ABOUT AUTHORS:
Sanjay Singh Bhandari *, Mahaveer Prasad Kabra, Raman Gupta, Ami Sharma
Kota College of Pharmacy, SP-1, RIICO Industrial Area, Ranpur,
Jhalawar road, Kota, Rajasthan, India – 324009
*bhandarisanjay001@gmail.com
ABSTRACT:
Damage to cells caused by free radicals is believed play a central role in the aging process and in disease progression. Antioxidants are our first line of defense against free radical damage, and are critical for maintaining optimum health and well being. The need for antioxidants becomes even more critical with increased exposure to free radicals. Pollution, cigarette smoke, drugs, illness, stress, and even exercise can increase free radical exposure. Because so many factors can contribute to oxidative stress, individual assessment of susceptibility becomes important. Many experts believe that the Recommended Dietary Allowance (RDA) for specific antioxidants may be inadequate and, in some instances, the need may be several times the RDA. As part of a healthy lifestyle and a well-balanced, wholesome diet, antioxidant supplementation is now being recognized as an important means of improving free radical protection.
REFERENCE ID: PHARMATUTOR-ART-1762
INTRODUCTION:
Definition:
Antioxidants are chemical compounds that can bind to free oxygen radicals preventing these radicals from damaging healthy cells.
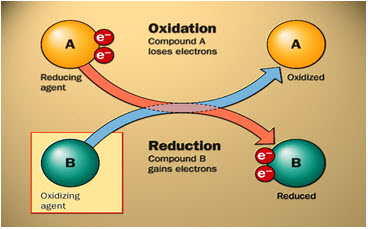
A) Oxidizers:
Substances that have the ability to oxidize other substances are said to be oxidative and are known as oxidizing agents, oxidants, or oxidizers. Put another way, the oxidant removes electrons from another substance, and is thus itself reduced. And, because it "accepts" electrons, it is also called an electron acceptor.
Oxidants are usually chemical substances with elements in high oxidation numbers (e.g., H2O2, MnO−4, CrO3, Cr2O2−7, OsO4) or highly electronegative substances that can gain one or two extra electrons by oxidizing a substance (O, F, Cl, Br).(1)
B) Reducers:
Substances that have the ability to reduce other substances are said to be reductive and are known as reducing agents, reductants, or reducers. In other words, the reductant transfers electrons to another substance, and is thus itself oxidized. And, because it "donates" electrons it is also called an electron donor.
Reductants in chemistry are very diverse. Electropositive elemental metals, such as lithium, sodium, magnesium, iron, zinc, or aluminium, are good reducing agents. These metals donate or give away electrons readily. Hydride transfer reagents, such as NaBH4 and LiAlH4, are widely used in organic chemistry, primarily in the reduction of carbonyl compounds to alcohols. Another method of reduction involves the use of hydrogen gas (H2) with a palladium, platinum, or nickel catalyst. These catalytic reductions are primarily used in the reduction of carbon-carbon double or triple bonds.(2)
C) A brief look at chemical bonding:
To understand the way that free radicals and antioxidants interact, you must first understand a bit about cells and molecules. So here's a (very) brief refresher course in Physiology/Chemistry 101: The human body is composed of many different types of cells. Cells are composed of many different types of molecules. Molecules consist of one or more atoms of one or more elements joined by chemical bonds.
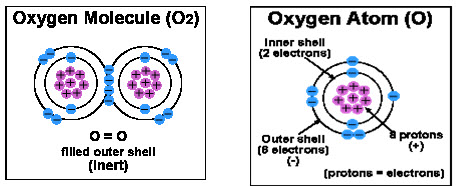
As you probably remember from your old high school days, atoms consist of a nucleus, neutrons, protons and electrons. The number of protons (positively charged particles) in the atoms nucleus determines the number of electrons (negatively charged particles) surrounding the atom. Electrons are involved in chemical reactions and are the substance that bonds atoms together to form molecules. Electrons surround, or "orbit" an atom in one or more shells. The innermost shell is full when it has two electrons. When the first shell is full, electrons begin to fill the second shell. When the second shell has eight electrons, it is full, and so on.
