{ DOWNLOAD AS PDF }
 About Authors:
About Authors:
*R. Natarajan., Abirami. M, , Pavan kumar J, G. Murugananthan
Department of Pharmaceutics & research
Swamy Vivekanandha College of pharmacy
Tamil Nadu, India
*svcpnatarajan@gmail.com
ABSTRACT
The work was carried out to formulate and evaluate the matrix transdermal patches of Lamivudine for the controlled delivery of drug in the body. The transdermal patches were prepared by the solvent casting method using Span-80 as a permeation enhancer and were prepared in different drug: polymer (Lamivudine: HPMC and Lamivudine: EC) ratios of 1:2.5, 1:5, without permeation enhancers and with permeation enhancers. The prepared transdermal patch were found to be good physicochemical properties, shows no skin irritation on the rat skin and subjected to in vitro drug permeation study by using rat skin in phosphate buffer pH 7.4 for 24 hours. The comparative statistical analytical data (ANOVA) showed ‘p’ value < 0.0001 which suggest that the prepared formulation are extremely significant for transdermal delivery. The formulation (FPE2) ratio 1:5 of (Lamivudine: HPMC) patch with permeation enhancer showed best result among all the formulation. This formulation (FPE2) was subjected to in-vivo studies by using rat. The in-vivo studies of the patches were administrated transdermally to rat skin and while a standard solution of 10μg/ml was used as a control, and collected the plasma drug sample at different time interval and analyzed by HPLC. The in-vitro in-vivo correlation studies shows the regression value (R) 0.863 and correlated and to be linear.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2506
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 5, Issue 7 Received On: 04/04/2017; Accepted On: 04/04/2017; Published On: 01/07/2017 How to cite this article: Natarajan R, Abirami M, , Pavankumar J, Murugananthan G;A novel transdermal patch of Lamivudine: Invitro-Invivo characterization; PharmaTutor; 2017; 5(7); 54-62 |
INTRODUTION
Transdermal drug delivery is the non-invasive delivery of medications from the surface of the skin through its layers, to the circulatory system. The current transdermal delivery system has evolved as a successful alternative to systemic drug delivery. Most transdermal patches are designed to release the active ingredient at a zero-order rate for a period of several hours to days following application to the skin.1
Acquired immune deficiency syndrome (AIDS), which threatens to cause a great plague in the present generation, was first identified in California in 1981.AIDS is considered to be an epidemic disease. The annual number of AIDS deaths can be expected to increase for many years to come, unless more effective and patient compliant anti-retroviral medications are available at affordable prices. The major drawbacks of anti-retroviral drugs for the treatment of AIDS are their adverse side effects during long-term therapy, poor patient compliance and huge cost of the therapy.2
Lamivudine (3TC), an anti-retro viral is commonly used in the treatment of HIV infected patients. Most of the anti-retro viral including Lamivudine are virustatic in nature and they must be administered for the life span of the patient. Despite being quite effective on oral administration, 3TC exhibits dose dependent toxic side effects. Side effects often require dosage reduction or even cessation of treatment, since conditions like lactic acidosis may even be fatal. The problems associated with oral administration of 3TC led us to explore the possibilities of designing novel drug delivery system for Lamivudine with an alternative route of administration. Novel drug delivery carriers such as transdermal patches are very versatile to suit the delivery of various drug molecules. Thus it was proposed that a non invasive zero-order delivery such as the transdermal route is desirable.3
MATERIALS AND METHODS
MATERIALS
Lamivudine were procured from Aurobindo Pharma Pvt Ltd., Hyderabad. Hydroxy propyl methyl cellulose E15LV, Ethyl cellulose, Span80, Chloroform was purchased from Loba Chemie Pvt Ltd., Mumbai. Glycerol was purchased from Spectrum reagents and chemicals Pvt Ltd., Cochin. Equipments used were Digital weighing balance, Dial calliper, pH meter, Orbitek shaker, Franz diffusion cell (60m
MATERIALS AND METHODS
MATERIALS
Lamivudine were procured from Aurobindo Pharma Pvt Ltd., Hyderabad. Hydroxy propyl methyl cellulose E15LV, Ethyl cellulose, Span80, Chloroform was purchased from Loba Chemie Pvt Ltd., Mumbai. Glycerol was purchased from Spectrum reagents and chemicals Pvt Ltd., Cochin. Equipments used were Digital weighing balance, Dial calliper, pH meter, Orbitek shaker, Franz diffusion cell (60ml), UV/Visible Spectrophotometer, FTIR and HPLC Spectrophotometer, Stability chamber, REMI CPR-24 centrifuge, Magnetic stirrer.
