 About Authors:
About Authors:
SINGH GAURAV*, GOKULAN DR P.D., KINIKAR DHANANJAY, KUSHWAH MANOJ
Shri Ramnath Singh Institute Of Pharmaceutical Science and Technology,
Sitholi, Gwalior, M.P
*gaurav.robby@gmail.com
ABSTRACT
Glibenclamide is an oral hypoglycemic drug with very low aqueous solubility. The drug is completely metabolized in liver, its principle metabolite being very weakly active, buccal film may be more efficacious for the treatment of diabetes. The objective of this work is to investigate the feasibility of obtaining the slow release, obtaining relatively constant levels of Glibenclamide from buccal films. The films were fabricated by solvent casting technique with different polymers HPMC, Sodium CMC and PVP and systematically evaluated for in vitro and ex vivo performance. Propylene Glycol is employed as plasticizer and penetration enhancer. Hydroxypropyl methyl cellulose is acting as film forming polymer, Sodium CMC and PVP are employed as mucoadhesive polymer. The formulation containing HPMC and Sodium CMC (F2) showed better controlled results correlated with ex vivo permeation studies.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1267
INTRODUCTION
One of the most widespread chronic disorders affecting mankind is Diabetes Mellitus. Mucoadhesive materials have been employed recently to deliver drugs to or via mucous membrane.[1] Mucoadhesion is adhesion of substrate to mucous or mucous membrane. Mucoadhesive polymers have been investigated and these consist of hydrophilic macromolecules which contain numerous hydrogen bond forming groups. They hydrate and swell when they come in contact with aqueous medium. They become adhesive in their hydrated form. Mucoadhesive dosage forms are being developed for ocular, nasal, rectal and vaginal cavity for local or systematic delivery. Nasal delivery has already achieved commercial status. Buccal delivery has been extensively investigated for delivery of few drugs like nicotine, isosorbide dinitrate, β blockers etc..[2] Mucoadhesive drug delivery system offers advantages like prolonging the drug action, targeting the drug to a localized site, avoidance of degradation of drug in gastrointestinal tract, to deliver high molecular weight proteins and peptides systemically and to avoid first pass metabolism.[3] Buccal delivery offers advantages like good accessibility, robust epithelium, quick and easy removal of the dosage form in case of need and good patient compliance. Mucoadhesive buccal films are preferred over tablets as they are flexible. They also overcome the short residence time of oral gels in the mucosa, which are easily washed away and removed by saliva.[4]
Glibenclamide is a potent second generation sulfonylurea drug, used in the treatment of maturity onset diabetes as an oral hypoglycaemic. Glibenclamide, chemically is 1-(p-(2-(5-chloro-O-anisamido) ethyl), phenyl), sulfonyl)-3-cyclohexyl urea. The various physicochemical properties of Glibenclamide such as low molecular weight (494.0), dissociation constant (5.3), short biological half life (3-5 hrs) necessitates multiple dosing for maintaining therapeutic effect throughout the day. Absence of objectionable taste and odor make it a suitable candidate for buccal administration [5, 6]. The present study is an attempt to formulate and evaluate mucoadhesive buccal films of Glibenclamide with different polymers viz. HPMC, PVP and Sodium CMC. Drug Interaction study was performed to confirm that the drug does not react with the polymers used.
[adsense:468x15:2204050025]
MATERIALS AND METHODS
Materials
Glibenclamide was received as a gift sample from Ingla Laboratories Pvt. Ltd. Mumbai. Hydroxypropyl methyl cellulose (5 cps), Sodium Carboxy methyl cellulose 1500-400cps (SCMC), Polyvinylpyrrolidone (PVP 40 000) was procured from Central Drug House, Mumbai. Propylene Glycol was procured from E. Merck (P) Ltd, Mumbai. Other chemicals employed were of analytical grade and used without further purification.