The most important structural feature of an atom for determining its chemical behavior is the number of electrons in its outer shell. A substance that has a full outer shell tends not to enter in chemical reactions (an inert substance). Because atoms seek to reach a state of maximum stability, an atom will try to fill its outer shell by:
Gaining or losing electrons to either fill or empty its outer shell Sharing its electrons by bonding together with other atoms in order to complete its outer shell Atoms often complete their outer shells by sharing electrons with other atoms. By sharing electrons, the atoms are bound together and satisfy the conditions of maximum stability for the molecule.
D) How free radicals are formed:
Normally, bonds don’t split in a way that leaves a molecule with an odd, unpaired electron. But when weak bonds split, free radicals are formed. Free radicals are very unstable and react quickly with other compounds, trying to capture the needed electron to gain stability. Generally, free radicals attack the nearest stable molecule, "stealing" its electron. When the "attacked" molecule loses its electron, it becomes a free radical itself, beginning a chain reaction. Once the process is started, it can cascade, finally resulting in the disruption of a living cell.
Some free radicals arise normally during metabolism. Sometimes the body’s immune system’s cells purposefully create them to neutralize viruses and bacteria. However, environmental factors such as pollution, radiation, cigarette smoke and herbicides can also spawn free radicals. Normally, the body can handle free radicals, but if antioxidants are unavailable, or if the free-radical production becomes excessive, damage can occur. Of particular importance is that free radical damage accumulate.(3, 4)
E) How antioxidants may prevent against free radical damage:
The vitamins C and E are thought to protect the body against the destructive effects of free radicals. Antioxidants neutralize free radicals by donating one of their own electrons, ending the electron-"stealing" reaction. The antioxidant nutrients themselves don’t become free radicals by donating an electron because they are stable in either form they act as scavengers, helping to prevent cell and tissue damage that could lead to cellular damage and disease.
Vitamin E: - The most abundant fat-soluble antioxidant in the body. One of the most efficient chain-breaking antioxidants available. Primary defender against oxidation. Primary defender against lipid per oxidation (creation of unstable molecules containing more oxygen than is usual).
Vitamin C: - The most abundant water-soluble antioxidant in the body. Acts primarily in cellular fluid. Of particular note in combating free-radical formation caused by pollution and cigarette smoke. Also helps return vitamin E to its active form.
Oxidants occur naturally as part of the normal body process; however harmful oxidants or free radicals, which are forms of oxygen, can cause damage to body cells. If free radicals are not neutralized they can work against the immune system and develop degenerative diseases.(5, 6)
High levels of oxidants are contained in substances such Drugs-
The desire to take medicine is perhaps the greatest failure which distinguishes man from animals.
Chemicals contained in food i.e.:-
Antibiotics- Used to supplement animal feeds as animal growth promoters
Pesticides, fungicides, herbicides and chemical fertilizers(e.g. nitrates) -Used on fruit and vegetables, some fruit and vegetables are sprayed up to seven times before harvesting Emulsifiers, flower treatment agents, mould growth retardants.
Preservatives and improvers- Used as additives in bread
Colorants preservatives and artificial sweeteners –Used in processed canned food and drinks e.g. Aspartame
Salt–This added either as salt, or in the form of sodium, to many processed foods
Chemicals in food packaging –Chemicals which can leach into food are added to plastic to make them soft and flexible and fire retardant. e.g. Bisphenol
A. They are also found in plastic seals inside metal twist–on, twist–off lids e.g. Semicarbazide
Chemicals in the air - Industrial pollution or exhaust fumes which can contain Toluene, a toxic chemical.
Chemicals in water– Chlorine, fluoride and ethyl oestradiol a synthetic oestrogen used in the formulation of many contraceptive pills. Some chemicals mimic the oestrogen in the body and interfere with systems that regulate the body’s oestrogen and other sex linked hormones.
Alcohol, cigarette smoke, caffeine chemicals in solvents glues inks and paint and fingernail polish – such as toluene.Detergents, cleaners, polishes, fresh air sprays and laundry products– Some contain toxic chemicals such as xenoestrogens. Paper products – Chlorine is used to bleach paper.
Frightening
Antioxidants counteract the effects of oxidants and control the build up of free radicals. The main antioxidants are, beta-carotene converted by the body into vitamin A, (beta-carotene and its related carotenoids form a vital part of the body's protection system, one of their rolls is to protecting individual cells) vitamins, C and E., and minerals such as Zinc and Selenium. These are found in sources such as red, orange and yellow vegetables, fruit and lentils. To reduce the risk of toxins buy organic fruit and vegetables and avoid purchasing products that contain chemicals wherever possible.
