 About Authors:
About Authors:
Sailesh Narayan*, Ankit Diwan
Sagar institute of Pharmacy and Technology,
Gandhi Nagar, Bhopal
*saileshcology@yahoo.co.in
INTRODUCTION
A tablet is a pharmaceutical dosage form. It comprises a mixture of active substances and excipients, usually in powder form, pressed or compacted from a powder into a solid dose. The excipients can include diluents, binders or granulating agents, glidants and lubricants to ensure efficient tabletting; disintegrants to promote tablet break-up in the digestive tract; sweeteners or flavours to enhance taste; and pigments to make the tablets visually attractive. A polymer coating is often applied to make the tablet smoother and easier to swallow, to control the release rate of the active ingredient, to make it more resistant to the environment or to enhance the tablet's appearance. These are compressed tablets. formulated to deliver an accurate dosage to a specific site; it is usually taken orally, but can be administered sublingually, buccally, rectally or intravaginally. Medicinal tablets were originally made in the shape of a disk of whatever color their components determined, but are now made in many shapes and colors to help distinguish different medicines. Tablets are often stamped with symbols, letters, and numbers, which enable them to be identified.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1596
1) Introduction Calpol tablets
Calpol is a liquid or meltaway pain reliever intended for children that is widely available in the United Kingdom without a prescription. In its generic form, Calpol is called paracetamol, which is sold in the United States under the name acetaminophen. Calpol is commonly given to children to lower fevers, treat aches and pains that accompany influenza and alleviate pain caused by teething, sore throats and headaches. Despite its effectiveness, Calpol is not for everyone, as it presents several risks for side effects and complications in some patients.
Mechanism of action
Paracetamol acts by inhibiting cyclo-oxygenase(COX) enzyme brain, thus inhibiting the formation of prostaglandins. Paracetamol is poor inhibitor of PG synthesis in peripheral tissues. The central Analgesic action of paracetamol is that, it retains pain threshold, but has weak perripheral anti-inflammatory component. Paracetamol is a good and promptly acting antipyretic. Analgesic action of aspirin and paracetamol is additive.
Indication: Pain and Fever
Dosage :
Adult: 500 mg 4-6 hourly as and when needed.
Children: 40-60mg/kg/day 4-6 hourly 0r 15mg/kg/day/dose 4-6 hourly
Side Effects: Paracetamol is relatively safe. It may however lead to Nausea and epigastric distress. Acute hepatic toxicity may occur usually in children and alcoholics.
Pregnancy Usage: May be used in pegnancy.
Calpol Side Effects
Some side effects of Calpol are not normally serious in nature, pose little discomfort and occur infrequently. Some children develop minor skin rashes after taking the drug. The meltaway or "Fast Melt" version of Calpol occasionally causes increased defecation, functioning similarly to a laxative. Unlike other over-the-counter pain relievers, Calpol typically does not cause stomach upset or gastrointestinal bleeding.
2) IntroductionMetacin Tablet
Synonym : Metacinum iodide.
White or white, with a slightly yellowish sheen crystalline powder. It is soluble in water (1:200). Aqueous solutions (pH 4, 0, 5, 0) + 100 to sterilize 'C for 30 min.
[adsense:468x15:2204050025]
Metacin is very active m-cholinolytic funds. As a quarternary ammonium compounds,it does penetrates through the blood-brain barrier and is therefore selective peripheral holinolitikom force.
At peripheral holinoretseptory metacin is stronger than atropine and spazmolitin. The effect on bronchial muscles more active than atropine. Most suppresses the secretion of salivary glands and bronchitis. Oesophagus relaxes smooth muscle, stomach, intestines. However, it has a much smaller midriaticheskoe effect than atropine, which apparently stems from the fact that it is difficult to penetrate through gematooftalmichesky barrier.