METHODS
COMPATIBILITY STUDY OF DRUG AND POLYMER
FTIR absorption spectra 2 mg of the substance being examined was dried in hot air oven at 500C for one hour to remove moisture and triturated with 300 mg to 400mg of finely powdered and dried potassium bromide. The mixture was grinded carefully, spread uniformly in a suitable disc of 13 mm diameter and put in vacuum to a pressure of about 800 Mpa(8t.cm-2). The same procedure was repeated for the polymers and the physical mixture of drug and the polymers.
PREPARATION OF STANDARD CURVE
Stock solution was prepared by dissolving 100 mg of lamivudine in 100 ml of phosphate buffer ph 7.4.
Standard solution 10 ml of stock solution was made to 100 ml with ph 7.4. From this solution 1-8 ml were transferred into 10 ml standard flask and make up with buffer. Thus the final concentration ranges from 10-60 µg/ml and measure the absorbance at 270 nm.
DOSE DESIGNING
Based on the Pharmacokinetic parameters of Lamivudine, the amount of drug required to achieve the effective plasma concentration was calculated using the equation4,
Kp = (Cplasma )ss x Kel x Vd
Where, (Cplasma)ss - The drug level at steady state,
Kel - Elimination rate constant
Vd - Volume of distribution of drug.
FABRICATION OF TRANSDERMAL PATCHES
Technique: Solvent casting method.
Four types of formulations were prepared by using two different polymers in different ratios. First two formulations were prepared by using HPMC alone having Drug & Polymer ratio 1:2.5, 1:5 using water as a solvent and the next formulations were formulated using EC having Drug & Polymer ratio 1:2.5, 1:5 using chloroform as a solvent with Span 80 (1%) as permeation enhancer, Glycerol (3%) was used as a plasticizer. The films were cast on to suitable designed and fabricated glass mould and then dried in hot air oven at 40ºC for 6 hrs. The films were removed by using sharp blade by inserting along the edges of the film. The dried films were wrapped in aluminium foils and stored in a closed container in cool place away from light.
FABRICATION OF LAMIVUDINE TRANSDERMAL PATCHES OF 3.14 SQ CM
|
S.No |
Ingredients |
F1W |
F2W |
F3W |
F4W |
FPE1 |
FPE2 |
FPE3 |
FPE4 |
|---|---|---|---|---|---|---|---|---|---|
|
1. |
Lamivudine |
5.0 |
5.0 |
5.0 |
5.0 |
5.0 |
5.0 |
5.0 |
5.0 |
|
2. |
Hydroxy Propyl Methyl Cellulose (mg) |
12.5 |
25 |
_ |
_ |
12.5 |
25 |
_ |
_ |
|
3. |
Ethyl cellulose (mg) |
_ |
_ |
12.5 |
25 |
_ |
_ |
12.5 |
25 |
|
4. |
Span 80 |
_ |
_ |
_ |
_ |
1% |
1% |
1% |
1% |
|
5. |
Glycerol |
3% |
3% |
3% |
3% |
3% |
3% |
3% |
3% |
PHYSICOCHEMICAL EVALUATIONS
Thickness of the patch Thickness of the patch was measured by using dial gauge in mm.
Folding endurance The folding endurance was measured manually for the prepared film .A strip of film was cut evenly and folded at the same place till it broke .The number of times the film could be folded at the same place without breaking gave the exact value of folding endurance5,6,7,8,9.
Percentage of moisture absorption10 To check the physical stability of the film in high humidity condition, accurately weighed film were placed in a desiccators containing saturated solution of aluminum chloride (79.5% relative humidity) for 3 days The film were reweighed and percentage moisture absorption were calculated using the formula.
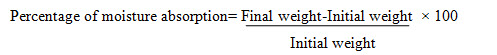
Percentage of moisture loss10 To check the extent of moisture loss from freshly prepared film. Accurately weighed film were placed in a dessiccator containing fused anhydrous calcium chloride for 72 hrs .After 72 hrs the film were reweighed and percentage moisture loss was calculated using the formula .
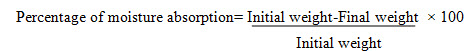
Water Vapour transmission rate
The patches were fixed over the edge of the glass vial containing 3g of fused calcium chloride as a desiccant by using an adhesive. Then the vial was placed in a desiccator containing saturated solution of potassium chloride. The vial was taken out periodically and weighed for a period of 72 hours7, 11, 12
Drug content uniformity
The prepared patch was cut into small piece and put into 100ml dissolution or diffusion medium used respectively and Stirred continuously by using a mechanical stirrer and sample was withdrawn at the end of three hours and the drug content was determined, spectrophotometrically at 270 nm5.
