 About Authors:
About Authors:
Singh Deep Hussan*, Roychowdhury Santanu
Sri Sai college of pharmacy, badhani,
pathankot
*hussannijjar@gmail.com
Abstract:
Mucoadhesion is the prevalent interest in the design of drug delivery systems. Mucoadhesion can be defined as the state in which two materials, at least one of which is biological (mucosa) in nature, are held together for a prolonged time period by means of interfacial forces. This mucoadhesive system offers many advantages as it allows for reduction in daily administrations and daily drug dosage and is suitable for the treatment of irritation, pain, and discomfort associated with gingivitis, sore throats, laryngopharyngitis, cold, and periodontal surgery. Moreover, it adheres well to the gum and is very simple to apply, which means that patient compliance is improved. Mucoadhesive drug delivery system are designed to enable prolonged residence time of the dosage form at the site of application or absorption and facilitate an intimate contact of the dosage form with the underline absorption surface. The present review describes mucoadhesion, mucoadhesive polymers and their classification, stages, mechanism and theories of mucoadhesion and factors affecting them along with its evaluation. However, the research on mucoadhesives, is still in its early stage and further advances need to be made for the successful translation of the concept into practical application in controlled drug delivery.
Reference Id: PHARMATUTOR-ART-1444
Introduction:
The interest in different routes of drug administration occurs from their capability to enhance the bioavailability of drugs impaired by the narrow absorption window in the gastrointestinal tract. Drug delivery via the buccal route using bioadhesive dosage forms offers such a novel route of drug administration. The concept of mucosal adhesive or mucoadhesive was introduced in the early 1980’s, into the field of control drug delivery [1]. Problems like first-pass metabolism and drug degradation in the harsh GI environment can be circumvented by administering the drug via the buccal route [2,3]. Moreover, buccal drug delivery offers a safe and easy method of drug utilization, because drug absorption can be promptly terminated in cases of toxicity by removing the dosage form from the buccal cavity.
Here, permeation problem was overcome by using natural gum as penetration enhancers. There is a possibility for mucosal (local effect) and transmucosal (systemic effect) drug administration via buccal route. As well as in buccal mucosa maxillary artery, blood flow is faster (2.4 ml/min/cm2) than that in the sublingual, gingival and palatal regions, thus facilities passive diffusion of the drug molecules across the mucosa. The thickness of the buccal mucosa is 500-800 μm and rough texture, hence suitable for retentive delivery systems [4].
Classification of mucoadhesion:
Mucoadhesion or bioadhesion can be defined as the state in which two materials, at least one of which is biological in nature, are held together for a prolonged time period by means of interfacial forces. Bioadhesion can be classified into 3 types in biological systems: -
· Type 1- adhesion between two biological phases. Eg: - platelet aggregation and wound healing.
· Type 2- biological phase adherence to an artificial substrate. Eg: - tissue, cell adhesion to culture dishes and biofilm formation on prosthetic devices and inserts.
· Type 3- adhesion of an artificial substance to a biological substrate. Eg: - adhesion of synthetic hydrogels to soft tissues.
For drug delivery purpose, the term “bioadhesion” indicates attachment of a drug carrier system to a specific biological location. On the surface of a tissue, the biological surface can be epithelial tissue or the mucus coat. When the adhesive attached to a mucous coat, then the phenomenon is referred to as “Mucoadhesion”. Mucoadhesion is defined as the interaction between synthetic/natural polymer and a mucin surface [5]. Bioadhesion can be adopted after a bacterial attachment to tissue surfaces and mucoadhesion can be adopted after the adherence of mucus on epithelial tissue [6].
Characteristics of an ideal mucoadhesive polymer: -
1. The polymer and its degradation products should be non-toxic, non-irritant to the mucus membrane and should be non-absorbable from the GI tract.
