{ DOWNLOAD AS PDF }
 ABOUT AUTHOR
ABOUT AUTHOR
Sagar Vishwanath Sonawane*
Dept. of Pharmaceutical Sciences and Technology,
Institute of Chemical Technology,
Matunga, Mumbai, Maharashtra, India.
*sssagarvs@gmail.com
ABSTRACT
Brain is the highly protected organ of the body by the sanctuary known as blood brain barrier (BBB). BBB comprises of tight junctions formed by the closed fenestration network of astrocytes, pericytes microglial cells and endothelial cells. Thus it is very difficult for drug molecules to cross BBB. Thus various approaches are discussed to overcome this barrier. Disruption of BBB was conventional invasive approach. The permeability of BBB increases in various diseases. This increased permeability of BBB is made use for drug trafficking. With advances in nanotechnology, various nanocarrier systems including Polymeric nanoparticles, Micelle, Dendrimer, Liposomes, Solid lipid nanoparticles, Nanostuctured lipid carriers, nanogels, etc. emerged as potential delivery systems.
Administration of inhibitors of p-glycoprotein with drug is another approach. Making lipophilic prodrugs of active drug substances can also be done.
Recently PEGylation of nanoparticles can be done to improve its half life in the body, known as the stealth technology. Further various receptor mediated processes are made use of for transport of nanocarrier across BBB. In this method the nanocarrier is attached to the targeting ligand thus making it receptor specific. The newer concept of molecular Trojan horses is also discussed. Finally the advantages of intranasal drug delivery are emphasized.
INTRODUCTION
Brain is a delicate organ and evolution built very efficient ways to protect it. Unfortunately, the same mechanisms that protect it against intrusive chemicals can also frustrate therapeutic interventions. Many existing pharmaceuticals are rendered ineffective in the treatment of cerebral diseases due to our inability to effectively deliver and sustain them within the brain. General methods that can enhance drug delivery to the brain are therefore of great interest.
Brain disorders are of significant global, social and economic concern with about 1.5 billion people affected globally. Neurological disorders, inflammatory and infectious conditions of brain, brain cancer and brain stroke are rapidly escalating. HIV infection is now pandemic and around 33.2 million people suffer worldwide. Further, statistics of brain cancer patients reveal a grim picture, with the incidence of afflicted population slowly increasing and survival rates of less than 25% after 5 years. [1]
More than 95% of drugs for delivery to the brain are limited by the formidable blood-brain barrier (BBB). Administration of high dose to attain adequate brain drug concentration results in severe side effects. Overcoming the BBB therefore represents an important step in brain targeting. The direct approach is the invasive approach which could permit direct administration of drugs into the brain tissue. This however necessitates drilling a hole in the cranium and involves surgical intervention. Non-invasive strategies could provide a lower risk and a more patient friendly approach. This has triggered enormous interest in colloidal drug delivery systems for delivery to the brain. Micelles, polymeric nanoparticles, lipid nanoparticles and liposomes have been evaluated and it has been generally observed that lipidic carriers can traverse the BBB more rapidly.
More new methods involving receptor mediated transport, carrier mediated transport are newly dealt with.
RATIONALE
With the newer advances in nanotechnology the newer approaches are coming up with the virtue of delivery of drugs across BBB. The idea behind improving BBB penetration is either to lipidise the drug or load it into suitable nanocarrier system.
This nanocarrier will then act as a transporting vehicle to the brain on account of its nanosize and lipophilic properties.
The recent advances include coupling of a ligand resembling to the endogenous substance to the nanocarrier. It will thus enable the nanosystem to target selectively to the receptors at the brain endothellium.these strategies include receptor mediated endocytosis, adsorptive endocytosis, etc.
Thus it can be said that ‘the delivery system would resemble a travelling vehicle, Drug being the passenger nanocarrier as the vehicle and targeting ligand as the driver.”
ANATOMY AND PHYSIOLOGY
BRAIN
The brain is an integral part of the central nervous system acting as a major regulating and communicating organ to maintain the body’s homeostasis in response to changes in both the external and internal environment.
The primary cells of the brain include nerve cells (neurons) and supporting glial cells (glia). An adult human has approximately 100 billion neurons and 1 trillion glialcells. The neurons and glial cells organize into specialized structures within the brain that can be characterized by both a unique architecture and function. Structures can be classified as either gray or white matter, areas of the brain dominated by cell bodies and axonsrespectively.
The three major subdivisions of the brain are the cerebrum, cerebellum, and the brain stem. The cerebrum is the largest section and is easily divided into the right and left hemispheres along the mid-sagittal plane. These hemispheres are made up of an outer layer of gray matter, the cerebral cortex, which is responsible for functions such as language and information processing. The cortex, made up of nearly 25 billion neurons, is highly convoluted, increasing the surface area to ¨2300 cm2. Cerebral cortex cells communicate with each other and with the spinal cord via the underlying cerebral white matter; communication between the two cerebral hemispheres primarily occurs via a major white matter tract called the corpus callosum. The cerebellum contains a similar gray and white matter organization but at a smaller scale, functioning primarily to control balance and coordinated movement. The brain stem, responsible for involuntary functions such as heart rate and breathing, connects the brain to the spinal cord. It contains both gray and white matter regions but they are not organized into an inner and outer layer as in the cerebrum and cerebellum.
The majority of brain tumours occur in the parenchyma space of the cerebrum. However, more so than in any other tissue, getting drugs into the brain is much more difficult than getting drugs out of the brain. Brain tissue is protected externally by the skull, which constrains the volume and regulates intracranial tissue pressure. Internally, the BBB greatly limits permeability and transport across the endothelial cell membranes of the blood vessels

Fig.1 Human brain
a) major parts of brain
b) composition of brain in terms of volume[2]
Blood Brain Barrier
BBB was first noticed by Paul Ehrlich in 1885 and later confirmed by Edwin Goldmann. BBB is located at the level of brain capillaries, where there is a convergence of different cell types endothelial cells, pericytes, astrocytes and microglias (perivascular macrophages). The brain micro vessel endothelial cell (BMEC) that form the BBB, display important morphological characteristics such as the presence of tight junctions between the cells, the absence of fenestrations and a diminished pinocytics activity, that together help to restrict the passage of compounds from the blood into the extra cellular environment of the brain. This barrier permits the exchange of essential gases and nutrients between the bloodstream and the brain, while blocking larger entities such as microbes, immune cells and most drugs from entering.