It eliminates motor dysfunction of stomach and duodenal ulcers, normalizes motor; Superior has an effect of atropine. Metacin can be used in treatment of patients ulcers in combination with other drugs. Metacin a highly effective tool for edema and renal colic liver.
Storage : A. In a list ukuporennyh banks of the dark glass; Tablets and capsules, in the dark place.
Active ingredients- Paracetamol
Our body generates Prostaglandins when it succumbs to injury or certain ailments. Te chemical produced sensitizes nerve ending, so when the body is injured it stimulates pain. The administration of paracetamol reduces the secretion of the chemical Prostaglandins, thus reduces sensitizing of the nerve endings. So even though paracetamol doesn’t heal the injury but it reduces the pain sensation by inhibiting the action of Prostaglandins. This is the analgesic function of the OTC medication.It reduces fever.
Metacin uses
Metacin Tablets are broadly used OTC drugs with antipyretic and analgesic properties. It is generally used for relief from moderate to severe headaches, fever and other minor muscular pain. It also is used for treating symptoms associated to common cold and flu.
3) Introduction of Pyrigesic tablet
Introduction
Pyrigesic tablets for oral administration provide paracetamol, a widely used, non-narcotic, non-salicylate, analgesic and antipyretic of high efficacy. When administered in usual therapeutic dosages, has virtually little side effects
Mechanism of Actions
Analgesic action of Pyrigesic (paracetamol) is believed to be due to inhibition of brain prostaglandin synthetase activity and through a peripheral action by blocking pain impulse generation. The peripheral action is also believed to be due to either inhibition of prostaglandin synthesis or inhibition of actions or synthesis of some other substances responsible for sensitizing pain receptors to chemical or mechanical stimulation. Antipyretic action of Pyrigesic (paracetamol) is believed to be due to its action on the hypothalamic heat-regulating centre producing peripheral vasodilatation and thus resulting in increased blood flow through the skin, sweating and heat loss. This action probably involves inhibition of prostaglandin synthetase activity in the hypothalamus.
Indications
Pyrigesic (tablets & syrup) is indicated in fever associated with bacterial or viral infections, mild to moderate pain including muscular pain, joint pains, sprains and injuries, wryneck, toothache, headache, earache, neuralgia, post-immunisation reaction, post tonsillectomy pain, menstrual discomfort, discomforts of cold and flu, pharyngitis, laryngitis and tracheobronchitis.
Adverse Reactions
With normal therapeutic doses of Pyrigesic hypersensitivity reactions like erythematous or urticarial skin rashes, mucosal lesions, drug, fever, etc. may occur rarely.
Precautions
Care should be taken for patients with impaired liver or kidney functions
Drug Interactions
Co-administration of alcohol and Pyrigesic (paracetamol), at least in high therapeutic doses, may be harmful. With chloramphenicol, half-life of the antibiotic is prolonged which may cause increased toxic effects of the antibiotic, probably due to competitive metabolism that takes place between the two compounds.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
4) Introduction of Crocin tablets
Crocin is a common over-the-counter (OTC) drug, a mild to moderate pain reliever and a fever reducer. Crocin relieves headaches, menstrual pain and tooth ache, as well as backache and muscular aches and pain. It is also used to treat arthritis and the symptoms of a cold. Talk with your pharmacist or your doctor about the appropriate dosage and frequency at which you should take this OTC drug because of its potential for liver damage. Corcin is available in tablet or suspension form.
Side Effects of Crocin
Crocin is an analgesic and an anti-pyretic drug that goes under several brand names depending on the country that it is being marketed. In Brazil, Canada and South Korea and in the United States, Crocin is called Tylenol and Panadol. In the United Kingdom and in India it is called Crocin. Corcin contains paracetamol, which is also called acetaminophen in North America. Crocin is produced mainly in India and leads the market in its production.
Function
Crocin is a common over-the-counter (OTC) drug, a mild to moderate pain reliever and a fever reducer. Crocin relieves headaches, menstrual pain and tooth ache, as well as backache and muscular aches and pain. It is also used to treat arthritis and the symptoms of a cold.
