{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Tabassum I. Hangad *, Rani S. Potawale
Department of Pharmaceutical Chemistry,
M. C. E. Society's Allana College of Pharmacy, Azam Campus ,Camp, Pune 411001.
ABSTRACT
Ivabradine is a specific heart rate lowering agent, acting by reducing the rate of pacemaker
activity in the sinoatrial node. Ivabradine is a novel heart rate lowering medicine for the symptomatic management of stable angina pectoralis and symptomatic chronic heart failure. In multicenter clinical trials, it has been proved that Ivabradine is superior to beta-blocking agents during complex therapy of chronic heart failure accompanied with its beneficial effects related to cardiac remodeling, improvement of the currency of heart failure and diminution of patients rehospitalisation. It is suggested that Ivabradine as a newer agent is a valuable perspective drug for the treatment of congestive heart failure. This review is useful for the future study for researcher involved in formulation development and quality control of Ivabradine.
This review article represents the various analytical methods which have been reported for estimation of Ivabradine in pharmaceutical dosage form. The spectrophotometric techniques and Q-absorbance ratio method were reported by the various authors. Many researchers also worked in chromatographic areas like Thin layer chromatography, High performance liquid chromatography, and High performance thin layer chromatography. Ivabradine is also studied by various hyphenated techniques .We reviewed and reported almost all analytical methods with more emphasis on chromatographic mrthodsfor Ivabradine.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2612
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 10 Received On: 01/08/2018; Accepted On: 28/08/2018; Published On: 01/10/2018 How to cite this article: Potawale, R. and Hangad, T. 2018. A Review on Analytical Methods for Ivabradine determination in Pharmaceutical Dosage Forms. PharmaTutor. 6, 10 (Oct. 2018), 26-30. DOI:https://doi.org/10.29161/PT.v6.i10.2018.26 |
INTRODUCTION
Ivabradine is a specific heart rate lowering agent, acting by reducing the rate of pacemaker activity in the sinoatrial node. Chemically 3-[3-({[(7S)-3,4-dimethoxybicyclo[4.2.0]octa-1, 3, 5-trien-7yl] methyl} methylamino) propyl]-1, 3, 4, 5-tetrahydro-7,8-dimethoxy-2H-3-benzazepin -2-One and available in the form of hydrochloride salt Ivabradine is the new class of drugs that have the ability of slowing the depolarization slope, reducing heart rate and show the activity similar to that of β-blockers. Ivabradine is freely soluble in water, methanol, and acetonitrile. The drug is a basic drug and having pKa values≈ 8.02. (O’Neil MJ., 2006, Sulfi et al., 2006 , Seerapu et al., 2010).
An increase in heart rate is a common occurrence in cardiac pathophysiology, particularly in heart failure, mediated by β-adrenergic receptors (βARs) following activation of the sympathetic nervous system. Although an elevated heart rate may initially compensate for insufficient cardiac output, sustained tachycardia usually leads to adverse haemodynamic consequences. Ivabradine is a novel pharmacological agent specifically inhibiting the hyperpolarization-activated pacemaker If–current (If) that underlies the rate of spontaneous diastolic depolarization in sinoatrial pacemaker cells. This drug is introduced in medical practice in the last decade is a pure heart rate-slowing agent. A large number of studies in patients with cardiovascular disease have demonstrated that heart rate is a very important and major independent risk factor for prognosis, because lowering of heart rate reduces cardiac work and diminished myocardial oxygen requirement. It was shown that ivabradine a selective inhibitor of the hyperpolarisation activated sodium channel (If) is involved in pacemaker generation and responsiveness of the sino-atrial node resulting in the heart rate reduction without negative inotropic action. Ivabradine in chronic heart failure improves diastolic function and attenuates cardiac tissue hypoxia. Long-term reduction of heart rate induced by Ivabradine reduced remodeling and preserved nitric oxide (NO) bioavailability, resulting from processes triggered early after reduction of heart rate. (Sulfi et al., 2006).