Preparation of mucoadhesive buccal films
Buccal films were prepared by the solvent casting technique. Polymeric composition of single cast film of various formulations is mentioned in Table 1. Buccal films were prepared by using HPMC (5cps) alone and in combination with PVP, Sodium CMC (High viscosity grade). The calculated amounts of polymers were dispersed in ethanol. 55 mg of Glibenclamide was incorporated in the polymeric solution after levigation with 30% w/w propylene glycol which served as plasticizer and penetration enhancer. The medicated gels were left overnight at room temperature to obtain clear bubble free gels. [7]
Tables 1
|
Patch code |
Total polymer (mg) |
Drug (mg) |
HPMC (5 cps) (mg) |
NaCMC (mg) |
PVP (mg) |
Propylene Glycol (ml) |
Solvent (Ethanol) ml |
|
F1 |
900 |
55 |
900 |
- |
- |
0.5 |
20 |
|
F2 |
1200 |
55 |
900 |
- |
300 |
0.5 |
20 |
|
F3 |
1150 |
55 |
900 |
250 |
- |
0.5 |
20 |
|
F4 |
1150 |
55 |
900 |
- |
250 |
0.5 |
20 |
|
F5 |
1200 |
55 |
900 |
300 |
- |
0.5 |
20 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Characterization of mucoadhesive buccal films
a) Weight Uniformity and thickness
Three samples of each patch (1.5 cm×1.9 cm) were randomly taken and each patch was weighed individually. The data was analyzed for mean weight and standard deviation. Weight variation of patch is in Table 2. Thickness of samples from each patch was measured in triplicate and average values were reported in Table 2.
Table 2
|
S. No. |
Formulation |
Surface pH |
Weight Uniformity |
Thickness (mm) |
Folding |
Drug |
Bio adhesive |
Residence time |
|
1 |
F1 |
6.67±0.04 |
162.26±0.56 |
0.195±0.032 |
300±5 |
98.18±1.36 |
10.2±0.6 |
330 |
|
2 |
F2 |
6.64±0.02 |
172.93±0.49 |
0.210±0.050 |
300±7 |
99.13±0.95 |
8.5±0.9 |
487 |
|
3 |
F3 |
6.65±0.03 |
184.48±0.47 |
0.260±0.023 |
257±6 |
97.25±1.27 |
6.8±0.7 |
364 |
|
4 |
F4 |
6.66±0.02 |
170.89±0.36 |
0.245±0.008 |
273±8 |
97.98±1.85 |
7.2±0.5 |
382 |
|
5 |
F5 |
6.67±0.03 |
187.75±0.29 |
0.265±0.028 |
300±4 |
98.54±1.14 |
9.3±0.8 |
432 |
b) Surface pH
The surface pH was determined for the films. A combined glass electrode was used for this purpose. The patches were kept in contact with 5 ml of distilled water for 1 hr. The pH was noted by bringing the electrode near the surface of the formulations and allowing it to equilibrate for 1 min. Surface pH of various formulations is shown in Table 2.
c) Folding Endurance
The folding endurance of patches was determined by repeatedly folding one patch at the same place till it broke or up to 300 times without breaking. The experiments were performed in triplicate, and average values were reported in Table 2.
d) Content Uniformity
Drug content uniformity was determined by dissolving each patch in 10 ml of ethyl alcohol and filtering it with Whatman filter paper (0.45 μm). The filtrate was evaporated and drug residue dissolved in 100 ml of phosphate buffer (pH 6.8). The 5 ml solution was diluted with phosphate buffer (pH 6.8) up to 20 ml, filtered through a 0.45-μm Whatman filter paper, and analyzed at 229.5 nm using a UV spectrophotometer. The experiments were performed in triplicate, and average values were reported in Table 2.
e) Swelling Study
Buccal patches were weighed individually (designated as W1) and placed separately in 2% agar gel plates, incubated at 37°C ± 1°C, and examined for any physical changes. At regular 0.5 hr time interval until 2 hours, patches were removed from the gel plates and excess surface water was removed carefully using the filter paper. The swollen patches were then reweighed (W2) and the swelling index (SI) were calculated using the following formula:
SI = (W2 − W1) /W1 × 100
The experiments were performed in triplicate, and results were reported in Table 3.