Oxidation
Oxidation is a chemical reaction that transfers electrons from a substance to an oxidizing agent. In the body, oxidation reactions can produce free radicals, (highly reactive unpaired electrons), which start chain reactions that damage cells. Antioxidants terminate these chain reactions by removing free radical intermediates, and inhibit other oxidation reactions by being oxidized themselves. As a result, antioxidants are often reducing agents such as thiols or polyphenols.
Although oxidation reactions are crucial for life, they can also be damaging; hence, plants and animals maintain complex systems of multiple types of antioxidants, such as glutathione, vitamin C, and vitamin E as well as enzymes such as catalase, superoxide dismutase and various peroxidases. Low levels of antioxidants, or inhibition of the antioxidant enzymes, causes oxidative stress and may damage or kill cells.
These oxidants can damage cells by starting chemical chain reactions such as lipid peroxidation, or by oxidizing DNA or proteins. Damage to DNA can cause mutations and possibly cancer, if
not reversed by DNA repair mechanisms, while damage to proteins causes enzyme inhibition, denaturation and protein Degradation. Consequently, organisms contain a complex network of antioxidant metabolites and enzymes that work together to prevent oxidative damage to cellular components such as DNA, proteins and lipids. In general, antioxidant systems either prevent these reactive species from being formed, or remove them before they can damage vital components of the cell. Antioxidants can cancel out the cell-damaging effects of free radicals. Furthermore, people who eat fruits and vegetables, which are good sources of antioxidants, have a lower risk of heart disease and some neurological diseases, and there is evidence that some types of vegetables, and fruits in general, probably protect against a number of cancers. These observations suggest that antioxidants might help prevent these conditions. There is also evidence that antioxidants might help prevent diseases such as macular degeneration, suppressed immunity due to poor nutrition, and neurodegeneration. The brain is uniquely vulnerable to oxidative injury, due to its high metabolic rate and elevated levels of polyunsaturated lipids, the target of lipid peroxidation. Antioxidants prevent oxidative stress in neurons and prevent apoptosis and neurological damage.(3, 7, 8)
ANTIOXIDANT
An antioxidant is a molecule capable of slowing or preventing the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons from a substance to an oxidizing agent. Oxidation reactions can produce free radicals, which start chain reactions that damage cells. Antioxidants terminate these chain reactions by removing free radical intermediates, and inhibitother oxidation reactions by being oxidized themselves. As a result, antioxidants are often reducing agents such as thiols, ascorbic acid or polyphenols.
Although oxidation reactions are crucial for life, they can also be damaging; hence, plants and animals maintain complex systems of multiple types of antioxidants, such as glutathione, vitamin C, and vitamin E as well as enzymes such as catalase, superoxide dismutase and various peroxidases. Low levels of antioxidants, or inhibition of the antioxidant enzymes, causes oxidative stress and may damage or kill cells.
As oxidative stress might be an important part of many human diseases, the use of antioxidants in pharmacology is intensively studied, particularly as treatments for stroke and neurodegenerative diseases. However, it is unknown whether oxidative stress is the cause or the consequence of disease. Antioxidants are also widely used as ingredients in dietary supplements in the hope of maintaining health and preventing diseases such as cancer and coronary heart disease. Although initial studies suggested that antioxidant supplements might promote health, later large clinical trials did not detect any benefit and suggested instead that excess supplementation may be harmful. In addition to these uses of natural antioxidants in medicine, these compounds have many industrial uses, such as preservatives in food and cosmetics and preventing the degradation of rubber and gasoline.(9, 10)
THE OXIDATIVE CHALLENGE IN BIOLOGY:
A) Metabolites:
A paradox in metabolism is that while the vast majority of complex life on Earth requires oxygen for its existence, oxygen is a highly reactive molecule that damages living organisms by producing reactive oxygen species. Consequently, organisms contain a complex network of antioxidant metabolites and enzymes that work together to prevent oxidative damage to cellular components such as DNA, proteins and lipids. In general, antioxidant systems either prevent these reactive species from being formed, or remove them before they can damage vital components of the cell. However, since reactive oxygen species do have useful functions in cells, such as redox signaling, the function of antioxidant systems is not to remove oxidants entirely, but instead to keep them at an optimum level.