Structure of Ivabradine

Fig.1 Structure of Ivabradine
Mechanism of action-
Ivabradine blocks the hyperpolarization-activated cyclic nucleotide-gated (HCN) channel, and inhibit the If–current (If) which reduces the cardiac pacemaker activity of the sinus node which decreases heart rate without an effect on ventricular repolarization or myocardial contractility. Ivabradine is recommended for angina pectoralis and symptomatic chronic heart failure. Ivabradine inhibit the "funny" channel pacemaker current (If) in the sinoatrial node in a dose-dependent fashion, resulting in a lower heart rate and thus more blood to flow to the myocardium. When we use calcium channel blockers and beta blockers for lowering heart rate, they are good in actiot but show adverse effcts due to their negative ionotropic effects. Therefore, Ivabradine is designed as a "pure" heart rate-lowering drug. It causes no serious adverse effects. (Corlanor® is a first-in-class, HCN channel blocker that lowers heart rate).
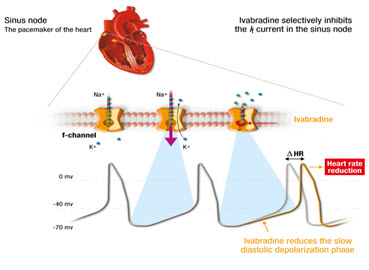
Fig.2 Mechanism of Ivabradine
Analytical methods
Chromatographic method
Table no. 1 Summary of chromatographic methods for Ivabradine
|
Sr.no |
Title |
Method |
Mobile phase |
Stationary Phase |
Wavelength |
Ref |
|
1 |
Development and validation of RP-HPLC method for the estimation of Ivabradine hydrochloride in tablets. |
RP-HPLC |
Methanol:25mm phosphate Buffer (60:40 v/v), adjusted to pH 6.5 with orthophosphoric acid |
SS Wakosil C18AR, 250×4.6 mm, 5 μm column |
285 nm |
Seerapu et al., 2010 |
|
2 |
Development and validation of stability-indicating HPTLC method for Ivabradine HCL.
|
HPTLC |
Chloroform: Methanol (1:1 v/v)
|
Aluminum Plate precoated with Silica Gel 60 F254 |
286 nm |
Damle et al., 2012
|
|
3 |
Development and validation of RP-HPLC method for analysis of Ivabradine hydrochloride in tablet dosage forms. |
RP-HPLC |
Buffer (pH-7.3), methanol and acetonitrile (55:15:30 v/v) |
C18 column (VP-ODS, 150 x 4.6 mm, 5 μm) |
----- |
Rahman et al., 2012 |
|
4 |
Chromatographic analysis of ivabradine on polar, nonpolar and chemically modified adsorbents by HPTLC.
|
HPTLC |
Aqueous (methanol–water and acetonitrile–water) and non-aqueous (methanol–acetonitrile and methanol–dimethyl sulfoxide) |
High-performance TLC plates |
----- |
Piotr P et al., 2013 |
|
5 |
Determination of the related substances in Ivabradine hydrochloride and its tablets by HPLC.
|
HPLC |
n-hexane and isopropanol (0.1% triethylamine) (60∶40) |
Chiral hplc column |
286 nm |
Xiaoli Y et al ., 2011
|
|
6 |
Development of validated RP-HPLC method for simultaneous estimation of Carvedilol and Ivabradine. |
RP- HPLC |
Acetonitrile: Phosphate Buffer pH 3 adjusted with o-Phosphoric acid (75:25) |
Hypersil ODS C18 in isocratic mode |
275 nm. |
Patel H et al .,2015 |
|
7 |
Quantitative determination and validation of Ivabradine HCL by stability indicating RP-HPLC method and spectrophotometric method in solid dosage form. |
RP-HPLC |
0.5% Formic Acid (pH=7.0): Acetonitrile (65: 35 v/v) |
Inertsil ODS-3V [250 mm × 4.6mm] 5m column |
286 nm |
Maheshwaria S et al., 2010 |
|
8 |
Determination of Ivabradine hydrochloride in the human plasma and the bioequivalence study by LC-MS/MS.