Table 3
|
Formulation code |
% swelling index |
||||
|
0 hr |
0.5 hr |
1 hrs |
1.5 hrs |
2hrs |
|
|
F1 |
0 |
12.14±0.63 |
22.83±0.74 |
36.75±63 |
42.39±0.32 |
|
F2 |
0 |
14.94±0.86 |
27.58±0.38 |
42.83±0.78 |
48.04±0.28 |
|
F3 |
0 |
16.72±0.37 |
37.49±0.67 |
48.82±0.63 |
53.64±0.76 |
|
F4 |
0 |
10.84±0.53 |
22.58±0.78 |
34.73±0.87 |
40.55±0.63 |
|
F5 |
0 |
20.48±0.87 |
33.72± 0.82 |
47.79±0.74 |
50.84±1.25 |
f) Ex VivoMucoadhesive Strength
Fresh goat buccal mucosa was obtained from a local slaughterhouse and used within 2 hours of slaughter. The mucosal membrane was separated by removing the underlying fat and loose tissues. The membrane was washed with distilled water and then with phosphate buffer (pH 6.8) at 37 °C ±1.
The patch’s bioadhesive strength was measured on a modified physical balance. Fresh goat buccal mucosa was cut into pieces and washed with phosphate buffer (pH 6.8). A piece of buccal mucosa was tied in the open mouth of a glass vial, filled with phosphate buffer (pH 6.8). This glass vial was tightly fitted in the center of a glass beaker filled with phosphate buffer (pH 6.8, 37°C ± 1) so it just touched the mucosal surface. The patch was stuck to the lower side of a rubber stopper with cyanoacrylate adhesive. Two pans of the balance were balanced with a 5 g weight on the right-hand side pan. The 5 g weight was then removed from the left-hand side pan, which lowered the pan along with the patch over the mucosa. The balance was kept in this position for 5 minutes of contact time. The water was added slowly at 100 drops/min to the right-hand side pan until the patch detached from the mucosal surface. The weight, in grams, required to detach the patch from the mucosal surface provided the measure of mucoadhesive strength. The experiments were performed in triplicate, and results were reportedin Table 2.
g) Ex Vivo Residence Time
The ex vivo mucoadhesion time was studied (n = 3) after application of patches on freshly cut goat buccal mucosa. The fresh goat buccal mucosa was fixed on the inner side of a beaker, about 2.5 cm from the bottom, with cyanoacrylate glue. One side of each patch was wetted with 1 drop of phosphate buffer (pH 6.8) and pasted to the goat buccal mucosa by applying a light force with a fingertip for 30 seconds. The beaker was filled with 200 ml of phosphate buffer (pH 6.8) and was kept at 37°C ± 1. After 2 minutes, at 50-rpm stirring rate was applied to simulate the buccal cavity environment, and patch adhesion was monitored for 12 hours. The time required for the patch to detach from the goat buccal mucosa was recorded as the mucoadhesion time and shown in Table 2.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
h)In-Vitro Drug Release
The US Pharmacopeia XXIII rotating paddle method was used to study drug release from the buccal patches; 200 ml of phosphate buffer (pH 6.8) was used as the dissolution medium, at 37 °C ± 0.5, and a rotation speed of 50 rpm was used. One side of the buccal patch was attached to the glass disk with instant adhesive (cyanoacrylate adhesive). The disk was put in the bottom of the dissolution vessel. Samples (5 ml) were withdrawn at half-hour intervals and replaced with fresh medium. The samples were filtered through 0.45-μm Whatman filter paper and analyzed UV spectrophotometrically at 229.5nm. The experiments were performed in triplicate, and average values were reported in Table 4.
