertile patients with chronic anovulation, and yet another one showed 5.4-fold incidence of breast cancer in pre-menopause women who were previously diagnosed with anovulation. Two other papers seem very interesting with respect to PCOS patients. One of them showed that in breast cancer patients suffering from ovulation problems the risk of breast cancer was 3.5- times higher. If the same patients had signs of hyperandrogenism (persistent acne, excess of body hair), the risk was 6.8 higher.. The second paper showed that in patients with obesity, hypertension and diabetes (conditions that frequently coincide with PCOS) the risk of breast cancer is 3-times higher. In a research risk of ovarian cancer in nullipara was 2.45-times higher compared to multipara and 1.27-times higher compared to unipara. The analysis of 12 case control studies showed 2.1- fold risk in nullipara with infertility history.
Obesity, which coincides with PCOS is a risk factor in all the above mentioned types of cancer. Meta-analysis of studies into coincidence of ovarian cancer and obesity showed that in 10 per 28 population-based studies correlation was statistically significant, and pooled effect estimate for adult obesity was 1.3 [3]. Recent epidemiologic studies have shown a positive relationship between BMI and breast cancer with a significant relative risk ranging from 1.26 to 2.52. Theoretical premises for correlation of PCOS and cancer are based on very specific observations. Unopposed stimulation of the endometrium in the setting of chronic anovulation in women with PCOS is an acknowledged factor leading to endometrial cancer development.
Endometrial cancer:
From both clinical and experimental research one can conclude that correlation between PCOS and endometrial cancer is the strongest. Endometrium of PCOS patients is known to be more sensitive to steroid hormones. Women with PCOS exhibit elevated endometrial androgen receptor expression compared to normal, fertile controls and endometrium of PCOS patients shows higher concentration of estrogen receptors. It was also proved that PCOS patients exhibit higher Cyr61 protein expression [4], protein which is integrin-binding, angiogenic factor, that also promotes cell migration and adhesion. This protein is also an apoptosis regulator and is associated with tumor genesis. Higher Cyr61 expression was also present in uterine myoma and in endometriosis. In the case of breast cancer its elevated expression was found both in tumor biopsy and in metastasis. In the course of PCOS endometrial homeostasis is disturbed. Apart from a change in Cyr61 production, a number of other modifications are identified; a change in Ki-67 expression, lower p53 production, higher cyclin D1 expression and higher bcl-2/bax ratio [5]. Disturbed homeostasis may result from intensified proliferation which may in turn lead to abnormal cell division possibly followed by tumorgenesis. However, clinical studies do not provide straightforward answer to the question about correlation between PCOS and endometrial cancer. This correlation was observed as early as 1949, 14 years after the first description of the syndrome. However, both this and some other studies on this problem [6] did not include study controls, which disqualifies explicit conclusions.
There was also an attempt to conclude that PCOS may provide better prognosis once endometrial cancer has been diagnosed [7], as histopathological research of preparations from PCOS patients showed well differentiated tumors or moderately well differentiated.
Ovarian cancer:
The most quoted paper showing correlation between ovarian cancer and PCOS is by Schildkraut et al. [8]. It showed 2.5-fold risk of ovarian cancer in PCOS. In the group of patients diagnosed with ovarian cancer there were only 7 PCOS women who accounted for 1.5% of the group, while in the study control there were 24 cases of PSOC, i.e. 0.06% of the control. Conclusions on correlation between ovarian cancer and PCOS seem inaccurate based on such a small group of PCOS patients in research groups. Most studies on correlation between PCOS and ovarian cancer failed to show a link.
Breast cancer:
In the case of correlation between breast cancer and PCOS proofs are also not convincing, and research results even show reverse correlation [9]. The basic problem underlying these studies was the fact that in the second study PCOS women accounted just for 0.94% of the study control and for 0.49% of breast cancer group, while in the first study for 1.35% of the overall study group. Based on available literature one may draw a conclusion that correlation between adenocarcinoma of endometrium and PCOS is likely to exist. This results both from clinical trials and papers on changes of endometrium in PCOS patients. However, despite single papers that report on the link, correlation between ovarian or breast cancer and PCOS is doubtful.
So we should pay attention how to get recovery from PCOS as well as endometrial cancer, PCOS is not responsible for ovarian cancer and breast cancer, so our aim will be the procurement of PCOS and endometrial cancer.