Effects of Crocin on the Liver
According to Interscience.wiley.com in an article written by William M. Lee, M. D., F.A.C.P, entitled "Acetaminophen and the U.S. acute liver failure study group: Lowering the risk of hepatic failure," Lee states, "Acetaminophen overdose is the leading cause of calls to Poison Control Centers and accounts for more than 56,000 emergency room visits." Dr. Lee also questions the over-the-counter availability of this medication.
Signs and Symptoms of Overdose
If you think you took too much Crocin, seek immediate emergency medical care and attention. The initial symptoms of a Crocin overdose may include nausea, vomiting and loss of appetite, or sweating with stomach pain, confusion and weakness. Other symptoms that may develop are pain in the stomach and signs of jaundice, such as yellowish discoloration of the skin and white of the eyes or clay colored stool and dark colored urine. Seek immediate medical care if you experience any of these symptoms.
Allergic Reaction
An allergic reaction is a serious medical emergency. The symptoms may include a ash or hives with itching along with swelling of the face, mouth and tongue or throat. In addition there may be wheezing, shortness of breath and difficulty breathing. Seek immediate emergency treatment if you experience any of these symptoms.
Drug Profile
5.1.1)Structure
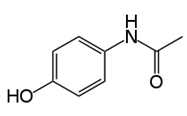
5.1.2)Table
|
IUPAC NAME |
N-(4-hydroxyphenyl)ethanamide |
|
PHARMACOKINETICS DATA |
|
|
BIOAVAILABILITY |
~100% |
|
METABOLISM |
90 to 95% Hepatic |
|
HALF-LIFE |
1–4 hour |
|
EXCRETION |
Ranal |
|
CLINICAL DATA |
|
|
ROUTE |
Oral, rectal, intravenous |
|
CHEMICAL DATA |
|
|
FORMULA |
C8H9NO2 |
|
MOL.MASS |
151.17 g/mol |
|
PHYSICAL DATA |
|
|
DENSITY |
1.263 g/cm³ |
|
MELTING POINT |
169 °C (336 °F) |
|
SOLUBILITY |
12.78 mg/mL (20 °C) |
5.1.3)Pharmacokinetics
It is well absorbed after oral administration. About 1/3rd of it gets protein bound and uniformly distributed in the body. The drug gets conjugated with glucuronic acid and gets rapidly excreted in urine. Plasma half life is about 2-3 hrs.Effect after a oral dose last for 3-5 hours.
5.1.4)Indications
Paracetamol has analgesic and antipyretic action with weak anti-inflammatory action. It is commonly used in fever and in mild to moderate pain.
5.1.5)Routes of Administration and Dosage
Oral dose (capsules, powders, suspension, or tablets) and rectal suppositories: For pain or pyrexia in Adults : 325 or 500 milligrams (mg) every three or four hours, 650 mg every four to six hours, or 1000 mg every six hours as needed. For short-term treatment (up to ten days), the total dose should not be more than 4000 mg a day. For long-term treatment, the total dose should not be more than 2600 mg a day. Children : Acetaminophen dose is based on the child's age - in Infants up to 3 months of age: 40 mg every four hours as needed, Infants 4 to 12 months of age: 80 mg every four hours as needed, Children 1 to 2 years of age: 120 mg every four hours as needed, Children 2 to 4 years of age: 160 mg every four hours as needed, Children 4 to 6 years of age: 240 mg every four hours as needed, Children 6 to 9 years of age: 320 mg every four hours as needed, Children 9 to 11 years of age: 320 to 400 mg every four hours as needed, Children 11 to 12 years of age: 320 to 480 mg every four hours as needed.
Contra Indications
Paracetamol is contraindicated in hypersensitivity, analgesic nephropathy, renal and hepatic impairment.