Table 4 In vitro drug release study of various formulations
|
Time |
% cumulative drug release |
||||
|
(Hrs) |
F1 |
F2 |
F3 |
F4 |
F5 |
|
0 |
0 |
0 |
0 |
0 |
0 |
|
1 |
27.3 ±1.21 |
19.25±1.52 |
15.57±1.37 |
12.48±1.75 |
23.53±1.36 |
|
2 |
38.27±1.11 |
30.1±1.35 |
22.83±1.34 |
20.57±1.76 |
28.84±1.81 |
|
4 |
54.58±1.19 |
46.35±1.24 |
42.75±1.88 |
38.35±1.31 |
48.45±1.39 |
|
6 |
77.27±1.80 |
69.89±1.61 |
58.28±1.79 |
53.42±1.51 |
63.35±1.84 |
|
8 |
92.34±1.31 |
98.47±1.82 |
92.73±1.69 |
88.18±1.64 |
97.15±1.31 |
i) Ex-VivoBuccal Permeation Study
The in vitro buccal permeation study of Glibenclamide through the goat buccal mucosa was performed using a Keshary-Chien type glass diffusion cell at 37°C ± 0.2. Goat buccal mucosa was obtained from a local slaughterhouse and used within 2 hours of slaughter. Freshly obtained goat buccal mucosa was mounted between the donor and receptor compartments. The patch was placed on the mucosa, and the compartments were clamped together. The donor compartment was filled with 2 ml of phosphate buffer (pH 6.8). The receptor compartment (10-mL capacity) was filled with isotonic phosphate buffer (pH 7.4), and the hydrodynamics in the receptor compartment were maintained by stirring with a magnetic bead at 50 rpm. At predetermined time intervals, a 1-ml sample was withdrawn and analyzed for drug content at 229.5 nm.[8]
Results and Discussion
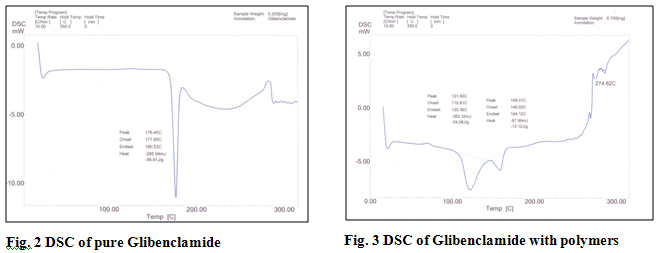
In this study Glibenclamide mucoadhesive buccal films were prepared using mucoadhesive polymers HPMC (5cps), Sodium CMC and PVP. DSC graph showed that the drug does not form any new compound. The shift in peaks can be attributed to the dilution of pure drug by polymers and alteration in physical geometry of crystalline drug during sample preparation (trituration). Propylene Glycol was used as the plasticizer as well as penetration enhancer. The drug delivery system was formulated as matrix. The films were characterized for their physical characteristics, bioadhesive performance, release characteristics, surface pH, thickness, folding endurance, drug content uniformity and percent swelling (Table 1). The film thickness was observed to be in the range of 0.195±0.032 mm to 0.265±0.028 mm and weight was found to be in the range of 162.26±0.56 mg to 187.75±0.29 mg. The principle cause of irritation to buccal mucosa can be the acidic or alkaline pH of the film. The surface pH of the films was measured and was found to be in the range of 6.64±0.04 to 6.67±0.03.
Folding endurance was measured manually. Folding endurance gives the idea of flexibility of films. Formulation F1, F2, F5 showed folding endurance above 300. F3 showed minimum folding endurance. However, all the films showed satisfactory flexibility.
Percentage drug content in formulations was uniform with an average of 98%. On this basis, it was concluded that drug was dispersed uniformly throughout the film.
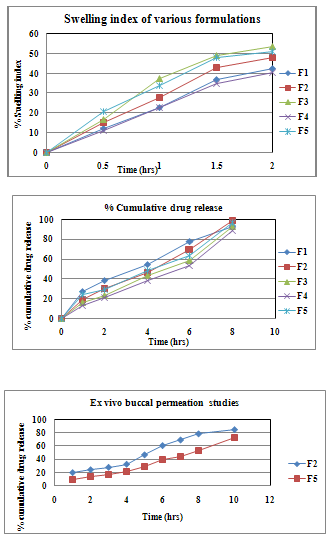
Mucoadhesive polymers on hydration expand and create macromolecular mesh of sufficient size. Polymer swelling permits a mechanical entanglement by exposing the bioadhesive sites for hydrogen bonding and/or electrostatic attraction between the polymer and mucous network.[9] The addition of the water-insoluble drug increased the water uptake of the film. This is possibly due to micronized drug particles which exist between the polymer chains allowing each chain to hydrate freely, resulting in weak hydrogen bonding areas around Glibenclamide molecules. These areas may increase the strength of the swollen layer followed by an obvious increase in the amount of penetrated water.[10] The swelling index of the films was found to be in the range of 32.4±0.32 to 48.64±0.76. The comparative percentage swelling for various formulation was in order F3>F5>F2>F4>F1. The films containing Sodium CMC showed higher percent swelling due to the presence of more hydroxyl groups.