The reactive oxygen species produced in cells include hydrogen peroxide (H2O2), hypochlorous acid (HOCl), and free radicals such as the hydroxyl radical (·OH) and the superoxide anion (O2−). The hydroxyl radical is particularly unstable and will react rapidly and non-specifically with most biological molecules. This species is produced from hydrogen peroxide in metal-catalyzed redox reactions such as the Fenton reaction. These oxidants can damage cells by starting chemical chain reactions such as lipid peroxidation, or by oxidizing DNA or proteins. Damage to DNA can cause mutations and possibly cancer, if not reversed by DNA repair mechanisms, while damage to proteins causes enzyme inhibition, denaturation and protein degradation. (11, 12)
The use of oxygen as part of the process for generating metabolic energy produces reactive oxygen species. In this process, the superoxide anion is produced as a by-product of several steps in the electron transport chain. Particularly important is the reduction of coenzyme Q in complex III, since a highly reactive free radical is formed as an intermediate (Q·−). This unstable intermediate can lead to electron "leakage", when electrons jump directly to oxygen and form the superoxide anion, instead of moving through the normal series of well-controlled reactions of the electron transport chain. Peroxide is also produced from the oxidation of reduced flavoproteins, such as complex I. However, although these enzymes can produce oxidants, the relative importance of the electron transfer chain to other processes that generate peroxide is unclear. In plants, algae, and cyanobacteria, reactive oxygen species are also produced during photosynthesis, particularly under conditions of high light intensity. This effect is partly offset by the involvement of carotenoids in photo inhibition, which involves these antioxidants reacting with over-reduced forms of the photosynthetic reaction centers to prevent the production of reactive oxygen species.
B) Ascorbic acid:
Ascorbic acid or "vitamin C" is a monosaccharide antioxidant found in both animals and plants. As one of the enzymes needed to make ascorbic acid has been lost by mutation during human evolution, it must be obtained from the diet and is a vitamin. Most other animals are able to produce this compound in their bodies and do not require it in their diets.(14) In cells, it is maintained in its reduced form by reaction with glutathione, which can be catalyzed by protein disulfide isomerase and glutaredoxins. Ascorbic acid is a reducing agent and can reduce, and thereby neutralize, reactive oxygen species such as hydrogen peroxide. In addition to its direct antioxidant effects, ascorbic acid is also a substrate for the antioxidant enzyme ascorbate peroxidase, a function that is particularly important in stress resistance in plants. Ascorbic acid is present at high levels in all parts of plants and can reach concentrations of 20 millimolars in chloroplasts.
C) Glutathione:
Glutathione is a cysteine-containing peptide found in most forms of aerobic life It is not required in the diet and is instead synthesized in cells from its constituent amino acids. Glutathione has antioxidant properties since the thiol group in its cysteine moiety is a reducing agent and can be reversibly oxidized and reduced. In cells, glutathione is maintained in the reduced form by the enzyme glutathione reductase and in turn reduces other metabolites and enzyme systems, such as ascorbate in the glutathione-ascorbate cycle, glutathione peroxidases and glutaredoxins, as well as reacting directly with oxidants. Due to its high concentration and its central role in maintaining the cell's redox state, glutathione is one of the most important cellular antioxidants. In some organisms glutathione is replaced by other thiols, such as by mycothiol in the actinomycetes, or by trypanothione in the kinetoplastids.(13, 15)
D) Melatonin:
Melatonin is a powerful antioxidant that can easily cross cell membranes and the blood-brain barrier. Unlike other antioxidants, melatonin does not undergo redox cycling, which is the ability of a molecule to undergo repeated reduction and oxidation. Redox cycling may allow other antioxidants (such as vitamin C) to act as pro-oxidants and promote free radical formation. Melatonin, once oxidized, cannot be reduced to its former state because it forms several stable end-products upon reacting with free radicals. Therefore, it has been referred to as a terminal (or suicidal) antioxidant.