Precautions
Some brands of Paracetamol contain aspartame, which can make Phenylketonuria worse. The chance of serious adverse effects may be increased in alcohol abuse, renal disease and in Hepatitis.
Interactions
It can increase the effect of anticoagulants, it can increase hepatic damage in alcoholics. Pethidine and propantheline can reduce the absorption of paracetamol.
5.1.6)Specific forms and uses
Analgesics are frequently used in combination, such as the paracetamol and codeine preparations found in many non-prescription pain relievers. They can also be found in combination with vasoconstrictor drugs such as pseudoephedrine for sinus-related preparations, or with antihistamine drugs for allergy sufferers.While the use of paracetamol, as well as aspirin, ibuprofen, naproxen, and other NSAIDS concurrently with weak to mid-range opiates has been said to show beneficial synergistic effects by combatting pain at multiple sites of action.
5.1.7)First Synthesis of paracetamol
Paracetamol was first synthesised by Morse in 1878 by the reduction of p-nitrophenol with tin in glacial acetic acid. The p-aminophenol produced by the reducing action of the tin was acetylated in situ by the acetic acid.
Vignolo simplified the synthesis by using p-aminophenol as the starting material which he acetylated with acetic acid.
Friedlander modified this process slightly by acetylating the p-aminophenol (from p-nitrophenol) with acetic anhydride in place of acetic acid.
Many preparative methods have since been described, mostly employing the acetylation of p-aminophenol with acetic anhydride, but other routes have also been discovered and used commercially.
6.2.8)Medical uses
Fever
Paracetamol is approved for reducing fever in people of all ages. The World Health Organization (WHO) recommends that paracetamol only be used to treat fever in children if their temperature is greater than 38.5 °C (101.3 °F).The efficacy of paracetamol by itself in children with fevers has been questionedand a meta-analysis showed it to be less effective than ibuprofen. Paracetamol has a well-established role in pediatric medicine as an effective analgesic and antipyretic.
Pain
Paracetamol is used for the relief of pains associated with many parts of the body. It has analgesic properties comparable to those of aspirin, while its anti-inflammatory effects are weaker. It is better tolerated than aspirin in patients in whom excessive gastric acid secretion or prolongation of bleeding time may be aconcern.
Paracetamol can relieve pain in mild arthritis but has no effect on the underlying inflammation, redness, and swelling of the joint. It is as effective as the nonsteroidal anti-inflammatory drug ibuprofen in relieving the pain of osteoarthritis of the knee.
Paracetamol has relatively little anti-inflammatory activity, unlike other common analgesics such as the NSAIDs aspirin and ibuprofen.
Regarding comparative efficacy, studies show conflicting results when compared to NSAIDs. A randomized controlled trial of chronic pain from osteoarthritis in adults found similar benefit from paracetamol and ibuprofen.
the efficacy of paracetamol when used in a combination form with weak opioids (such as codeine) has been questioned by recent data studies; the small amount of data available have made reaching a conclusion hard.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Metabolism
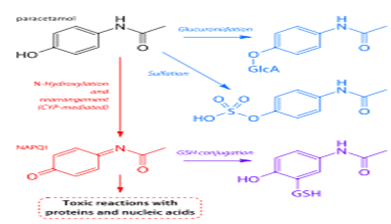
• Main pathways of paracetamol metabolism Pathways shown in blue and purple lead to non-toxic metabolites; the pathway in red leads to toxic NAPQI.
• Paracetamol is metabolised primarily in the liver, into non-toxic products. Three metabolic pathways are notable:
• Glucuronidation is believed to account for 40% to two-thirds of the metabolism of paracetamol.
• Sulfation (sulfate conjugation) may account for 20–40%.
• N-hydroxylation and rearrangement, then GSH conjugation, accounts for less than 15%. The hepatic cytochrome P450 enzyme system metabolizes paracetamol, forming a minor yet significant alkylating metabolite known as NAPQI (N-acetyl-p-benzo-quinone imine). NAPQI is then irreversibly conjugated with the sulfhydryl groups of glutathione.