Stability Study
The prepared patches were subjected to stability study by storing the patches at storage conditions specified in the ICH guidelines. The patches were stored for three months at temperature 400C ± 20C and at a relative humidity of 75% ± 5%. The stability study was conducted with regard to tensile strength, moisture content and drug content. The patches, which retained their physical properties, were further subjected to in-vitro permeation studies5,6.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
IN VITRO SKINPERMEATION STUDIES OF TRANSDERMAL PATCH
IN VITRO SKIN PERMEATION STUDIES
The Ethical clearance for the handling of experimental animals was obtained from the Institutional animal ethical committee (IAEC) formed for this purpose. The protocol of the animal study was approved by the Institutional Animal Ethical committee, Protocol Number: SVCP/ IAEC/ Ph.d/02/2009 dated, 7th November 2009. The experiment was conducted according to the guidelines of CPCSEA (Committee for the purpose of control and supervision of experiment on animals).
These studies were carried out using a mammalian rat skin of required thickness. The permeation cell used for this study was a specially fabricated “Modified Franz diffusion cell “were used 5,6,8,13.
Modified Franz diffusion cell
Modified Franz diffusion cell is a skin permeation system developed by Franz and commercialized by crown glass has been frequently used for the studying the kinetics of percutaneous absorption. The modified Franz diffusion cell has receptor compartment with an effective volume approximately 60ml and an effective surface area of permeation of 3.14cm2. A rod shaped magnetic stirrer driven by a 3w synchronized motor stirs the solution and the stirring magnet rotates at constant rpm in a low viscosity receptor compartment14,15,16.
Operation of diffusion cell
The skin was mounted and clamped between the receptor and donor compartment .The surface area exposed to the drug patch was 3.14 Sq. cm. The cell was maintained at 320 ±0.50c using thermostatically controlled water bath. Water from the water bath was circulated through the cell, which was maintained at constant flow. The diffusion cell of 60ml capacity was filled with Phosphate buffer pH 7.4 and fixed to a thermostatically controlled magnetic stirrer, which was also maintained at the temperature 320 ±0.50C. At each sampling interval, samples were withdrawn and were replaced by equal volumes of fresh receptor fluid on each occasion. Samples withdrawn were analysed spectrophotometrically at 270 nm.
KINETIC CHARACTERISTICS OF THE DRUG RELEASE
To know the mechanism of the drug release from the patches, the results obtained from the In-vitro dissolution process were fitted into different kinetic equations as follows:
1. Zero - order drug release: Cumulative % drug release Vs Time.
2. First Order drug release: Log cumulative % drug retained Vs Time.
3. Higuchi’s classical diffusion equation: Cumulative % drug release Vs Square root of time.
4. Peppa’sKorsemeyer Exponential equation: Cumulative % drug release Vs Log time
THE STUDY OF IN VITRO SKIN PERMEATION KINETICS
Permeability coefficient (P) is the velocity of drug passage through the membrane in µg/cm 2/h. The permeability coefficient was calculated from the slope of the graph of percentage of drug transported versus time as,
P = Slope x Vd/ S
Where, Vd is the volume of donor solution and S is the surface area of tissue.
Flux (J) is defined as the amount of material flowing through a unit cross-sectional barrier in unit time. It is calculated by17, 18,
Flux (J) = P x CD
Where, CD = concentration of donor solution and P= permeability.
Enhancement ratio (Er) was used to evaluate the effect of permeation enhancer on diffusion and permeation of selected drug molecules. It is calculated by,

SKIN IRRITATION TEST
The skin irritation test was done on healthy rabbit weighing between 2-3 kg.The rabbits were divided into 5 groups with three each. On the previous day of the experiment, the hair on the backside of the rabbit was removed. Drug loaded polymeric film of 3.14 cm2was placed on the left dorsal surface of the rabbit. The skin was examined for erythema/ edema6, 7,8,19.
Group – I : Served as normal, without any treatment
Group – II : Control- applied with marketed official adhesive tape in SP
Group – III: Blank – applied with drugless polymeric patches
Group – IV: Test – applied with drug loaded polymeric patches
Group – V: Applied with 0.8%v/v aqueous solution of formalin as standard irritant
STATISTICAL ANALYSIS BY ANOVA
The in-vitro permeation data were statistically compared and analysed using Graph Pad Instat – 3 software version. The Tukey – Kramer multiple comparisons test was utilized to find the ‘q’ and ‘p’ values and the significance of the formulations were studied. If the ‘p’ is <0.05, the formulations are said to be significant.
INVIVO STUDIES OF TRANSDERMAL PATCHES
Six Wister rats of 180-220gm weight are to be utilized for the study. The rats are to be allowed to acclimatize for 1 week before the day of administration. The rats were fasted,but allowed access to water on the day before the study20.
Standard concentration solution of lamivudine
A mixture of acetonitrile and water (65:35%V/V) was used as the mobile phase and C18 column as the stationary phase. A stock solution of the pure drug was prepared by dissolving 50mg of Lamivudine in 50ml of the mobile phase. This stock solution was further diluted with the mobile phase to obtain the concentration of 10μg/ml3.