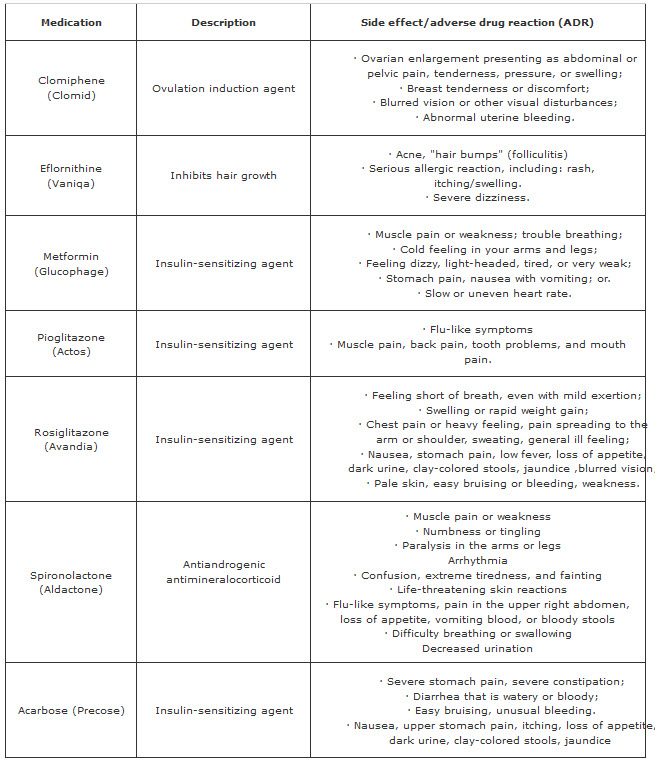
Synthetic Drugs used in PCOS and their major side effects (ADR)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Synthetic drugs used in endometrial cancer and their major side effects (ADR)

Those are the synthetic drugs which mainly used in the treatment and procurement of PCOS and endometrial cancer but here the problem is those drugs has lots of side effect/Adverse effect so we should go with the natural product Pulsatilla Sp. which have no major side effect and most important it is very much effective on both PCOS as well as endometrial cancer.
Action of Pulsatilla on PCOS:
Pulsatilla is a natural medicine for treating PCOS. It is especially beneficial for treating PCOS cases in females who suffer from suppressed periods for a long duration. In women requiring Pulsatilla, the periods when they appear remain very scanty and extremely painful. Pulsatilla removes the obstacles that cause the periods to be suppressed and the periods start to flow normally. Pulsatilla increases the body’s power and directs its path towards removing the obstacle, thus re-establishing the menstrual flow. Pulsatilla is of great help for young girls at puberty who suffer from PCOS with irregular periods. Though the mechanism of action is not known clearly but the effect is well known proven.
Pulsatilla and its antitumor activity:
The Pulsatilla genus, Pulsatilla koreana Nakai (Ranuculaceae) in Korea is an important herb in traditional medicine that has been used to treat amoebic dysentery and malaria. It had been reported that plants from the Pulsatilla genus contain ranunculin, anemonin, protoanemonin, triterpenes, and saponins (9 %), mainly the oleanane and lupane-type saponins. A paper also reported that ranunculin has cytotoxicity against KB cells by inhibiting DNA polymerase.[10] Recently, deoxypodophyllotoxin, which was isolated from the roots of P. koreana, was found to inhibit the tube-like formation of HUVECs (human umbilical venous endothelial cells) and have a potent antitumor effect. Previously, it was reported that the anticancer preparation called SB31®,[11] which was produced from the roots of Pulsatilla koreana. Therefore, its major ingredients, the roots of P. koreana, might be an important component that is responsible for its potent antitumor activity.
In vitro cytotoxic assay:
In a study the level of cytotoxicity was measured using the sulforhodamine B (SRB) method.[12] Viable cells kept in the growth medium (180 μL) into 96 well microtiter plates (3-4×104 cells per each well) and allowed to attach overnight. The test sample (Pulsatilla saponin D) was dissolved in DMSO and adjusted to a final sample concentration ranging from 0.3 ng/mL to 10 μg/mL by a dilution with the growth medium. Each sample was prepared in triplicate. The final DMSO concentration was then adjusted to <0.1%. After 72 h incubation, the medium was removed and the remaining cells were fixed with 10% trichloroacetic acid (TCA) for 1 h at 4oC. The TCA-treated cells were washed extensively with water and dried in air. Subsequently, 50 μL of the SRB solution (0.4% in AcOH) was added to each well at room temperature. After standing for 1 h, the wells were washed 3-4 times with 1 % AcOH and dried in air. The bound dye was dissolved in Tris base (100 μL of 10 mM). The absorbance of the Tris solution was measured using a micro-plate reader at 520 nm. The ED50 value was defined as the Pulsatilla saponin D concentration needed to reduce the absorbance relative to the vehicle-treated controls by 50%.
In vivo anti-tumor activity assay:
In study the antitumor activity was measured according to Teruhiros method.[13] 2×2×2 mm3 tumors were transplanted into the auxiliary region of the BDF1 mice. Twenty-four hours after tumor transplantation, the mice were divided into several groups consisting of 5 mice each. The samples, WT, SPX fractions, and Pulsatilla saponin D were then dissolved in saline, and injected intraperitoneally on the 1st to 7th and 9th to 14th day at a dose of 280 mg/kg, 70 mg/kg, and 6.4 mg/kg of Pulsatilla saponin D, respectively. The experimental mice were weighed twice a week in order to evaluate the toxicity of Pulsatilla saponin D.
The tumor volume (TV) was measured on the 15th and 16th day, and calculated according to the following formula:
TV = L (mm) × W2 (mm2) / 2
where L and W represent the length and the width of the tumor mass, respectively.