2. With the mucin–epithelial cell surfaces, it should preferably form a strong non-covalent bond.
3. It should adhere quickly to most tissues and should possess some site specific characteristics.
4. It should offer no hindrance to its release by allowing easy incorporation of the drug.
5. During the shelf life of the dosage form or on storage, the polymers must not decompose.
6. The cost of the polymer should be low so that the prepared dosage form remains competitive [7].
The mucoadhesive drug delivery system may include the following[8]:
Gastrointestinal delivery system, Sublingual delivery system, Vaginal delivery system, Nasal delivery system, Ocular delivery system, Rectal delivery system, Buccal delivery system.
Need of mucoadhesive drug delivery system [9]:
1. Controlled release 2. Target & localized drug delivery 3. By pass first pass metabolism 4. Avoidance of drug degradation 5. Prolonged effect 6. High drug flux through the absorbing tissue 7. Reduction in fluctuation of steady state plasma level.
Advantages of mucoadhesive drug delivery systems[10]:
· At the site of drug action or absorption, a prolonged residence time can be achieved.
· Termination of therapy is easy.{except gastrointestinal}
· Can be administered to unconscious patients. {except gastrointestinal}
· A significant dose reduction can be achieved thereby reducing dose related side effects.
· Unlike rectal and transdermal routes, the presence of saliva ensures relatively large amount of water for drug dissolution.
· As an alternative for the administration of various hormones, narcotic, analgesic, steroids, enzymes, cardiovascular agents etc.
· As buccal mucosa is highly perfused with blood vessels so it offers a greater permeability than the skin.
· Safety margin of high potent drugs can be increased due to better control of plasma levels.
· Due to less frequent drug administration it improves patient compliance and convenience.
· Reduction of intensity of local or systemic side effects and also disease conditions are better controlled by reducing the fluctuation in steady state levels [11].
· By this route, drugs which are unstable in the acidic environment and which are destroyed by enzymatic or alkaline environment of intestine can be administered. E.g. Buccal, sublingual, vaginal [12].
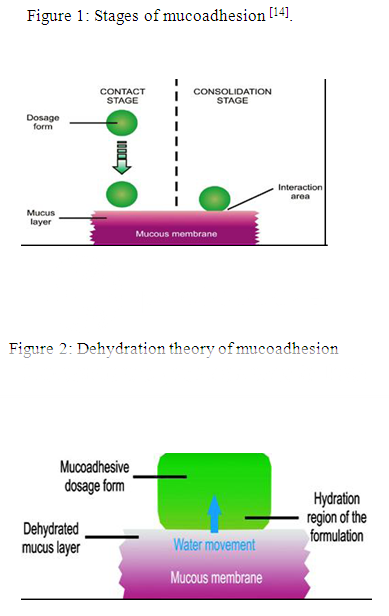
Stages of mucoadhesion:
The stages of mucoadhesion are generally divided in two steps:
I. Contact stage
II. Consolidation stage
In the contact stage (Fig.1), there is a contact between the mucoadhesive and the mucous membrane, initiating its deep contact with the mucus layer with spreading and swelling of the formulation. In some cases like ocular or vaginal formulations, the delivery system is mechanically attached over the membrane.
In other cases, promotion of the deposition takes place by the aerodynamics of the organ to which the system is administered, such as for the nasal route. On the other hand, in the GIT direct formulation attachment over the mucous membrane is not feasible [13].
In the consolidation step (Fig. 1), by the presence of moisture, the mucoadhesive materials are activated. Moisture plasticizes the systems, which allow the mucoadhesive molecules to break free and to link up by weak Vander Waals and hydrogen bonds.
Essentially, there are two theories which explain the consolidation step:
1) Diffusion theory2) Dehydration theory
According to diffusion theory, by means of interpenetration of their chains, the mucoadhesive molecules and the glycoproteins of the mucus mutually interact and the building of secondary bonds occurs. For this to take place, the mucoadhesive device must have features which favor both chemical and mechanical interactions.