|
LC-MS/MS |
methanol: water ( containing 5 mol·L-1ammonium formate and 0. 1% formic acid) |
METHODS C18column (150 mm × 4. 6 mm, 3. 5 μm) |
---- |
Yan-yan et al ., 2014 |
|
9 |
Validated stability-indicating high performance thin layer chromatographic method for determination of Ivabradine hydrochloride in bulk and marketed formulation: An application to kinetic study. |
HPTLC |
Ethyl acetate: 0.389 M ammonium acetate in methanol (1:5, v/v) as solvent system
|
Precoated silica gel 60 F254 aluminuim plates (10×10 cm 100 um thickness) |
287 nm |
Motisariya M.H.,2013 |
|
10 |
Validated stability indicating chromatographic methods for determination of Ivabradine Hydrochloride in the presence of its acidic degradation Product.
|
TLC
HPLC |
Methanol: chloroform: water (8:1:1 v/v)
Methanol: acetonitrile: phosphate buffer pH 3 (50:40:10 v/v) |
Aluminium sheet of silica gel 60 F254
C18 column |
286 nm
230nm |
Nadia M. M et al.,2016 |
|
11 |
Characterization of degradation products of Ivabradine by LC-HR-MS/MS: a typical case of exhibition of different degradation behavior in HCl and H2SO4 acid hydrolysis. |
LC-HR-MS/MS |
Ammonium Formate (10 mm, pH 3.0) and acetonitrile |
Phenomenex Luna C18 (250 × 4.6 mm, 5.0 µm) column |
286 nm |
Patel et al., 2015 |
|
12 |
Simultaneous determination of ivabradine and its metabolites in human plasma by liquid chromatography--tandem mass spectrometry. |
Tandem Mass Spectrometry |
Liquid chromatography--tandem mass spectrometry
|
C18 column |
---- |
MaryseFrançois-Bouchard et al.,2000 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
UV Spectroscopic Method
First order derivative spectroscopy and Area Under the curve spectroscopic technique was developed for simultaneous determination of Ivabradine was developed
Table no 2. Summary of Spectroscopic methods for Ivabradine
|
Sr.No |
Title |
Method |
Wavelength |
Linearity (ug/ml) and R2
|
Recovery
|
Ref |
|
1 |
Rapid and selective UV spectrophotometric and RP-HPLC methods for dissolution studies of Ivabradine controlled release formulations. |
UV |
286 nm |
5-60 μg/ml 0.9998 |
----- |
Panda et al., 2014 |
|
2 |
Q-absorbance ratio spectrophotometric method for simultaneous determination of atenolol and ivabradine hydrochloride in synthetic mixture |
Q-absorbance ratio |
286.04 nm and 276 nm |
2-10 μg/ml 0.998 |
100.47 ± 0.348
|
Patil et al., 2016 |
DISCUSSION
The presented systematic review covers the current analytical methods for the determination of Ivabradine and its combination in pharmaceutical and biological samples like serum and plasma. HPLC method were found to be most widely use for Ivabradine. Various chromatographic conditions are presented in table.
CONCLUSION
The sensitivity, specificity, and better separation efficiency enable HPLC to be used frequently for simultaneous qualitative and quantitative determination of Ivabradine. The presented information is useful for the future study for researcher involved in formulation development and quality control of Ivabradine.
REFERENCES
1. Corlanor® is a first-in-class, HCN channel blocker that lowers heart rate1, 2.
2. Damle M. C , Bagwe R. A.(2012); “ Development And Validation Of Stability-Indicating Hptlc Method For Ivabradine Hcl” International journal of Pharmaceutical Invention June; 43-52.
3. Difrancesco D, Camm JA;(2004); “Heart rate lowering by specific and selective I(f) current inhibition with ivabradine Drugs”; 64(16);1757-65.
4. http://www.chemspider.com/Chemical-Structure.117373.html (accesed 7 July 2018)
5. https://en.wikipedia.org/wiki/Ivabradine (accesed 7 July 2018)
6. Maheshwaria S., Khandharb A., Jaina A (2010); “ Quantitative Determination and Validation of Ivabradine HCL by Stability Indicating RP-HPLC Method and Spectrophotometric Method in Solid Dosage Form”; Eurasian Journal of Analytical Chemistry ; 5 (1); 53-62.