This barrier system is a perfectly logical arrangement, since the brain is the most sensitive and complex organ in the human body and it would not make sense for it to become the battleground of infection and immune response. This biological demilitarization zone is enforced by an elaborate and dense network of capillary vessels that feeds the brain and removes waste products. Each capillary vessel is bound by a single layer of endothelial cells, connected by tight junction-zona occludens thereby making it very difficult for most molecules to exit the capillaries and permeate into the brain. Tight junctions provide significant transendothelial electrical resistance (TEER) to BMEC and impede the penetration of potential therapeutic agents such as oligonucleosides, antibodies, peptides and proteins. Furthermore, BMEC express a variety of enzymes, both cytosolic and on the extra cellular membrane which also contribute to the restrictive nature of the BBB. P-glycoprotein (P-gp) is also present in the luminal plasma membrane of BMEC.This is an ATP-dependant efflux pump and a member of a family of intrinsic membrane proteins.P-gp is known to prevent the intracellular accumulation of an extensive variety of chemotherapeutic agents and hydrophobic compounds.[3]
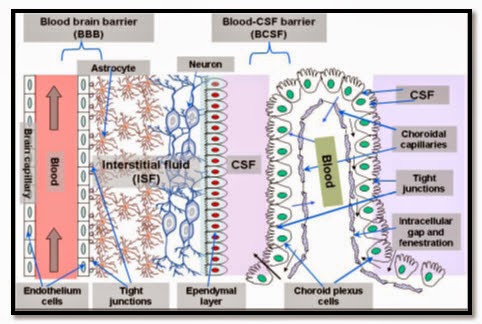
Fig.2 Overview of the two main barriers in the CNS. blood-brain barrier and Blood cerebrospinal fluid barrier (BCSF).[3]
Blood-Cerebrospinal Fluid Barrier
The second barrier that a systemically administered drug encounters before entering the CNS is known as the Blood-cerebrospinal fluid barrier (BCB). Since the CSF can exchange molecules with the interstitial fluid of the Brain parenchyma, the passage of blood-borne molecules into the CSF is also carefully regulated by the BCB. Physiologically, the BCB is found in the epithelium of the choroids plexus, which are arranged in a manner that limits the passage of molecules and cells into the CSF.
The choroid plexus and the arachnoid membrane act together at the barriers between the blood and CSF. On the external surface of the brain the ependymal cells fold over onto themselves to form a double layered structure, which lies between the dura and pia, this is called the arachnoid membrane. Within the double layer is the subarachnoid space, which participates in CSF drainage. Passage of substances from the blood through the arachnoid membrane is prevented by tight junctions. The arachnoid membrane is generally impermeable to hydrophilic substances, and its role is forming the Blood-CSF barrier is largely passive.
The choroid plexus forms the CSF and actively regulates the concentration of molecules in the CSF. The choroid plexus consist of highly vascularised. The preponderance of choroid Plexus is distributed throughout the fourth ventricle near the base of the brain and in the lateral ventricles inside the right and left cerebral hemispheres. The cells of the choroidal epithelium are modified and have epithelial characteristics. These ependymal cells have microvillus on the CSF side, basolateral interdigitations, and abundant mitochondria. The ependymal cells, which line the ventricles, form a continuous sheet around the choroid plexus. While the capillaries of the choroid plexus are fenestrated, non-continuous and have gaps between the capillary endothelial cells allowing the free-movement of small molecules, the adjacent choroidal epithelial cells form tight junctions preventing most macromolecules from effectively passing into the CSF from the blood. However, these epithelial-like cells have shown a low resistance as compared the cerebral endothelial cells, approximately 200 .cm2 between blood and CSF.
In addition, the BCB is fortified by an active organic acid transporter system in the choroids plexus capable of driving CSF-borne organic acids into the blood. As a result a variety of therapeutic organic acids such as the antibiotic penicillin, the anti-neoplastic agent methotrexate, and the antiviral agent zidovudine are actively removed from the CSF and therefore inhibited from diffusing into the brain parenchyma.
Furthermore, substantial inconsistencies often exist between the composition of the CSF and interstitial fluid of the brain parenchyma, suggesting the presence of what is sometimes called the CSF-brain barrier. This barrier is attributed to the insurmountable diffusion distances required for equilibration between the CSF and the brain interstitial fluid. Therefore, entry into the CSF does not guarantee a drug’s penetration into the brain.[3]
TRANSPORT MECHANISMS AT THE BLOOD–BRAIN BARRIER
A.Paracellular (aqueous) diffusion: Small water-soluble molecules can diffuse through the BBB by apparently passing through the tight junctions
B.Transcellular (lipophilic) diffusion: In the transcellular lipophilic pathway, lipid-soluble substances such as O2, CO2, alcohol and steroid hormones can penetrate endothelial cells easily by dissolving in their lipid plasma membrane
C.Saturable (carrier-mediated) transport: this transport system involves transfer of solute by means of a carrier. This is a active type of transport requiring ATP. This process is saturable.
D. Receptor mediated endocytosis-this involves coupling of the solute molecule with the receptor and further this complex is endocytosed and transported to the other end. The complex then dissociates and solute molecule is exocytosed.
E. Adsorptive mediated endocytosis-this transport is similar to RME but it involves electrostatic attraction between solute and receptor.
F.Efflux transport pathways-this involves only removal of solute molecules from brain to outside. It mainly involves members of ABC family, multi-drug transporters; multi-drug resistance protein (MRP), P-glycoprotein (Pgp) and the multi-specific organic anion transporter (MOAT),which belong to the members of the ABC cassette (ATP-binding cassette) of transport protein. [4]

Fig.3 Different transport mechanism across BBB
DISEASES OF BRAIN
This group of diseases includes Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis, lysosomal storage diseases, HIV infection of the brain, brain tumours, and CNS intoxications, for instance, with organophosphorous compounds.
Brain tumours include gliomas (50.4%), meningiomas (20.8%), pituitary adenomas(15%)nerve sheath tumours(8%). [1]
Thus these diseases have a significant impact on the longevity of human being.
Various drug molecules are available for these diseases but major hurdle is the transport of these drugs to their site of action.