· All three pathways yield final products that are inactive, non-toxic, and eventually xcreted by the kidneys. In the third pathway, however,
· the intermediate product NAPQI is toxic. NAPQI is primarily responsible for the toxic effects of paracetamol; this constitutes an example of toxication.
metabolisers, and when paracetamol is taken at very large doses. At usual doses, NAPQI is quickly detoxified by conjugation. Following overdose, and possibly also in extensive and ultrarapid metabolizers, this detoxification pathway becomes saturated, and, as a consequence, NAPQI accumulates.
Experiment work
Manufacture of the tableting
· In the tablet pressing process, the main guideline is to ensure that the appropriate amount of active ingredient is in each tablet. Hence, all the ingredients should be well-mixed.
· If a sufficiently homogenous mix of the components cannot be obtained with simple blending processes, the ingredients must be granulated prior to compression to assure an even distribution of the active compound in the final tablet.
· Two basic techniques are used to granulate powders for compression into a tablet: wet granulation and dry granulation.
· Powders that can be mixed well do not require granulation and can be compressed into tablets through direct compression.
Wet granulation
Wet granulation is a process of using a liquid binder to lightly agglomerate the powder mixture. The amount of liquid has to be properly controlled, as over-wetting will cause the granules to be too hard and under-wetting will cause them to be too soft and friable.Aqueous solutions have the advantage of being safer to deal with than solvent-based systems but may not be suitable for drugs which are degraded by hydrolysis.
Procedure
Step 1: The active ingredient and excipients are weighed and mixed.
Step 2: The wet granulate is prepared by adding the liquid binder–adhesive to the powder blend and mixing thoroughly. Examples of binders/adhesives include aqueous preparations of cornstarch, natural gums such as acacia, cellulose derivatives such as methyl cellulose, gelatin, and povidone.
Step 3: Screening the damp mass through a mesh to form pellets or granules.
Step 4: Drying the granulation. A conventional tray-dryer or fluid-bed dryer are most commonly used.
Step 5: After the granules are dried, they are passed through a screen of smaller size than the one used for the wet mass to create granules of uniform size.
Dry granulation
Dry granulation processes create granules by light compaction of the powder blend under low pressures. The compacts so-formed are broken up gently to produce granules (agglomerates).
This process is often used when the product to be granulated is sensitive to moisture and heat. Dry granulation can be conducted on a tablet press using slugging tooling or on a roll press called a roller compactor.
Dry granulation equipment offers a wide range of pressures to attain proper densification and granule formation. Dry granulation is simpler than wet granulation, therefore the cost is reduced.
After granulation, a final lubrication step is used to ensure that the tableting blend does not stick to the equipment during the tableting process. This usually involves low shear blending of the granules with a powdered lubricant, such as magnesium stearateor stearic acid.
Manufacture of the tablets
First, the powder is filled into the die from above. The mass of powder is determined by the position of the lower punch in the die, the cross-sectional area of the die, and the powder density. At this stage, adjustments to the tablet weight are normally made by repositioning the lower punch. After die filling, the upper punch is lowered into the die and the powder is uniaxially compressed to a porosity of between 5 and 20%.The compression can take place in one or two stages and for commercial production occurs very fast (500–50 msec per tablet).Finally, the upper punch is pulled up and out of the die (decompression), and the tablet is ejected from the die by lifting the lower punch until its upper surface is flush with the top face of the die. This process is simply repeated many times to manufacture multiple tablets.
Evalution Parameter of tablet
All the prepared tablets were evaluated for following parameters.
· Hardness test
· Friability test
· Drug content
· Weight variation
· Dissolution study
Hardness
The tablet was placed between two anvils of hardness tester (Monsanto) and increasing amount of force (kg) was applied. The reading at the marked scale was recorded for the pressure which is required to break the tablet.