The ex vivo mucoadhesive strength of the formulation was found to be dependent on the type of polymers employed. The order of bioadhesive strength for all formulation was F1>F5>F2>F4>F3. The adhesion strength varied between 10.2±0.6g to 6.8±0.7g.
Presence of micronized drug in polymer matrix can affect the residence time of formulation profoundly. Dissolution of water soluble polymers like SCMC causes porosity of matrix. The void volume so created gets occupied by the external solvent which diffuses into the film and accelerates the dissolution of gel.[11] The ex vivo residence time of the film was in order F2>F5>F3>F3>F1.
The in vitro release studies of various formulations were performed using pH 6.8 phosphate buffer as dissolution medium and measuring drug concentration spectrophotometrically at 229 nm. The differences in release pattern of Glibenclamide films were significant. In 8 hrs the percentage cumulative drug release was observed as 92.34, 98.47, 92.73, 88.18 and 97.15. Marked increase in surface area during swelling can promote drug release but increase in diffusion path length of the drug may also delay the release.[12]
The ex vivo permeation studies were carried out on the formulations which showed almost complete release of drug. Formulation F2 and F5 were chosen for permeation studies. Formulation F2 and F5 showed 84% and 73% cumulative drug permeation respectively, for a period of 10 hrs. Thus it was conclude that F2 was the best formulation which gave sustained release of drug with good mechanical properties.
Conclusion
The study clearly demonstrated that Glibenclamide can be successfully delivered through buccal route. The patches were non-irritating with favorable film properties and showed sufficient mucoadhesive potential until the drug is absorbed from the formulation. The further studies are required for better utilization of the drug, so that complete drug from formulation can go into systemic circulation. In vivo studies need to be designed and executed to substantiate further in-vitro in-vivo correlation.
REFERENCES
1. Goudanavar PS, Bagli RS, Patil SM, Chandashkhara S. Formulation and In-Vitro Evaluation of Buccal films Of Glibenclamide. Der Pharmacia Lettre. 2010, 2(1), 382-387.
2. Shojaei AH. Buccal Mucosa as A Route for Systemic Drug Delivery: A Review. J Pharm Pharmaceut Sci, 1998, 1, 15-30.
3. Rathbone J, Michael Ed. Marcell Dekker, Inc, New York, 1996.
4. Nafee NA, Ismail FA, Boraia NA, Mortuda LM. Mucoadhesive buccal patches of miconazole nitrate: in vitro/ in vivo performance and effect of ageing. Int. J. Pharm. 2003, 264, 1-14.
5. Tripathi KD. Jaypee Brothers medical publication (P) Ltd, New Delhi. 2001,278.
6. Gillman, AG, Hardman, JG, Limbird, LE. The Pharmacological basis of therapeutics, Tenth edition. 1964.
7. Semalty, M, Semalty A, Kumar G. Development of mucoadhesive buccal films of glipizide. Int. J.Pharm. Sci. & Nanotech. 2008, 1(2), 184-190.
8. Parmar HG, Jain JJ, Patel. Buccal Note: A technical note. Int. J. of Pharm. Sci. Rev. & Res. 2010, 4, 178-182
9. Gu JM, Robinson JR, Leung SHS. Binding of acrylic polymers to mucin/ epithelial surfaces: structure property relationship. Crit. Rev. Ther. Drug Carr. Sys. 1998, 5, 21-67.
10. Panomusk SP, Hatanaka T, Aiba T, Katayama K, Koizumi T. A study of hydrophilic matrix: effect of drug on the swelling properties. Chem. Pharm. Bull. 1996, 44, 1039-1042.
11. Samuelov Y, Donbrow M, Friedman M. Sustained release of drugs from ethyl cellulose-polyethylene glycol films and kinetics of drug release. J. Pharm. Sci. 1979, 68, 325-329.
12. Rodriguez CF, Bruneau N, Barra J, Alfonso D, Doelkar E. Hydrophillic derivatives as drug delivery carriers: influence of the substitution type on the properties of compressed matrix tablets. In Wise, D.L. (eds). Handbook of Pharmaceutical Controlled Release Technology Vol. 1. Marcell Dekker, New York. 2000, 1-30.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