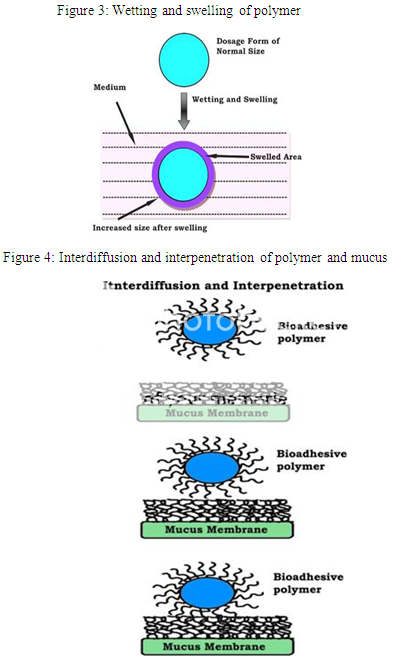
According to dehydration theory (Fig. 2), materials which are able to readily gelify in an aqueous environment when placed in contact with the mucus can cause its dehydration due to the difference of osmotic pressure. The difference in concentration gradient draws the water into the formulation until the osmotic balance is reached. This process leads to the mixture of formulation and mucus and can thus increase contact time with the mucous membrane. Therefore, it is the water motion that leads to the consolidation of the adhesive bond, and not the interpenetration of macromolecular chains. However, the dehydration theory is not applicable for solid formulation or highly hydrated form.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Mechanism of mucoadhesion:
The mechanisms responsible in the formation of bioadhesive bond formation as a three step process:-
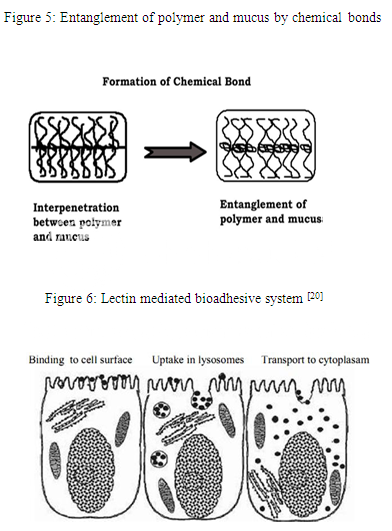
Step 1: Polymer wetting and swelling. Step 2: Interpenetration between the mucosal membrane and the polymer chains. Step 3: Chemical bonds formation between the entangled chains.
Step 1: In this step (Fig. 3), when the polymer spreads over the surface of biological substrate or mucosal membrane, the wetting and swelling step occurs in order to develop an intimate contact with the substrate [15]. Bioadhesives are able to adhere to or bond with biological tissues with the help of surface tension and forces that exist at the site of adsorption or contact. Swellings of polymers occur because the components within the polymers have an affinity for water [16].
Step 2: The surface of mucosal membranes are composed of high molecular weight polymers known as glycoproteins. In this step (Fig. 4) inter-diffusion and inter-penetration take place between the chains of mucoadhesive polymers and the mucous gel network creating a great area of contact [17]. The strength of this bond depends on the degree of penetration between the two polymer groups. In order to form strong adhesive bonds, one polymer group must be soluble in the other and both polymer types must be of similar chemical structure [18].
Step 3: In this step (Fig. 5), entanglement and formation of weak chemical bonds as well as secondary bonds between the polymer chains mucin molecule occur. The types of bonding formed between the chains include primary bonds such as covalent bonds and weaker secondary interactions such as Vander Waals interactions and hydrogen bonds. Both primary and secondary bonds are utilized in the manufacture of bioadhesive formulations in which strong adhesions between polymers are formed.