7. MaryseFrançois-Bouchard, GillesSimonin, Marie-JeanneBossant, ClaireBoursier-Neyret(2000) “Simultaneous determination of ivabradine and its metabolites in human plasma by liquid chromatography--tandem mass spectrometry”; Journal of Chromatography B: Biomedical Sciences and Applications; 745(2); 261-9
8. Motisariya M.H. ,GovindbhaiPatel K.,ShahP.A.(2013): “Validated stability-indicating high performance thin layer chromatographic method for determination of Ivabradine hydrochloride in bulk and marketed formulation”; An application to kinetic study .Bulletin of Faculty of Pharmacy ,Cairo university; 51(2) ; 233-241.
9. Nadia M. M, Fayez Y. M., Farid J., Abd El-Aziz B. Abd El-Alim by Piotr Pikul*(2016) ;Joanna Nowakowska, Krzesimir Ciura : “Validated Stability Indicating Chromatographic Methods for Determination of Ivabradine Hydrochloride in the Presence of Its Acidic Degradation Product”; International Journal of Research and Reviews in Pharmacy and Applied sciences ; (1) ; 1370-1380.
10. O’Neil MJ. (2006);The Merck Index, an Encyclopedia of Chemicals, Drugs and Biologicals. 4th Ed. Whitehouse Station: Merck Research Laboratories, Division of Merck and Co., Inc.; 907.
11. Panda S., Patra S.(2014); “Rapid and Selective UV Spectrophotometric and RP-HPLC Methods for Dissolution Studies of Ivabradine Controlled release formulations”;PharmaTutor; 2 (8); 201-213.
12. Patel H., Jivani N.2(2015); “Development Of Validated Rp-Hplc Method For Simultaneous Estimation Of Carvedilol And Ivabradine”; World Journal of Pharmacy and Pharmaceutical Sciences ; 4(5) ; 630-639.
13. Patel P.N., Borkar R.M., Kalariya P.D., Gangwal R.P., Sangamwar A.T., Samanthula G, Ragampeta S;(2015); “ Characterization of degradation products of Ivabradine by LC-HR-MS/MS: a typical case of exhibition of different degradation behavior in HCl and H2SO4 acid hydrolysis”;Journal of Mass Spectrometry Feb 2015; 50 (2): 344-53.
14. Patil P, Hasumati A. Raj,Sonora G. (2016); “Q-absorbance ratio spectrophotometric method for simultaneous determination of atenolol and ivabradine hydrochloride in synthetic mixture”. Pharmaceutical and Biological Evaluations; 3(2) ; 224-230.
15. Piotr P., Nowakowska J., Krzesimir Ciura (2013); “Chromatographic analysis of ivabradine on polar, nonpolar and chemically modified adsorbents by HPTLC”Journal of Food and drug analysis; 21(2) ; 165-168
16. Rahman Md. Rezowanur, Asaduzzaman Md. , Ashraful Islam S. M.(2012); “Development and validation of RP-HPLC method for analysis of Ivabradine Hydrochloride in tablet dosage forms “Research Journal of Pharmaceutical, Biological and Chemical Sciences”; 3(3) ; 1032-1043.
17. Seerapu S , Srinivasan B. P. (2010); “Development and Validation of RP-HPLC Method for the Estimation of Ivabradine Hydrochloride in Tablets”.International journal of Pharmaceutical Sciences; 72 (5): 667–671.
18. Sulfi S..Timmis A.D.(2006); “Ivabradine – the first selective sinus node If channel inhibitor in the treatment of stable angina”;International Journal of Clinical Practice; 60(2);222-8.
19. Xiaoli Y., Hui L.,Cui L.(2011); “ Determination of the Related Substances in Ivabradine Hydrochloride and Its Tablets by HPLC”; Chinese Pharmaceutical Affairs.
20. Yan-yan, Cheng-tao LU,Song Y.,Ding L.,YANG J., Chen M., Xue-qin, SONG Wei,ZHOU Lun,FENG Zhi-jun,WEN Ai-dong(2014) ; “ Determination of Ivabradine hydrochloride in the human plasma and the bioequivalence study by LC-MS/MS JIA “; West China Journal of Pharmaceutical Sciences ;02.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