Impact of pathological conditions on the properties of the Blood–brain barrier
The properties of the BBB undergo significant changes when the brain is developing a neurological disorder, in inflammatory conditions or under attack by pathogens. These changes affect the integrity as well as the functions of the BBB, including transport pathways. In vitro studies have shown that altered drug delivery under pathological Conditions may be mediated through changes in a number of transport pathways including paracellular transport and transcellular transport (RMT and adsorptive transcytosis) . Indeed, it has been anticipated that the impaired BBB may provide a window of opportunity for those drugs which normally are unable to traverse the BBB to reach the target in the diseased brain.
Table no.1 Pathological conditions, their impact on the blood–brain barrier[5]
|
Pathological condition |
Impact on the blood–brain barrier |
|
Multiple sclerosis |
Disruption of Tight Junction; enhanced leukocyte activity; release of inflammatory cytokines/chemokines. |
|
Alzheimer's disease |
BBB disruption and permitted the greater access of peripheral IgG to the CNS. |
|
Parkinson's disease |
BBB disruption. |
|
HIV |
Increase in the diameter of cortical vessels, thinning of basal lamina, loss of glycoproteins, apoptosis of endothelial cells and tight junction disruption. |
|
Infectious disease |
Leukocyte invasion, elevated CSF-to-serum albumin ratio, and BBB impairment. |
|
Inflammation |
Increased BBB permeability. |
|
Stroke |
BBB disruption Up regulation of Diphtheria toxin receptor. |
|
Brain tumour |
Decreased TJ proteins and BBB perturbation Loss of the tight junctions in the tumour vascular system, enhanced retention effect Over expression receptors of folate, insulin and transferrin. |
|
Ischemia/seizure |
Up regulation of Diphtheria toxin receptor |

Fig.4 BBB in healthy and diseased state.
REQUIREMENT FOR CROSSING BBB:-
· Only small molecules(500 Da),
· lipid-soluble, log P<5
· electrically neutral molecules
· weak bases are able to diffuse passively across the BBB
ROUTE OF ADMINISTRATION IN VARIOUS DISEASES
Table no.2 Routes of administration in various diseases.
|
Intravenous |
Oral |
Intranasal |
|
Brain Cancer |
Brain Tuberculosis |
Migrane |
|
Brain Tuberculosis(critical) |
AIDS |
Parkinson’s diseases |
|
AIDS(critical) |
Multiple sclerosis |
Insomnia |
|
Multiple sclerosis |
Parkinson’s disease, |
|
QUANTITATIVE MEASURE OF CNS UPTAKE [6]
Rule of 2-
The hypnotic activity of a number of congeneric series of CNS depressants reached a maximum when log octanol–water partition coefficient (log Po/w) was near to 2. Various researchers confirmed this finding and the “rule of 2” became generally accepted.
Brain uptake index (BUI)
The brain uptake index is a more rigorous measure of brain uptake in which there is a relative measure of brain uptake by intra-carotid injection of a mixture of 14Clabeled compound and 3H-labeled water (i.e. a saline solution in 3H-labeled water). The radioactivity in brain tissue is recorded 15 seconds after administration, and a brain uptake index (BUI) is defined in equation-
BUI = 100 X ( 14C/3H)tissue/(14C/3H)Saline
where the BUI for water is 100. Although, the BUI is useful as a rank order index of brain uptake, is not easily amenable to analysis by physicochemical method
Permeability-surface area product (PS) or Permeability coefficient (PC)
A more well-defined measure of rapid brain uptake is the permeability, expressed either as a permeability-surface area product (PS) or as a permeability coefficient (PC), obtained by intravenous injection and measurement of the drug profile in arterial blood. Both the PS product and PC are quantitative measures of the rate of transport obtained by in-situ vascular perfusion technique and so are amenable to analysis through standard physicochemical procedures. An advantage of the perfusion technique as a measure of brain uptake is that the time scale for determination of PS products is very short, so that back transport and biological degradation are minimized.
Brain tissue accumulation (Cbrain)
Cbrain= PS * AUC
Where PS-is the brain capillary permeability surface area product, an expression equivalent to the organ clearance
AUC- is the area under the plasma concentration time curve.
METHODS OF DRUG TRANSPORT ACROSS THE BBB
Targeting active molecules to specific sites in the body had been pursued actively ever since Ehrlich first envisaged the use of 'magic bullets' for the therapy of various diseases. Interest in this concept has increased significantly in recent decades with the innovations of nanomedicine Targeted delivery can be achieved by either passive or active targeting.
Passive Targeting
Passive targeting is achieved by loading drug into a nanocarrier that reaches the target organ passively. Passive targeting of tumours takes advantage of hyper-permeable cells owing to their rapid vascularisation. This rapid vascularization results in leaky, defective cells and impaired lymphatic drainage. Nanoparticles ranging from 10 to 100 nm then begin to accumulate within tumours because of their ineffective lymphatic drainage. This results in a phenomenon known as the enhanced permeation and retention (EPR) effect. The size and surface properties of a nanomedicine is vital for passive targeting.
Active Targeting
Recent advances have led to the transformation from passive to active targeting. Active targeting of a drug is achieved by conjugating a nanocarrier system (drug loaded) to a tissue- or cell-specific targeting ligand. Active targeting has raised the importance of nanomedicine and this can now be achieved by a number of specific interactions, such as ligand-receptor and antibody-antigen binding. These specific interactions result in preferential accumulation of nanomedicine into molecular targets. [7]
Drug transport by BBB disruption
Osmotic imbalance
The thought behind this approach was to break down the barrier momentarily by injecting mannitol solution into arteries in the neck. The resulting high sugar concentration in brain capillaries takes up water out of the endothelial cells, shrinking them thus opening tight junction. The effect lasts for 20-30 minute, during which time drugs diffuse freely, that would not normally cross the BBB. This method permitted the delivery of chemotherapeutic agents in patients with cerebral lymphoma, malignant glioma and disseminated CNS germ cell tumours. Physiological stress, transient increase in intracranial pressure, and unwanted delivery of anticancer agents to normal brain tissues are the undesired side-effects of this approach in humans.
Intraventricular / Intrathecaldelivery
Here using a plastic reservoir which implanted subcutaneously in the scalp and connected to the ventricles within the brain by an outlet catheter. Drug injection into the CSF is a suitable strategy for sites close to the ventricles only.