Table 1: Different theories explaining the mechanism of mucoadhesion [19].
|
Theory |
Mechanism of Mucoadhesion |
Comments |
|
Wetting Theory |
Ability of bioadhesive material to spread and develop intimate contact with the mucus membranes. |
Spreading coefficient of polymer must be positive and contact angle between the polymer and the cells must be near to zero. |
|
Electronic Theory |
Attractive electrostatic forces between glycoproteins mucin network and the bioadhesive material. |
Electron transfer occurs between the two forming a double layer of electrical charge at the interface. |
|
Adsorption Theory |
Surface forces resulting in the semi-permanent physical/ chemical bonding. |
Strong primary forces: covalent bonds Weak secondary forces: ionic bonds, hydrogen bonds and Vander Waals forces |
|
Diffusion Theory |
Physical entanglement of mucin strands at the flexible polymer chain and interpenetration of mucin strands into the porous structure of the polymer substrate. |
For maximum diffusion and the best bioadhesive strength: solubility parameters of the bioadhesive material and the mucus glycoproteins must be similar. |
|
Fracture Theory |
Analyses the maximum tensile strength developed during detachment of bioadhesive drug delivery systems from the mucosal surface. |
Does not require the physical entanglement of the bioadhesive polymer chains and mucin strands, hence appropriate to study the bioadhesion of hard polymers which lacks the flexible chains |
Classification of mucoadhesive polymers:
Traditional non-specific mucoadhesive polymers (first-generation)
First-generation mucoadhesive polymers may be divided into three main subsets, namely:
(1) Anionic polymers
(2) Cationic polymers
(3) Non-ionic polymers.
Of these, anionic and cationic polymers have been shown to exhibit the greatest mucoadhesive strength.
1. Anionic polymers
Due to their high mucoadhesive functionality and low toxicity, anionic polymers are the most widely employed mucoadhesive polymers within pharmaceutical formulation. Such polymers are characterized by the presence of carboxyl and sulphate functional groups that give rise to a net overall –ve charge at pH values which exceed the pKa of the polymer. Examples are polyacrylic acid (PAA) and its weakly cross-linked derivatives and sodium carboxymethylcellulose (NaCMC). PAA and NaCMC possess excellent mucoadhesive characteristics due to the formation of strong hydrogen bonding interactions with mucin.
Availability of PAA polymers in a wide range of molecular weights, form transparent, easily modified gel networks, is non-irritant, non-toxic and are considered safe for oral use by the FDA. Furthermore, gel formation occurs as a result of electrostatic repulsion between anionic groups.
2. Cationic polymers
Of the cationic polymer systems, chitosan is the most extensively investigated within the current scientific literature. Chitosan is a cationic polysaccharide, produced by the deacetylation of chitin, the most abundant polysaccharide in the world, next to cellulose. The intriguing properties of chitosan have been known for many years with many examples of its use in agriculture, industry and medicine. Agriculturally, chitosan has been utilized as an antipathogenic and industrially, investigated as a metal-recovering agent. Chitosan has been noted for its film-forming properties and has used extensively in cosmetics.
Furthermore, chitosan has been employed as a dye binder for textiles, a strengthening additive in paper and as a hypolipidic material in diets. Among presently explored mucoadhesive polymers, chitosan is gaining increasing importance due to its good biocompatibility, biodegradability and due to their favourable toxicological properties.
The major benefit of using chitosan within pharmaceutical applications has been the ease with which various chemical groups may be added, in particular to the C-2 position allowing for the formation of novel polymers with added functionality. Using such modifications, the properties of chitosan may be tailored to suit the requirements of specific pharmaceutical-technological challenges.
Novel second-generation mucoadhesive polymers
The major disadvantage in using traditional non-specific mucoadhesive systems (first generation) is that adhesion may occur at sites other than those intended. Unlike first-generation non-specific platforms, certain second-generation polymer platforms are less susceptible to mucus turnover rates, with some species binding directly to mucosal surfaces; more accurately termed ‘‘cytoadhesives”.