E) Tocopherols and tocotrienols (vitamin E)
Vitamin E is the collective name for a set of eight related tocopherols and tocotrienols, which are fat-soluble vitamins with antioxidant properties. Of these, α-tocopherol has been most studied as it has the highest bioavailability, with the body preferentially absorbing and metabolizing this form. It has been claimed that the α-tocopherol form is the most important lipid-soluble antioxidant, and that it protects membranes from oxidation by reacting with lipid radicals produced in the lipid peroxidation chain reaction.(16, 17) This removes the free radical intermediates and prevents the propagation reaction from continuing. This reaction produces oxidized α-tocopheroxyl radicals that can be recycled back to the active reduced form through reduction by other antioxidants, such as ascorbate, retinol or ubiquinol. This is in line with findings showing that α-tocopherol, but not water-soluble antioxidants, efficiently protects glutathione peroxidase 4 (GPX4)- deficient cells from cell death.GPx4 is the only known enzyme that efficiently reduces lipid-hydroperoxides within biological membranes.(13, 18, 19)
However, the roles and importance of the various forms of vitamin E are presently unclear, and it has even been suggested that the most important function of α-tocopherol is as a signaling molecule, with this molecule having no significant role in antioxidant metabolism. The functions of the other forms of vitamin E are even less well-understood, γ-tocopherol is a nucleophile that may react with electrophilic mutagens, and tocotrienols may be important in protecting neurons from damage.(20)
CONCLUSION:
In brief antioxidants destroy free radicals in the body. Free radicals are byproducts of oxygen metabolism that can damage cells and are among the causes of many degenerative diseases, especially diseases associated with aging. They are also associated with the aging process itself. As a person ages, cell damage accumulates and supplementing the diet with extra antioxidant-rich can help slow the oxidative damage done to cells. Scientific studies validate the role of antioxidants in preventing many diseases. Although studies have shown lower rates of cancer and heart disease in people who eat a recommended amount of fruits and vegetables, recent clinical studies have shown that supplementation of diet with antioxidant vitamin therapy does not lower risk of cardiovascular or certain other diseases.(7, 13, 21)
Antioxidants are chemical compounds that can bind to free oxygen radicals preventing these radicals from damaging healthy cells. Antioxidant is a chemical that reduces the rate of particular oxidation reactions in a specific context, where oxidation reactions are chemical reactions that involve the transfer of electrons from a substance to an oxidizing agent.
Antioxidants are particularly important in the context f organic chemistry and biology. All contain complex system of antioxidant chemicals and enzymes to prevent chemical damage to the cells components by oxidation.Priliminary studies have suggested that antioxidants are useful in a number of ways in regards to cancer. For instance they may improve the effectiveness of chemotherapy, decrease side-effects of chemotherapy and radiotherapy and prevent some types of cancer.(22, 23)
Cell membrane is a rich source of PUFA (Poly-unsaturated fatty acids), which are readily attacked by oxidizing agent, a process that is called lipid peroxidation. The breakdown of lipid hydro-peroxides often involves transition metal ion catalysis. Protein and nucleic acids seems to be less susceptible then PUFA’s to free radicals, there seems to be less possibility in the formation of rapidly processing chain reactions. Oxidizing radicals readily attack DNA; it is therefore a vulnerable and these reactions cause several diseases.(5, 24)
REFERENCES:
1.Badami S., Prakash, Dongre Sh, Suresh B., In Vitro antioxidant property of Solanum pseudo capsicum leaf extract. Indian Journal of Pharmacology, 2005, 34(4)’251-252.
2.Balu M., Paneerselvam C., Age associated macromolecular damages in central nervous system of aged rats had been determined: Role of grape seed extract, Chemistry Biology Interface, Synergistic New Frontiers, NewDelhi, November 21-26, 2004, 23-25.
3.Bangalore D.V., McGreen W., Effect of beta-cyclodextrin in improving the correlation between Lycopene concentration and ORAC value, Jornal of Agricultural & food Chemistry, 2005, 53(6), 1878-1883.
4.Bao J., Cai Y.,Wang G., Anthocyanins, flavanols, and free radical scavenging, activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability, Journal of Agricultural & food Chemistry, 2005, 53(6), 2327-2332.
5.Bauziz M.Grarey R.J.,Damak M.,Identification and antioxidant potensial of flavanoids and low molecular weight in olive cultivure chaemlali growing in Tunisia,Journal of Agricultural & Food Chemistry, 2005, 53(2), 236-55.
6.Biondi D.M.,Rocco C.,Ruberto G.,New dihydrostilbene derivatives from the leaves of Glycyrrhiza glabra and evaluation of their antioxidant activity,Indian Journal of Natural Products, 2003,66(4), 477-480.