The inhibition ratio (IR) was calculated according to the following formula:
IR (%) = [(Mean TV of control group − Mean TV of treatedgroup) / Mean TV of control group] × 100
RESULT AND DISCUSSION
The 50% aqueous EtOH fraction (WT fraction) of the roots of P. koreana was evaluated in a BDF1 animal model. The WT fraction was injected at the maximum tolerated dose of 280 mg/kg/day and Adriamycin, which was used as the positive control, was administered at 0.5 mg/kg/day to the right groin of the BDF1 mice. The results, which are expressed as the inhibition ratio (IR, %), are the WT fraction exhibited significant antitumor activity with an IR of 56 % (day 14) and 55 % (day 15), although it was less than that of Adriamycin (60% and 64%).
The Pulsatilla saponin D was further evaluated for their antitumor activity in the same animal tumor model, and for their cytotoxic activity against three cancer cell lines (human lung cancer, human melanoma, and human breast cancer). The Pulsatilla saponin D showed moderate cytotoxic activity (ED50, 6.3 μg/mL to >10 μg/mL) against the cancer cell lines. Considering its low cytotoxic activity, it is remarkable that the Pulsatilla saponin D has such a potent antitumor effect (IR, 82 %) on a solid tumor, which is higher than Adriamycin (IR, 64%).
From another study it can be reported that 23-Hydroxybetulinic acid synthesized from Pulsatilla chinensis also shows anti tumoral activity. 23-Hydroxybetulinic acid has been found to have cytotoxicity against a variety of tumor cell lines, [14] but the exact mechanism of action is still unclear. In addition, it is not as potent as the classical antitumor agents but it’s also has advantages that it has not any major side effect.
CONCLUSION
So here we can conclude that various species of pulsatilla has potent anti tumoral effect and it’s also used in poly cystic syndrome. So the Pulsatilla sp. May used in PCOS as well as various types of cancer which may or may not stimulated by PCOS. Pulsatilla sp. Show low potency in various cancer but by structural modification of the saponin present in Pulsatilla, we may prepare a potent anti tumoral agent and most important is that we may prepare an anti tumoral agent which will show the least side effect. Now a day there are various anti tumoral drugs are present in the market , they are mostly potent but also they have maximum side effects so if we can prepare a ponent anti tumoral agent with least side effect then that will open a new gate of chemotherapy.
REFERENCES:
1. Adams J, Polson DW, Franks S. Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. BMJ 1986; 293:355-9.
2. Brinton LA, Berman ML, Mortel R. Reproductive, menstrual, and medical risk factors for endometrial cancer: results from a case-control study Am J Obstet Gynecol. 1992; 167(5):1317-25.
3. Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: a systematic review and meta-analysis. Eur J Cancer. 2007; 43(4):690-709.
4. MacLaughlan SD, Palomino WA, Mo B, Lewis TD, Lininger RA, Lessey BA. Endometrial expression of Cyr61: a marker of estrogenic activity in normal and abnormal endometrium. Obstet Gynecol. 2007; 110(1); 146-54.
5. Villavicencio A, Bacallao K, Gabler F; Deregulation of tissue homeostasis in endometria from patients with polycystic ovarian syndrome with and without endometrial hyperplasia; Gynecol Oncol; 2007; 104(2); 290-5.
6. Gallup DG, Stock RJ; Adenocarcinoma of the endometrium in women 40 years of age or younger; Obstet Gynecol; 1984; 64(3); 417-20.
7. Jafari K, Javaheri G, Ruiz G; Endometrial adenocarcinoma and the Stein-Leventhal syndrome; Obstet Gynecol. 1978; 51(1); 97-100.
8. Schildkraut JM, Schwingl PJ, Bastos E; Epithelial ovarian cancer risk among women with polycystic ovary syndrome; Obstet Gynecol; 1996; 88(4 Pt 1); 554-559.
9. Gammon MD, Thompson WD; Polycystic ovaries and the risk of breast cancer; Am J Epidemiol; 1991; 134(8); 818-824.
10. Li, R. Z. and Ji, X. J.; The cytotoxicity and action mechanism of Ranunculin in vitro; Yao Xue Xue Bao; 1993; 28(5); 326-331.
11. Kim, S. Y. and Kim, S. B.; Anti-tumor effects of extracts of Pulsatilla koreana (SB-31®) in vitro; J. Kor. Cancer Assoc.; 1994; 26(6); 959-963.
12. Skehan P., Storeng R., Scudiero D; New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening; J. Natl. Cancer Inst. 1990; 82(13); 1107-1112.
13. Teruhiro U, Jiro S, Yoshikazu S; Antitumor activity of a novel podophylotoxin derivatives (TOP53) against lung cancer and lung matastatic cancer; Cancer Res; 1996; 56(12); 2809-2814.
14. Ji, Z., Ye, W., Liu, G., Hsiao, W; 23-Hydroxybetulinic acid-mediated apoptosis is accompanied by decreases in bcl-2 expression and telomerase activity in HL- 60 Cells; Life Sciences; 2002; 72(1), 1-9.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