1. Lectins
Lectins (involving cells and proteins) are the naturally occurring proteins that play a fundamental role in biological recognition phenomena. For example: - during infection, some bacteria use lectins to attach themselves to the cells of the host organism. Enhancement of mucosal delivery may be obtained through the use of appropriate cytoadhesives that can bind to mucosal surfaces. The most widely investigated of such systems in this respect are lectins. Lectins (Fig. 6) belong to a group of structurally diverse proteins and glycoproteins that can bind reversibly to specific carbohydrate residues. After initial mucosal cell-binding, lectins can either remain on the cell surface or in the case of receptor-mediated adhesion, possibly become internalized via a process of endocytosis. Such systems could offer duality of function in that lectin based platform could not only allow targeted specific attachment but additionally offer a method of controlled drug delivery of macromolecular pharmaceuticals via active cell-mediated drug uptake.
Although lectins offer significant advantages in relation to site targeting, many are toxic or immunogenic, and the effects of repeated lectin exposure are largely unknown.
2. Thiolated polymers
Thiolated polymers (thiomers) come under the category of second generation mucoadhesive which are derived from hydrophilic polymers such as polyacrylates, chitosan or deacetylated gellan gum. Examples are Chitosan–iminothiolane (250-fold improved mucoadhesive properties), Polyacrylic acid–cysteine (100-fold improved mucoadhesive properties) etc. The formation of covalent bonds with cysteine-rich sub domains of the mucus gel layer occurs due to the presence of “thiol” groups, which leads to increased residence time and improved bioavailability. In this respect thiomers mimic the natural mechanism of secreted mucus glycoproteins that are also covalently anchored in the mucus layer by the formation of disulphide bonds. While first generation mucoadhesive platforms are facilitated via non-covalent secondary interactions, the covalent bonding mechanisms involved in second- generation systems lead to interactions that are less susceptible to changes in ionic strength and/or the pH. Moreover the presence of disulphide bonds may significantly alter the mechanism of drug release from the delivery system due to increased rigidity and cross-linking. In such platforms, a diffusion-controlled drug release mechanism is more typical, whereas in first generation polymers, irregular transport of API into bulk solution is more common [21].
Techniques to evaluate Mucoadhesion [22]:
1. In vitromethods:
a) Tensile strength measurement b) Shear strength measurement c) Modified physical balance d) Detachment force method e) Microbalance method f) Ex-vivo mucoadhesion g) Falling film method h) Swelling index i) Wash off method j) Colloidal gold staining k) Adhesion number l) Viscometric method m) Everted sac technique n) Drug permeation o) Fluorescent probe method p) Mucoadhesion time q) Surface pH study r) Scanning Electron microscopy (SEM) s) Novel Rheological Approach t) Texture analyzer.
2. In vivomethods:
a) Use of radioisotopes b) Use of gamma scintiography c) X-ray studies d) In vivo evaluation of mucoadhesive studies e) Isolated loop technique.
3. In vitroas well as in vivomethod:
Biacore (Surface Plasmon Resonance).
Factors Affecting Mucoadhesion:
1. Polymer-related factors:
a. Molecular Weight of the Polymer: The optimum molecular weight for maximum mucoadhesion depends upon the type of mucoadhesive polymer and tissue. Mucoadhesiveness of a polymer increases with the increase in the molecular weight (MW) of the polymer chain. In general, polymers having MW ≥ 100,000 have been found to have adequate mucoadhesive property for biomedical applications. For example, polyethylene glycol (PEG), having 20,000 molecular weight has little adhesive character, whereas PEG with 200,000 molecular weight has enhanced, and a PEG with 400,000 has superior adhesive properties [23,24]. Interpenetration is more critical for lower molecular weight polymers to be an excellent bioadhesive, whereas Entanglement is important for higher molecular weight polymers [25].
b. Concentration of Active Polymers: There is an optimum concentration of polymer corresponding to the best bioadhesion. In extremely concentrated systems, beyond the optimum level, the adhesive strength drops significantly because the coiled molecules become separated from the medium so that the chains available for interpenetration become limited [26]. It affects the availability of long polymer chains for penetration into the mucus layer. Thus it is important mainly for liquid and viscous drug delivery system [13].