· Gliadel wafer®-implanted intracavitary
· Ommaya reservoir®-catheter with pump delivers intermittent bolus injections into CSF. [6]
Table no.3 methods of BBB disruption [5],[8]
|
Biological |
Physical |
Chemical |
|
Zonula occludens toxin |
Ultrasound |
Cyclodextrin |
|
Vasoactive peptides |
Microwave |
Polaxamers |
|
Histamine |
Electromagnetic field |
Cell penetrating peptides |
|
Bradikinin |
|
|
|
VEGF |
|
|
Prodrug Approach
Few drugs do not have CNS penetrations in active form hence they are converted into prodrugs.These prodrugs after crossing BBB are converted back to the original form of the active drug.
Dopamine does not have active CNS penetration hence it is administered in its prodrug form namely levodopa in cases of Parkinson’s diseases. after crossing BBB it is again converted into its active form that is dopamine.GABA, Niflumic acid, valproate or vigabatrin are coupled to diglycerides or modified diglycerides [6]
Nanocarrier transport system based drug transport
Polymeric Nanoparticles
Nanoparticles for pharmaceutical purposes as defined by the Encyclopaedia of Pharmaceutical Technology are solid colloidal particles ranging in size from 1 to 1000 nm , consisting of macromolecular materials in which the active principle is dissolved, entrapped, or encapsulated, or to which the active principle is adsorbed or attached. The use of nanoparticles for the transport of drugs across the BBB was already suggested in the early 1980s by Prof Speiser at the ETH (Swiss Federal Institute of Technology) in Zurich , who was also the first to systematically develop nanoparticles for drug delivery purposes in the late 1960s and early 1970s.
Based on arrangement of drug and polymer matrix Nanoparticles are classified into two types5:
1. Nanospheres: drugs are either absorbed or entrapped inside the polymer matrix.
2. Nanocapsules: drug present in the inner liquid core, and external surface of nano particles are covered by the polymeric membrane.
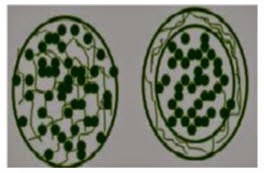 Fig.5. Schematic diagram of Nanosphere (A), and Nanocapsule (B) .
Fig.5. Schematic diagram of Nanosphere (A), and Nanocapsule (B) .
Drugs can be entrapped in the matrix, encapsulated in the core, or attached to the surface of nanoparticles. The most frequently used nanoparticles formulation for brain drug delivery consists of poly(butyl cyanoacrylate) (PBCA) . In order to overcome the uptake in RES of the liver and spleen, nanoparticles can be coated with surfactants or, PEGylated like liposomes. As described later in this review, coating with the surfactant polysorbate 80 also indirectly promotes brain uptake of the nanoparticle contents.
Mechanism of the Delivery of Drug across the Blood-Brain Barrier with Nanoparticles
Presently, the mechanism of the delivery of drugs with nanoparticles across the BBB is not totally elucidated. A number of possibilities were suggested for this mechanism
1. An increased retention of the nanoparticles in the brain blood capillaries combined with an adsorption to the capillary walls. This could create a higher concentration gradient that would enhance the transport across the endothelial cell layer, and as a result, the delivery to the brain.
2. The polysorbate 80 used as the coating agent could inhibit the efflux system,
Especially P-glycoprotein (Pgp).
3. A general toxic effect on the brain vasculature, leading to the permeabilization of the brain blood vessel endothelial cells.
4. A general surfactant effect characterized by a solubilisation of the endothelial cell membrane lipids that would lead to membrane fluidization and an enhanced drug permeability through the blood-brain barrier.
5. The nanoparticles could lead to an opening of the tight junctions between the endothelial cells. The drug could then permeate through the tight junctions in Free, or together with the nanoparticles, in bound form.
6. The nanoparticles may be endocytosed by the endothelial cells, followed by the release of the drugs within these cells and the delivery to the brain.
7. The nanoparticles with bound drugs could be transcytosed through the endothelial cell layer.
All these mechanisms could also work in combinations. [9]
Solid lipid nanoparticles
SLN are composed of a solid lipid matrix stabilized by surfactants. The lipids used (triglycerides, complex glyceride mixtures and waxes) have the particularity of remaining in solid form at both room and physiological temperatures. These lipid nanoparticles are mainly produced by highpressure homogenization, via microemulsions and by solvent emulsification-evaporation or diffusion.
The physical stability of SLN, the use of bioacceptable and biodegradable lipids, the size in the nanometer range and the protection of the encapsulated drug are promising features for parenteral administration However, the drug loading capacity of conventional SLN is limited by the weak solubility of drugs in the lipid melt and the drug expulsion after polymorphic transition. [10]
Lipid nanocapsule
Nanostructured lipid carriers andlipid–drug conjugates (LDC) werethereafter developed leading to a significant increase of drug loading. Sterically stabilized SLN is prepared with PEG2000 conjugated to phospholipids and glycerides. Conversely, LNC are composed of a liquid, oily core (medium-chain triglycerides) surrounded by hydrophilic (PEG660-hydroxystearate) and lipophilic (phosphatidylethanolamine and phosphatidylcholine) surfactants. These nanocapsules are prepared by the phase-inversion temperature method (PIT method). In short, during three cycles of progressive heating and cooling, the O/W emulsion is inverted into W/O emulsion by passing through a phase-inversion zone. During the last cycle, rapid cooling with cold water is performed at the PIT, leading to the formation of submicron-scale particles. LNC are obtained without organic solvent and with pharmaceutically acceptable excipients.
Albumin nanoparticles
Albumin is playing an increasing role as a drug carrier in the clinical setting. Principally, three drug delivery technologies can be distinguished: coupling of low-molecular weight drugs to exogenous or endogenous albumin, conjugation with bioactive proteins and encapsulation of drugs into albumin nanoparticles.
Liposomes
Liposomes are phospholipids based vesicular system with an inner aqueous layer surrounded completely by a phospholipid membrane bilayer. Liposomes are nanosystem that exhibit in high biocompatibility, low toxicity, ease of preparation and commercial availability. Liposomes are therefore more extensively evaluated nanosystem for the delivery of drugs to the brain.

Fig.6 Types of liposomes used to transport drugs over the BBB. (A) Small unilamellar vesicles (SUV). (B) Multilamellar vesicles (MLV). (C) PEGylated or PEG coated liposomes.(D) PEGylated ligand/antibody bearing immunoliposomes. (E) Stimuli sensitive liposomes.