Friability
Ten tablets were weight and placed in the Roche friability apparatus and apparatus was rotated at 25 rpm for 5 minute. After revolutions the tablets were dedusted and weighed again. The observed value should not be more than 1%.The percentage friability was measured using the formula.
%F= {1-(Wt/W)}*100
Where, %F=Friability in percentage, W=Initial weight of tablet, Wt=weight of tablets after revolution.
Drug content
Twenty tablets for each batch was taken and triturated. Powder equivalent to 100mg of drug was weight and was transferred to breaker and 0.1N HCl was added and it was then shaken for 5 minutes and finally 0.1N HCl was added to make the volume upto 100ml and solution was then sonicated for 15 minutes and filtered through Whatman fiter paper.
Finally a solution was diluted suitably and the absorbance of resultant solution was measured spectrophotometrically at 290nm using UV/Visible spectrophotometer jasco V-530 against 0.1N HCl blank.15
Weight variation
Twenty tablets were randomly selected from each batch and individually weighed. The average weight and standard deviation of 20 tablets was calculated. The batch passes the test for weight variation test if not more than two of the individual tablet weight deviates by more than the percentage shown.
Percentage deviation allowed under weight variation
|
weight |
Percentage deviation |
|
<130mg |
10 |
|
130-324mg |
7.5 |
|
>324mg |
5 |
In-vitro dissolution study
· The release rate of Propranolol HCl floating tablets was determine using reported method in Indian Pharmacopoeia dissolution testing apparatus II (paddle method).the dissolution test was carried out under sink condition.
· At appropriate time interval (0.25,0.5,1,2,3,4,5,6,8 and 24 hrs)5ml samples were withdrawn from dissolution liquid and was replaced with fresh media to maintain the volume constant.
· After dilution and filtration, the sample solution was analysed by UV spectrophotometer at 290 nm. The amount of drug present in the sample was calculated by Cumulative percentage of drug release method reported in IP.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULT
Weight variation of tablets
|
Weight Variation of paracetamol Tablet |
|||
|
Sno. |
Tablets name |
Total weight |
Average weight |
|
1. |
Calpol |
12.340 mg |
0.617 mg |
|
2. |
Metacin |
11.680 mg |
0.584 mg |
|
3. |
Pyrigesic |
11.120 mg |
0.556 mg |
|
4. |
Crocin |
11.750 mg |
0.587 mg |
Hardness of tablets
|
Hardness of Tablets |
||
|
Sno. |
Tablets name |
Hardness |
|
1. |
Calpol |
5.4 kg/cm |
|
2. |
Metacin |
10.4 kg/cm |
|
3. |
Pyrigesic |
5.6 kg/cm |
|
4. |
Crocin |
5.6 kg/cm |
Friability of tablets
|
Friability of Tablets |
||||
|
Sno. |
Tablets name |
Total weight |
Weight after Friability |
Percentage |
|
1. |
Calpol |
6.340 mg |
6.280 mg |
0.94% |
|
2. |
Metacin |
6.080 mg |
6.000 mg |
1.31% |
|
3. |
Pyrigesic |
5.720 mg |
5.630mg |
1.57% |
|
4. |
Crocin |
6.070 mg |
6.040 mg |
0.49% |
Disintegration of tablets
|
Disintegration of Tablets |
||
|
Sno. |
Tablets name |
Disintegration Time |
|
1. |
Calpol |
1:2 Minute |
|
2. |
Metacin |
38:45 Minute |
|
3. |
Pyrigesic |
48:37 Minute |
|
4. |
Crocin |
34:60 Minute |
SUMMARY
* Comparitive studies of Paracetamol in varius product have done.