c. Flexibility of Polymer Chains: It is critical for interpenetration and entanglement. Mobility of individual polymer chains decrease as water-soluble polymers become crosslinked and thus the valuable length of the chain that can penetrate into the mucus layer decreases, which reduces mucoadhesive strength [27].
d. Spatial Conformation: Despite a high molecular weight of 19,500,000 for dextrans, spatial conformation of a molecule is also important. They have adhesive strength similar to that of polyethylene glycol, which has a molecular weight of 200,000. The helical conformation of electrons may shield many adhesively active groups, primarily responsible for adhesion unlike PEG polymers that have a linear conformation. Also the effect of polymer concentration is dependable on the physical state (solid / liquid) of the bioadhesive drug delivery systems; more is the polymer concentration results the higher bioadhesive strength in Solid BDDS while an optimum concentration is required for best bioadhesion in liquids [28].
e. Swelling (Hydration): Swelling characteristics are related to the mucoadhesive itself and its environment. Swelling depends upon the concentration of polymer, the ionic strength, and the presence of water. Maximum bioadhesion in vitro occurs with optimum water content during the dynamic process of bioadhesion. Formation of wet slippery mucilage without adhesion occurs due to over-hydration [26].
2. Environment Related Factors:
a. Applied Strength: It is necessary to apply a defined strength, if you want to place a solid bioadhesive system. The adhesive strength increases with the applied strength or with the density of its application up to an optimum. The depth of interpenetration is affected by the pressure initially applied to the mucoadhesive tissue contact site. If high pressure is applied for a satisfactory longer period of time, polymers become mucoadhesive even though they do not have attractive interaction with mucin [29,30].
b. pH at Polymer Substrate Interface: pH can influence the formal charge on the surface of the mucus as well as certain ionizable mucoadhesive polymers [23]. Mucus will have a different charge density on the basis of pH due to the difference in dissociation of functional groups on the carbohydrate moiety and the amino acids of the polypeptide backbone [26,31].
c. Initial Contact Time: Contact time between the mucoadhesive and mucus layer determines the extent of swelling and interpenetration of the mucoadhesive polymer chains. More mucoadhesive strength increases as the initial contact time increases [27].
3. Physiological Variables:
a. Mucin Turnover: The natural mucin turnover from the mucus layer is important for at least two reasons. First, Residence time of the mucoadhesive on the mucus layer is expected to be limited due to mucin turnover. Due to mucin turnover, mucoadhesives are detached from the surface, no matter how high the mucoadhesive strength is. The turnover rate differs in the presence of mucoadhesive.
Second, Substantial amount of soluble mucin molecules results from mucin turnover. Before these mucin molecules have a chance to interact with mucus layer, they first interact with mucoadhesives [32,33,34]. Mucin turnover may depend on the other factors such as presence of blood. Lehr et al. (1991) calculated mucin turnover time of 47-270 minutes [32]. In the nasal cavity, the ciliated cells are known to transport the mucus to the throat at a rate of 5mm/min. In the tracheal region, the mucociliary clearance has been found to be in the range of 4-10mm/min [29,31,33,34].
b. Disease States: Physicochemical properties of mucus are known to change during diseased states, such as common cold, gastric ulcers, ulcerative colitis, cystic fibrosis, bacterial and fungal infections of the female reproductive tract and inflammatory conditions of the eye. Under these conditions, the exact structural changes taking place in mucus are not clearly understood. The mucoadhesive property must be evaluated when the mucoadhesives are used in the diseased state [35,36,37].