PEGylation of liposome resulted in resulted in prolonged half life to to increased to in circulation time. [4]
· Long circulating stealth liposomes (caelyx®) have shown 13-19 times higher concentration of DOX in glioblastoma.
· Thus stealth liposomes can cross BBB in brain tumour.
· Liposomes have been used as carriers for radioisotopes and contrast agents to be used in diagnostic imaging. Liposomes carrying contrast agents accumulate more in surrounding cells than in tumour cells due to the low phagocytic activity of tumour cells. This can be used for tumour imaging,
· Specificity can be added to liposomes by coating their surface with targeting molecules. As an example, “immunoliposomes” can be formed by coating liposomes with a BBB-targeting antibody
Polymeric micelles
Polymeric micelles are formed from a spontaneous association of amphiphilic copolymers in an aqueous phase. They are characterized by a diameter not exceeding 100nm. The attractive force leading to micellisation is based on an interaction between the hydrophobic and electrostatically neutral parts of copolymers. Self-assembly starts when the copolymer concentration reaches a threshold value known as the critical micelle concentration (CMC) polyester: poly(lactic acid) (PLA), poly(e-caprolactone)(PCL); poly(L-amino acid): poly(aspartic acid) (PAsp), poly(glutamic acid) (PGlu); phospholipids: phosphatidylethanolamine (PE) are the polymers used. [11]
Dendrimer
Three-dimensional tree-like branched macromolecules possess some fascinating characteristics: a well-defined structure, a very narrow molecular weight distribution, a three-dimensional structure tuned by dendrimer generation and dendron structure, and flexibility for tailored functional groups with high density on the periphery.
These dendrimers can act as a carrier novel class of three-dimensional nanoscale, core-shell structures that can be precisely synthesized for a wide range of applications. They are most useful in drug delivery. Polyvalent dendrimers interact simultaneously with multiple drug targets. They can be developed into novel-targeted cancer therapeutics.
Nanogels
Microgels/nanogels are crosslinked polymeric particles, which can be considered as hydrogels if they are composed of water soluble/swellable polymer chains. They possess high water content, biocompatibility, and desirable mechanical properties. They offer unique advantages for polymer-based drug delivery systems (DDS): a tunable size from nanometers to micrometers, a large surface area for multivalent bioconjugation, and an interior network for the incorporation of biomolecules. [12]
Microemulsions and Nanoemulsions
ME are clear thermodynamically stable isotropic mixtures of oil, water and surfactant, frequently used in combination with cosurfactants.they are stable, single phase swollen micellar solutions that offer the advantage of spontaneous formation and ease of manufacture.
NE is kinetically stable, thermodynamically unstable mixture of oil water and surfactants. Co surfactant are not essential in NE. NE do not form spontaneously.
The size of oil droplet in NE and ME ranges from 20-200nm.
For brain delivery o/w ME and NE are preferred.Water free ME may be designed as self-microemulsifying drug delivery system (SMEDDS) which on dilution with an aqueous phase spontaneously microemulsify to form ME.[1]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table no. 4 Summary of drug carriers across BBB [13]
|
Carrier types |
Particle materials |
Drug(s) delivered |
Case studies |
|
1.Polymeric nanoparticles |
PBCA |
Methotrexate |
Drug levels in brain and cerebrospinal fluid increased, especially with smaller np (b100 nm) |
|
Dalargin |
BBB penetration by dalargin increased 3 fold when drug was loaded on np Oral use of PBCA np coated with polysorbate-80 and PEG-20000 caused antinociceptive effect |
||
|
MMA-SPM; PCBA |
Lamivodine,zidovodine |
PCBA np enhanced BBB permeability of 3TC and AZT by 10–18 and 8–20 fold, respectively |
|
|
PLGA/alginate |
Dexamethasone |
Extended drug release to 2 weeks; inflammation of glial cells significantly reduced |
|
|
|
PLA/PEG |
Vasoactive intestinal peptide |
Drug level in brain increased by 5.6 to 7.7 fold |
|
|
PLA |
Superoxide dismutase |
Infarct volume reduced 65%, greater survival (75% vs 0% at 28 days), regained most vital neurological functions in ischemic-reperfusion rat model |
|
2.Solid lipid nanoparticles |
Soya phosphatidyl-choline 95% |
Doxorubicin |
Drug accumulated in brain only in np groups; Stearic acid-PEG2000 modestly improve the circulation time and brain accumulation |
|
|
Stearic acid/ soy bean Lecithin |
Campothescin |
Drug release up to a week; Highest enhancement in camptothecin AUC was observed in brain when the drug was orally administered as SLN |
|
|
Stearylamine |
Paclitaxel |
Orally administered np led to>3-fold serum drug level and higher brain drug level |
|
|
Stearic acid |
Melatonin |
Oral melatonin-SLN led to delayed onset, higher AUC and longer half-life; Transdermally applied SLN maintained plasma drug level for 24 h |
|
|
|
|
|
|
3.Lipid nanocapsules |
Triglycerides of capric and caprylic acids, Solutol |
Etoposide |
LD50 of lipid nanocapsule formulation of etoposide in C6, F98, and 9 L glioma cell lines substantially lower than free etoposide
|
|
4.Albumin nanoparticles |
Albumin |
Loperamide |
Coating of ApoE improved BBB penetration by np; antinociceptive effects induced in CNS by np only |
|
5.liposomes |
Phospholipids, cholesterol |
Phenytoin, GABA |
Improved local action against epilepsy; reduced penicillin induced epileptic activity |
|
|
|
Cisplatin |
Increased drug concentration and cell killing in brain tumor invaded areas |
|
|
|
Horseradish peroxidise |
Increased permeability across BCEC culture; transferrin coated liposomes improved transcytosis |
|
6.Micelle |
Pluronic P85 |
Amphotericin |
Several-fold increase in brain uptake |
|
7.Dendrimer |
Mannosylated poly(propyl eneimine)
|
Lamivodine |
Dendrimer increased uptake of lamivudine into MT2 cells by 21-fold, and intracellular viral p24 levels decreased by 2.6-fold |
|
|
Polyether–copolyester |
Methotrexate |
Dendrimer significantly enhanced intratumoral drug transport in gliomas |
|
|
PEG-poly (amidoamine) |
Doxorubicin |
BBB transport of doxorubicin to brain tumour increased from 5% to 13.5% in dendrimer group |
|
8.Nanogel |
PEG-PEI |
Antisense oligonucliotide |
Increased in vitro uptake, intracellular oligonucleotide release and antisense activity |
|
NVP/ NIPAM |
5-fluorouracil |
Polysorbate coating of nanogels increased brain accumulation from 0.18% of the injected dose to 0.52% |
|
|
9.Nano-emulsion |
Edible oils |
Sequinavir |
Oral use of nanoemulsion increased serum and brain drug concentrations by 3- and 5-fold respectively |
|
|
Peanut oil |
Paclitaxel |
Nanoemulsion increased drug uptake, cytotoxicity and apoptotic activity in U-118 human glioblastoma |
ApoE: apolipoprotein-E; AUC: area- under-curve; MMA-SPM: methylmethacrylate–sulfopropylmethacrylate; np: nanoparticles; PEG: polyethylene glycol; PLA: polylactic acid;
PLGA: poly (lactic-co-glycolic acid).