* Paracetamol Tablet are form to be in evalution various parameter
* The Average weight of Paracetamol table are found to be:
* Calpol have Average weight ( 0.617Mg)
* Metacin have Average weight ( 0.584Mg)
* Pyrigesic have Average weight ( 0.556Mg)
* Crocin have Average weight( 0.587)
* The hardness of Paracetamol tablet are found to be :
* Calpol have hardness (5.4 Kg/cm )
* Metacin have hardness (10.4 Kg/cm)
* Pyrigesic have hardness (5.6 Kg/cm)
* Crocin have hardness (5.6 Kg/cm)
* The determine various category uses of Paracetamol used in various category like antipyretic.
CONCLUSION
* Comparitive studies of Paracetamol in varius product evalution parameter were found to be accurate.
* All the evaluation parameter were found within acceptance criteria.
* The parameter were and it’s derivatives
These approach has been succusfully used in the lab to evaluate parameter of tablet.
REFERENCE
1. R. D. Altman, M. C. Hochberg, R. W. Moskowitz, and T. J.Schnitzer, “Recommendations for themedical management ofosteoarthritis of the hip and knee: 2000 update,” Arthritis andRheumatism, vol. 43, no. 9, pp. 1905–1915, 2000.
2. K. M. Jordan, N. K. Arden, M. Doherty et al., “EULARrecommendations 2003: an evidence based approach to themanagement of knee osteoarthritis: report of a Task Forceof the Standing Committee for International Clinical StudiesIncluding Therapeutic Trials (ESCISIT),” Annals of theRheumatic Diseases, vol. 62, no. 12, pp. 1145–1155, 2003.
3. W. Zhang, M. Doherty, N. Arden et al., “EULAR evidencebased recommendations for the management of hiposteoarthritis: report of a task force of the EULAR StandingCommittee for International Clinical Studies Including Therapeutics(ESCISIT),” Annals of the Rheumatic Diseases, vol. 64,no. 5, pp. 669–681, 2005.
4. W. Zhang, M. Doherty, B. F. Leeb et al., “EULAR evidenceed recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT),” Annals of the Rheumatic Diseases, vol. 66,no. 3, pp. 377–388, 2007.
5. W. Zhang, J. Robertson, A. C. Jones, P. A. Dieppe, and M. Doherty, “The placebo effect and its determinants in osteoarthritis: meta-analysis of randomised controlled trials,”Annals of the Rheumatic Diseases, vol. 67, no. 12, pp. 1716–1723, 2008.
6. T. E. Towheed, L. Maxwell, M. G. Judd, M. Catton, M. C. [1] R. D. Altman, M. C. Hochberg, R. W. Moskowitz, and T. J. Schnitzer, “Recommendations for themedical management of osteoarthritis of the hip and knee: 2000 update,” Arthritis and Rheumatism, vol. 43, no. 9, pp. 1905–1915, 2000.
7. K. M. Jordan, N. K. Arden, M. Doherty et al., “EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT),” Annals of the Rheumatic Diseases, vol. 62, no. 12, pp. 1145–1155, 2003.
8. W. Zhang, M. Doherty, N. Arden et al., “EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT),” Annals of the Rheumatic Diseases, vol. 64,no. 5, pp. 669–681, 2005.
9. W. Zhang, M. Doherty, B. F. Leeb et al., “EULAR evidence based recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT),” Annals of the Rheumatic Diseases, vol. 66, no. 3, pp. 377–388, 2007.
10. W. Zhang, J. Robertson, A. C. Jones, P. A. Dieppe, and M. Doherty, “The placebo effect and its determinants in osteoarthritis: meta-analysis of randomised controlled trials,” Annals of the Rheumatic Diseases, vol. 67, no. 12, pp. 1716–1723, 2008.
11. T. E. Towheed, L. Maxwell, M. G. Judd, M. Catton, M. C. [1] R. D. Altman, M. C. Hochberg, R. W. Moskowitz, and T. J. Schnitzer, “Recommendations for themedical management of osteoarthritis of the hip and knee: 2000 update,” Arthritis and Rheumatism, vol. 43, no. 9, pp. 1905–1915, 2000.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