Conclusion:
Mucoadhesive drug delivery is a promising area for continued research with the aim of systemic delivery of orally inefficient drugs as well as a feasible and attractive alternative for non-invasive delivery of potent peptide and protein drug molecules. Mucoadhesive systems prolongs the residence time of the dosage form at the site of application or absorption and facilitates an intimate contact of the dosage form with the underline absorption surface and thus contributes to improved and/or better therapeutic performance of the drug. It is the developing area whose goal is the development of new devices and more “intelligent” polymers, as well as the creation of new methodologies that can better elucidate the mucoadhesion phenomenon. With the great influx of new molecules elucidate from drug research, mucoadhesive systems may play an increasing role in the development of new pharmaceuticals. Mucoadhesive drug delivery has been proposed as an alternative to parenteral administration of drugs. However, for safe and effective buccal permeation, absorption enhancer is a crucial component for a prospective future in the area of buccal drug delivery.
Reference:
1. Sharma Deepak., Singh Mankaran., Kumar Dinesh and Singh Gurmeet: Novel paradigms in mucoadhesive drug delivery system. IJPSR. 2012; 3(8): 2455-2471.
2. Nagai T, Konishi R: Buccal/gingival drug delivery systems. Journal of Control Release 1987; 6:353-60.
3. Harris D, Robinson JR: Drug delivery via the mucous membranes of the oral cavity. Journal of Pharmaceutical Sciences 1992; 81:1-10.
4. Gupta A, Garg S, Khar RK: Measerement of bioadhesive strength of mucoadhesive buccal tablets: design of an in-vitro assembly. Indian Drugs1992; 30:152-55.
5. Shaikh R, Singh TRR, Garland MJ, Donnelly RF. Mucoadhesive Drug Delivery Systems. Journal of Pharmacy and Bioallied Sciences 2011; 3(1): 89-100.
6. Longer MA, Robinson JR. Fundamental Aspects of Bioadhesion. Pharm. Int. 1986; 7: 114-117.
7. Asane GS, Nirmal SA, Rasal KB, Naik AA, Mahadik MS. Polymers for Mucoadhesive drug delivery system. Drug Development and Industrial Pharmacy 2011; 34: 1246-1266.
8. Jain NK. Controlled release and Novel Drug Delivery. 1st ed. CBS publishers and Distributors New Delhi; 1997. p. 353-370.
9. Abnawe SA. Mucoadhesive Drug Delivery System, Pharmainfo.net. 2009.
10. Alexander A, Ajazuddin, Tripathi DK, Verma T, Swarna, Maurya J, Patel S. Mechanism responsible for mucoadhesion of mucoadhesive drug delivery system: A review. Int. J. Appl. Bio. Pharm. Tech. 2011; 2(1): 434-441.
11. Ganga S. Mucosal Drug Delivery. Pharmainfo.net 2007; 5.
12. Sachan NK, Bhattacharya A. Basic and Therapeutic Potential of Oral Mucoadhesive Microparticulate Drug Delivery Systems. International Journal of Pharmaceutical and Clinical Research 2009; 1: 10-14.
13. Hagerstrom H. Polymer gels as pharmaceutical dosage forms: rheological performance and physicochemical interactions at the gel-mucus interface for formulations intended for mucosal drug delivery. Uppsala. 2003; 76.
14. Flavia CC, Marcos LB, Raul CE, Maria Palmira Daflon Gremião. Mucoadhesive drug delivery systems. Brazilian J. Pharm. Sci. 2010; 46(1): l-17.
15. Bhatt JH. Designing and evaluation of mucoadhesive microspheres of metronidazole for oral controlled drug delivery. Pharmainfo.net. 2009; 2: 1-13.
16. Sheila A. Bioadhesive Polymers, Power Point Presentation, Slide No.1-50.userweb.port.ac.uk/~roldom/Mucoadhesives/Bioadhesives.ppt.
17. Sharma HK, Sarangi B, Pradhan, Prasad S. Preparation and in-vitro evaluation of mucoadhesive microbeads containing Timolol Maleate using mucoadhesive substances of Dillenia indica L. Arch. Pharm. Sci. Res. 2009; 1: 181 -188.