NIPAM: N-isopropyl-acrylamide; NVP: N-vinyl-pyrrolidone;; PEI: polyethylenimine.
Drug transport by Pgp inhibition Pgp inhibitors if coadministered with the drug results in good amount of drug accumulation in brain compartment.The most studied Pluronic® P85 showed the ability to enhance the BBB permeability of a wide range of drugs, including doxorubicin,etoposide, taxol, 3′-azido-3′-deoxythymidine, valproic acid and loperamide, in the bovine brain microvessel endothelia cell monolayer.
Evidence suggests that the inhibition mechanisms of Pluronic® block copolymers on P-gp activity in the BBB involve 1) copolymer interaction with the cell membrane, producing a “membrane fluidization”effect; 2) inhibition of P-gp ATPase activity; 3) depletion of cellular ATP. [14]
FUNCTIONALISATION OF NANOCARRIERS
In order for a neuropharmaceutical to be delivered into the brain via the receptor-mediated mechanism, it must first be linked to the BBB delivery vector. The following section briefly reviews several strategies that have been used to link therapeutic cargo with BBB delivery vectors.
Stealth Technology
Nanocarriers like liposomes loaded with drugs have very short half life. Thus they are unable to retain the drug invivo until it reaches the site of action. Surface modification by grafting PEG chains stabilise the drug loaded liposomes by reducing their stearic hindrance and increasing their stay in vivo.A highly flexible and hydrated PEG chain attached to the liposomes surface is assumed to have an effective opsonins-resistant property due to its stearic repulsion effect. PEG-containing surfactants, poly (oxy-ethylene)-poly(oxy-propylene) block copolymers, (poloxamer 338 = Pluronic®F108 and poloxamine 908 = Tetronic®) were also found to be effective in prolonging the blood circulation time of nanoparticles. [15]
Chemical linkage
The key to any linkage strategy is to ensure that both the transport vector and pharmaceutical protein retain their functionality. Several well-established methods for covalent chemical conjugation have been used to achieve this goal. The most common approach is linkage via primary amines, principally lysine residues, of either the targeting vector or protein therapeutic.
Chemical functionalisation using Traut’s reagent (2-iminothiolane) yields a thiol that can subsequently be reacted with maleimide-functionalized drug or vector to form a stable thioether bond. Thiolated drug or vector can also be reacted with a free cysteine or reduced disulfide bond to yield a disulfide-bonded drug-vector conjugate. To further ensure functionality of the vector and protein, a chemical spacer (CH2)5NHCO(CH2)5NHCO or polyethylene glycol (PEG) moiety can be incorporated into the linkage to reduce stearic hindrance . [16]
Non-covalent streptavidin/biotin linkage
Due to the extremely high binding affinity between streptavidin and biotin , this non-covalent interaction can be used to couple BBB delivery vectors with therapeutics. To achieve this coupling, the therapeutics can be monobiotinylated at lysine residues using N-hydroxysuccinimide (NHS) analogs of biotin, or alternatively, biotin can be attached using biotin hydrazide which reacts with carboxylic acid moieties on glutamate and aspartate residues. Having multiple choices of amino acid residues where biotin can be attached can be helpful to ensure that the therapeutic activity is retained upon biotinylation.
In addition, as a result of streptavidin multivalency, it has been shown that monobiotinylation is necessary to prevent the formation of aggregates, and hence rapid clearance by the reticuloendothelial system (RES). The streptavidin can be coupled to the targeting vector via a thioether linkage. A BBB-targeted therapeutic can then be created simply by mixing the biotinylated therapeutic with the streptavidin-functionalized targeting vector. Again a PEG linkage can be used to better separate the therapeutic and targeting moiety, while also providing improved plasma residence time in some cases. [16]
CONCEPT OF MOLECULAR TROJAN HORSES
Various defence cells like macrophages, monocytes traverse the BBB about 80% turnover in three months. Although these cells have protective nature in many studies it has shown that many pathogenic organism make use of these cells to travel across BBB.here arises concept of molecular Trojan horses.
These defence cells can be made use for anticancer drug trafficking across BBB by cell mediated transcytosis directly into tumours.[5]
DRUG TRANSPORT BY TARGETING ENDOGENOUS PATHWAYS
Carrier mediated endocytosis
Brain capillary endothelium has various carrier mediated systems for transport of nutrients across BBB. These include amino acid transport, hexose transport, peptide transport, nucleoside, choline transport, etc. Most of the peptide drugs can be delivered by this route.Utilization of differences in the affinity and the maximal transport activity among these transport systems expressed at the BBB is an attractive strategy for controlling the delivery and retention of drugs into the brain. These protein macromolecular carrier systems are characterized by saturability and molecular selectivity.
Receptor mediated endocytosis
Major targeted receptors include
I. Transferrin receptor
II. Insulin receptor and insulin-like growth factor receptor
III. Low density lipoprotein receptor-related protein 1 and low density lipoprotein receptor related protein 2
IV. Diphtheria toxin receptor/ Heparin binding epidermal growth factor-like growth factor
Adsorbtive mediated endocytosis
Transport route for delivery of drugs into the brain via cationic proteins, and cell-penetrating peptides (CPPs).