7.Calliste C.A., Allais D.p., Duroux J.L., Castania sativa mill.Leaves reported as new sources of natural antioxidant: an electronic spin resonance study, Journal of Agricultural & food Chemistry, 2005, 53(2), 282-288.
8.Cerda B., Espin J.C., Metabolism of antioxidants and chemopreventive ellagitanins from strawberries, respberries, walnuts, and oak-aged wine in humans had been reported and Identification of biomarkers and individual variability, Journal of Medical & food Chemistry, 2005, 68(8), 1293.
9.Gagera H.P., Patel S.V.,Antioxidant properties of some therapeutically medicinal plants,Journal of Medicinal & Aromatic Plants, 2005, 27(1), 91-100. Gonzalex-Paramas A.M., Santos-Bullga c., Flavanol content and antioxidant activity in winery byproducts, Journal of Agricultural & food Chemistry, 2004, 52(2), 234-238.
10.Hiranpaich V.,Utaipat A.,Sato H.,Anti-oxidant effect of aques extracts from dried clays of Hibiscus sabdariffa Lin (Roselle) in vitro using rat low-intensity lipo-protein (LDL),Biological & Pharmaceutical Bulletin,2005,28(3),481-484.
11.Ka M.H., Choi E.H.,Chun H.S.,Lee K.G., Antioxidative activity of votalile extracts isolated from Angelica tenuissimae roots,peppermint leaves, pine needles and and sweet flag leaves, Journal of Agricultural & food Chemistry, 2005, 53(10), 4124-4129.
12.Kim J.P., Chon I.J., Cho H.K., The antioxidant and the antidiabetic effects of ethanol extracts from biofunctional fod prescriptions, Korean Journal of Pharmacognosy, 2004, 31(1), 98-103.
13.Kouam S.F., Krohn K.,Ajaz A., Chaudhary M.I.,Prenylated anthronoid antioxidants from the stem bark of Harungana madagaskariensis,Phytochemistry,2005,66(10),1174-1179.
14.Kuti J.O., Kunuru H.B., Antioxidant capacity and phenolic content had been reported in leaf extracts of tree spinach (cnidoscolus spp.), Journal of Agricultural & food Chemistry, 2004, 52(1),117-121.
15.Lee J.H., Jeon W.J.,Yoo.E.S., The chemical constituents and their antioxidant activity of the stem of Rhododendron mucronalotum,Natural Product Science. 2005, 11(2), 97-102.
16.McCune L.M. Johns t., Symptom-specific antioxidant activity of Boreal Diabetes treatments, Pharmaceutical Biology, 2003, 41(5), 362-370.
17.Padmaja M.V., Mrumugal P., Santhiya S.T., Estimation of total antioxidant activity in different parts of Annona Squamosa Linn. Had been determined by ABTS/ K2S208 dercoloration method, Chemistry Biology Interface, Synergistic New Frontiers, New Delhi, November 21-26, 2004, 23-42.
18.Padmaja S., Raju T.N., Antioxidant effect of curcumin in selenium induced cataracts of Wistar rats, Indian Journal Exp.Biology, 2004, 42(6), 601-603.
19.Prior R.L. Wu X., Standardized method for determination of antioxidant capacity and phenolies in foods and dietry suppliments, Journal of Agriultural & food Chemistry, 2005, 53(10), 4290-4302.
20.Ratansooriya W.D., Deraniyagala S.A., Hattiarachchi H.D.I., Leaf extract of crinumbul bispermum has antinociceptive activity had been carried out in rats, Journal of Ethnopharmacology 2005, 98(1-2), 195-200.
21.Ricci D.,Fraternale D.,Burini G.,Curini M., chemical composition antimicrobial and antioxidant activity of the essential oils of Teucrium marum Lamiaceae,journal of Ethanopharmacology, 2005,98(1-2),195-200.
22.Sabu M.C., Kuttan R., Antioxidant activity of Indian herbal drugs was determined in rats with aloxan induced diabetes, Pharmaceutical Biology, 2003, 41(7), 500-505.
23.Shriwaikar A., Rajendran K., Dinesh Kumar C., in vitro antioxidant studies of Annona squamosa Linn. Leaves, Indian Journal Exp. Biology, 2004, 42(8), 803-807.
24.Siddiqui M.R., Taha A.,Moorthy K.,Hussain M.E.,Bashir S.F., & Baquer N.Z., Journal BioSci.,2005,30(4),483.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