18. Smart, JD. The basics and underlying mechanisms of mucoadhesion. Sci. Dir. 2005; 57(11): 1556-1568.
19. Sachan NK, Bhattacharya A. Basics and Therapeutic Potential of Oral Mucoadhesive Microparticulate Drug Delivery Systems. Int. J. Pharm. Clin. Res. 2009; 1(1): 10-14.
20. Khairnar GA, Sayyad FJ. Development of buccal drug delivery system based on mucoadhesive polymers. Int. J. Pharm. Tech. Res. 2010; 2(1): 719-735.
21. Gavin PA, Laverty TP, Jones DS. Mucoadhesive Polymeric Platforms for Controlled Drug Delivery. European Journal of Pharmaceutics and Biopharmaceutics 2009; 71: 505-518.
22. Alexander A, Ajazuddin, Giri T, Swarna, Shukla P. Various evaluation parameters used for the evaluation of different mucoadhesive dosage forms: A Review. Int. J. Drug Form. Res. 2011; 2(2): 1-26.
23. Kumar RS, Bala P. Bioadhesive Polymeric Platforms for Transmucosal Drug Delivery Systems. Tropical J. Pharm. Res. 2010; 9: 91-104
24. Hoffman A. Pharmacodynamic aspects of sustained release preparations. Adv. Drug Del. Rev. 1998; 33:185-99.
25. Sachan NK, Bhattacharya A. Basics and Therapeutic Potential of Oral Mucoadhesive Microparticulate Drug Delivery Systems. Int. J. Pharm. Clin. Res. 2009; 1(1): 10-14.
26. Rajput GC, Majmudar FD, Patel JK, Patel KN, Thakor RS, Patel BP, Rajgor NB. Stomach specific mucoadhesive tablets as controlled drug delivery system - A Review work. Int. J. Pharm. Bio. Res. 2010; 1(1): 30-41.
27. Patel AR, Patel DA, Chaudhry SV. Mucoadhesive buccal drug delivery system. Int. J. Pharm. life Sci. 2011; 2(6): 848-856.
28. Vasir JK, Tambwekar K, Garg S. Bioadhesive microspheres as controlled drug delivery systems. Int. J. Pharm. 2003; 255: 13-32.
29. Chowdary KPR, Shrinivasrao Y. Mucoadhesive drug delivery systems: A review of current status. Indian Drugs. 2000; 37 (9): 400- 406.
30. Ponchel G, Irache JM. Specific and non-specific bioadhesive particulate systems for oral delivery to gastrointestinal tract. Adv. Drug Del. Rev. 1998; 34: 191-219.
31. Kamath KR, Park K. Mucosal adhesive preparations, In Encyclopedia of pharm. Tech. 1995; 12: 132-162.
32. Lehr CM, Poelma FGJ, Junginger HE, Tukker JJ. An estimate of turnover time of intestinal mucus gel layer in the rat in situ loop. Int. J Pharm. 1991; 70: 235-240.
33. Lehr CM. From sticky stuff to sweet receptors-achievements, limits and novel approaches to bioadhesion. Eur. J. Drug Meta. Pharm. 1996; 21: 139-148.
34. Lehr CM, Bouwstra JA, Schacht EH, Junginger HE. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int. J. Pharm. 1992; 78: 43-48.
35. Devarajan PV, Adani MH. Oral Transmucosal Drug delivery in Controlled and Novel Drug Delivery by Jain NK. 1st ed. New Delhi: CBS publishers.
36. Chien YW. Mucosal Drug Delivery: Potential Routes for Noninvasive Systemic Administration. Novel Drug Delivery Systems. 2nd ed. Marcel Dekker; 1999; 50:197-228.
37. Khar K, Ahuja A, Javed A: Mucoadhesive Drug Delivery, Controlled and Novel Drug Delivery by Jain NK. 1st ed. New Delhi: CBS publishers; 1997.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