Cell Penetrating Peptides-CPPs are positively charged peptides with amphipathic characteristics.CPPs refer to a group of short peptides of less than 30 amino acids that are able to penetrate cell membranes and transport their cargo into cells. [14]
Table no.5 examples of cell penetrating peptides [14]
|
model amphipathic peptide |
MAP |
|
sequence signal-based peptide |
SBP |
|
fusion sequence-based peptide; |
FBP |
|
HIV-1 trans-activating transcriptor |
TAT48-60 |
|
SybB1 &B2 |
|
Table no.6 Ligand targeting at various receptors [13]
|
STRATEGY |
TARGETING LIGAND |
CASE STUDIES |
|
1.Target transferrin receptor |
Transferrin |
Transferrin coating on liposomes significantly increased glioma uptake of doxorubicin |
|
2.Target folate receptor |
Folate |
Folate coated liposomes able to adjust the specificity to allow differentiating of astrogliomas which express low folate receptor level from normal brain tissues |
|
3.Target lactoferrin receptor |
Lactoferrin |
PEG-PLGA linked with lactoferrin increased coumarin level in brain by 3-fold after intravenous administration. No significant toxicity detected |
|
4.Target thiamine receptor |
Thiamine |
Wax nanoparticles coated with thiamine achieved increased kinetics taken up by thiamine transporters |
|
5.Antibody to target transferrin receptor
|
OX26 |
OX26 tagging to liposomes enhanced daunomycin and plasmid delivery to brain; OX26-linked polymersomes delivered peptide NC-1900 to improve scopolamine activity in rats |
|
5.Antibody to target insulin receptor |
83-14 antibody |
83-14-liposomes targeting insulin receptor delivering anti-EGF receptor oligonucleotides successfully suppressed the receptor expression in gliomas |
|
6.Cell-penetrating peptide |
TAT |
TAT-micelles improved delivery of ciprofloxacin across human brain endothelial cells and coumarin to rat brain; TAT-neuroglobin fusion protein crossed BBB to protect against focal cerebral ischemia in mice |
|
7.Target low-density lipoprotein receptor |
Apolipoproteins (e.g. ApoA, ApoE |
Nanocarriers (e.g. PBCA nanoparticles, SLN) directly linked or indirectly adsorbed to apolipoproteins via surfactants able to cross BBB and delivered different drugs (e.g. loperamide, methotrexate, dalargin) to brain; Direct method superior to indirect method |
|
8.Antibody to target EGF receptor |
Fab' fragments of cetuximab (IMC-C225) |
Only immunoliposomes with IMC-C255 internalized extensively within brain tumor cells (92% versus b5% for non-targeted liposomes) and improved delivery of doxorubicin, epirubicin, and vinorelbine |
|
9.deptheria toxin receptor |
CRM197, a nontoxic mutant of diphtheria toxin, |
|
ApoA: Apolipoprotein-A; ApoE: Apolipoprotein-E; EGF: Epidermal growth factor; PBCA: Poly(butyl cyanate); PLGA: PLGA: Poly(lactic-co-glycolic acid); TAT: Trans-activator of transcription.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
INTRA NASAL DRUG DELIVERY
An alternative CNS drug delivery strategy that has received relatively little attention is the intranasal route. This route involves the olfactory or trigeminal nerve systems for transport of exogenous substances from the nose to the brain. Olfactory or trigeminal nerve systems start from the brain and end in the nasal cavity at the olfactory neuro epithelium or respiratory epithelium respectively, the only portion of CNS exposed externally.
The olfactory region of the nasal cavity is involved in the detection of smell and comprises of three cell types namely olfactory receptor cells, epithelial (sustentacular) cells and basal cells. Olfactory receptor cells are neurons present in the scattered form among the sustentacular cells of the olfactory epithelium.
They originate from the olfactory bulb in the brain . The trigeminal nerve has three major branches, the ophthalmic nerve, maxillary nerve and mandibular nerve. The ophthalmic and maxillary branches pass directly through the nasal mucosa and hence play an important role in nose to brain delivery .Large surface area (150 cm2) is the other physiological advantage offered by the nasal route. Nose to brain transport of small molecular weight drugs have been reported.
In theory, this strategy could be effective in the delivery of therapeutic proteins such as brain-delivered neurotropic factor (BDNF) to the olfactory bulb as a treatment for Alzheimer’s disease. The nasal drug delivery to the CNS is thought to involve either an intraneuronal or extraneuronal pathway. Recent evidence of direct nose-to-brain transport and direct access to CSF of three neuropeptides bypassing the bloodstream has been shown in human trials, despite the inherent difficulties in delivery .
Disadvantage
The difficulties that have to be overcome include an enzymatic ally active, low pH nasal epithelium, the possibility of mucosal irritation or the possibility of large variability caused by nasal pathology, such as common cold.
Advantage
An obvious advantage of this method is that it is non-invasive relative to other strategies. In practice, however, further study is required to determine if therapeutic drug concentrations can be achieved following intranasal delivery
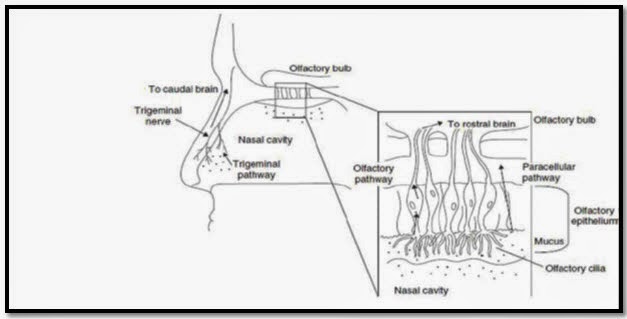
Fig.7Schematic illustration of transport pathways from nose to brain[1]
CNS DELIVERY MARKET ANALYSIS
Global brain tumour market-USD 1.094m-2009
USD 1.3billion-2016
Major players-Myriad genetics, Merck sereno, Merck F Hoffman La, Roche Ltd, Pfizer, Novartis, GSK.
Table no.7 MARKETED FORMULATIONS
|
Formulation |
Tradename |
Disease |
|
PEGylated liposome |
Caelyx® doxil® |
Malignanant glioma |
|
Transdermal patch |
Exelon® |
Alziemers |
|
Liposomal cytarabine |
Depocyt® |
Malignant lmphomatous malignancies |
Table no.8 Market share of various delivery system for CNS [4]
|
Drug delivery system |
CNS Drug market (USD billion ) |
||
|
|
2006 |
2010 |
2015 |
|
Controlled release |
2.8 |
3.1 |
5.5 |
|
Transdermal delivery |
1.3 |
1.9 |
3.2 |
|
Peptide/protein delivery technologies |
1.3 |
1.5 |
2 |
|
Injectable/implantable |
0.9 |
1.1 |
2 |
|
Intrathecal |
0.9 |
1.2 |
1.4 |
|
Transnasal delivery |
0.7 |
0.8 |
1.5 |
|
Liposomal drug delivery |
0.6 |
0.8 |
1.3 |
|
Intracerebrovascular &catheters |
0.5 0.5 |
0.7 0.7 |
1.1 1.2 |
|
Cell/gene therapy/RNAi |
None |
1 |
2 |
|
Inhalation |
0.5 |
0.8 |
1.5 |
|
Others |
0.2 |
1.9 |
2.8 |
|
Total |
10.2 |
15.5 |
25.5 |
CONCLUSION
Brain the supreme commander of human body is highly protected by the Sanctuary made up of blood brain barrier. This protection from unwanted substances also posed a problem for delivery of drug substance in various diseased conditions. Drug delivery to the brain is thus a major challenge in front of formulation scientists. the permeability of BBB increases in various diseases, the use of this is made to transport drug across BBB.
Disruption of tight junctions, developing lipophilic prodrugs, identifying Pgp inhibitors were some of the strategies
Primary condition for transport of drug across BBB is to make it lipophilic and of size less than 500Da. Thus various nanocarriers were designed including polymeric nanoparticles, liposomes micelles, dendrimers, lipidic nanoparticles, etc for traversing the drug across BBB.
Later came the era of coupling drug loaded nanocarrier to a stabilising agent like polyethylene glycol to improve its half life. With the advances in biotechnological research various receptor specific ligands like OX26 antibody to the transfferin receptor were identified. These ligands when coupled to the drug loaded nanocarrier resulted in receptor specific drug transport across BBB. Defence cells like leucocytes including macrophages and monocytes were made use as molecular Trojan horses for drug transport. Major route of drug delivery to CNS is the intranasal having multiple advantage like bypassing BBB.
FUTURE PROSPECTS
With the fast growing advances in the fields of nanotechnology and biotechnology the future of CNS drug delivery belongs to the specific molecular targets in the brain. With the evolution of pharmacogenomics delivery of siRNA, CPP, Gene delivery to the brain will be a challenge. The world is moving more towards the concept of personalised medicines Developing various biodegradable and biocompatible polymers, will be advancing with great speed. Thus there is a need of interdisciplinary research with the goal of Delivery of drug substances to the brain. Considering the complexity of the brain, in-depth and comprehensive toxicological studies of brain targeting nanomedicines are required.
The issues of drug safety and cost must not be underestimated.
REFERENCES
1. Shinde, R.; B. Jindal, A.; V. Devarajan, P. Microemulsions and Nanoemulsions for Targeted Drug Delivery to the Brain. Curr. Nanosci. 2011, 7, 119–133.
2. Huynh, G. H.; Deen, D. F.; Szoka, F. C. Barriers to Carrier Mediated Drug and Gene Delivery to Brain Tumors. 2006, 110, 236–259.
3. Bhaskar, S.; Tian, F.; Stoeger, T.; Kreyling, W.; de la Fuente, J. M.; Grazú, V.; Borm, P.; Estrada, G.; Ntziachristos, V.; Razansky, D. Multifunctional Nanocarriers for Diagnostics, Drug Delivery and Targeted Treatment Across Blood-Brain Barrier: Perspectives on Tracking and Neuroimaging. Part. Fibre Toxicol. 2010, 7, 3.
4. Brasnjevic, I.; Steinbusch, H. W. M.; Schmitz, C.; Martinez-Martinez, P. Delivery of Peptide and Protein Drugs over the Blood-Brain Barrier. Prog. Neurobiol. 2009, 87, 212–51
5. Chen, Y.; Liu, L. Modern Methods for Delivery of Drugs Across the Blood–brain Barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665.
6. Misra, A.; Ganesh, S.; Shahiwala, A.; Shah, S. P. Drug Delivery to the Central Nervous System: a Review. J. Pharm. Pharm. Sci. 2003, 6, 252–73.
7. Bhagwat, R. R, I.S. Vaidhya Novel Drug Delivery Systems: An Overview International Journal of Pharmaceutical Sciences and Research,2013, 4, 970–982.
8. Ningaraj, N. S. Drug delivery to brain tumours:challenges and progress Expert Opin. Drug Deliv. 2006, 499–509.
9. Prabha, K. S.; Muthu, P. A Review?: Nanoparticles As Specified Carriers In Targeted Brain Drug Delivery System.American journal of pharmaceutical sciences 2011, 1.
10. Reddy, A. R. N. Reddy et Al., solid lipid nanoparticles: an advanced drug delivery system ;international Journal of Pharmaceutical Sciences and Research,. 2013, 4, 161–171
11. Giulia bonacucina, marco cespi, monica misici-falzi, giovanni f. Palmieri, Journal Of Pharmaceutical Sciences, VOL. 98, NO. 1,2009
12. Jung Kwon, Ray Drumright,Daniel J. Siegwart,Krzysztof Matyjaszwski,Progress in Polymer Science,vol 33, Issue 4, April 2008.448-477.
13. Wong, H. L.; Wu, X. Y.; Bendayan, R. Nanotechnological Advances for the Delivery of CNS Therapeutics. Adv. Drug Deliv. Rev. 2012, 64, 686–700
14. Banks, W. a. Drug Delivery to the Brain in Alzheimer’s Disease: Consideration of the Blood-Brain Barrier. Adv. Drug Deliv. Rev. 2012, 64, 629–39
15. Pisal, D. S.; Kosloski, M. P.; Balu-iyer, S. V. Delivery of Therapeutic Proteins, Journal of Pharmaceuticall Sciences2010, 99, 2557–2575
16. Angela R. Jones Eric V. Shusta, Blood-Brain Barrier Transport of Therapeutics via eceptor-Mediation, NIH Public Access. 2009, 24, 1759–1771
REFERENCE ID: PHARMATUTOR-ART-2200
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 7 Received On: 05/05/2014; Accepted On: 11/05/2014; Published On: 01/07/2014How to cite this article: SV Sonawane; Brain as a Sancturian Site in Drug Delivery Approaches to Improve Bioavailability in Brain; PharmaTutor; 2014; 2(7); 63-82 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