Six groups of rats were taken for the animal studies and each group containing one rat. Rats are to be anesthetized before the experiment. The hair on the abdominal site is clipped before the experiment. The skin was gently wiped with water and alcohol swab and patted dry. The patch of 3.14sq.cm size patch containing 5mg lamivudine was fixed over the prepared skin. Blood samples of approximately 0.15ml were collected from the jugular vein in the dried heparinized tube at 0.5, 2, 4, 8, 16, 24 hrs after transdermal administration, and they are frozen at -20°C as soon as possible and stored until the analysis. The HPLC analytical reports of the blood samples collected at different hours were compared with that of standard concentration of Lamivudine.
IN-VITRO / IN-VIVO CORRELATION
Invitro-in vivo correlation is the demonstration of the direct relationship of in vitro dissolution rate / diffusion rate of drugs and there in vivo bioavailability. Correlation is used to ensure batch-to-batch consistency in the physiologic performance of a drug product by use of such in vitro values and to serve as a tool in the development of a new dosage form with desired in vivo performance.
There are two basic approaches by which a correlation between dissolution/diffusion testing and bioavailability can be developed21.
1. By establishing a relationship, between the in vitro dissolution/diffusion and the in vivo bioavailability parameters. If this relationship becomes linear with a slope of 1, then curves are super imposable, and there is a 1:1 relationship which is defined as point-to-point or level A correlation.
2. By using the data from previous bioavailability studies to modify the dissolution/diffusion methodology in order to arrive at meaningful in vitro-invivo correlate ion.
|
Time (hrs) |
Cumulative % of drug permeation |
|||||||
|
F1W |
F2W |
F3W |
F4W |
FPE1 |
FPE2 |
FPE3 |
FPE4 |
|
|
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
0.25 |
0.57 |
3.03 |
0.55 |
1.12 |
9.28 |
10.99 |
2.74 |
3.88 |
|
0.5 |
1.44 |
7.91 |
1.42 |
2.56 |
11.15 |
12.88 |
5.35 |
6.22 |
|
0.75 |
2.60 |
11.46 |
2.58 |
3.37 |
15.88 |
17.64 |
7.43 |
8.88 |
|
1 |
4.43 |
14.77 |
3.67 |
4.85 |
18.70 |
20.49 |
11.25 |
12.44 |
|
2 |
6.49 |
18.71 |
5.15 |
6.07 |
25.54 |
27.36 |
13.99 |
14.63 |
|
4 |
8.88 |
21.85 |
7.51 |
8.16 |
34.76 |
36.61 |
17.63 |
18.85 |
|
6 |
12.29 |
25.27 |
10.64 |
10.43 |
38.75 |
40.59 |
20.75 |
21.41 |
|
8 |
18.60 |
28.58 |
16.05 |
13.01 |
43.06 |
44.97 |
26.21 |
27.17 |
|
10 |
24.02 |
32.44 |
19.44 |
17.48 |
46.10 |
47.94 |
31.46 |
32.72 |
|
12 |
27.82 |
36.93 |
22.03 |
21.17 |
54.68 |
56.64 |
33.66 |
34.94 |
|
14 |
31.39 |
41.48 |
25.79 |
28.33 |
57.81 |
59.79 |
38.45 |
39.75 |
|
16 |
36.72 |
43.83 |
28.75 |
31.05 |
63.81 |
65.83 |
41.03 |
42.36 |
|
18 |
42.32 |
48.76 |
31.48 |
33.81 |
67.91 |
69.95 |
45.08 |
50.97 |
|
20 |
45.63 |
51.20 |
34.52 |
38.03 |
74.05 |
76.12 |
50.60 |
56.87 |
|
22 |
48.04 |
53.96 |
37.04 |
40.90 |
76.58 |
81.52 |
52.21 |
62.28 |
|
24 |
51.89 |
56.75 |
39.31 |
46.35 |
80.84 |
88.70 |
57.26 |
65.78 |
RESULTS IN-VITRO SKIN PERMEATION STUDIES OF VARIOUS FORMULATIONS
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
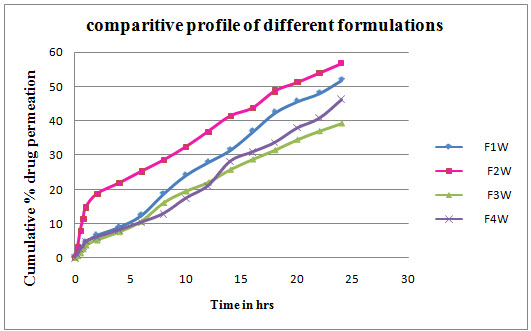
In-vitro skin permeation of various formulations (Without permeation enhancers)
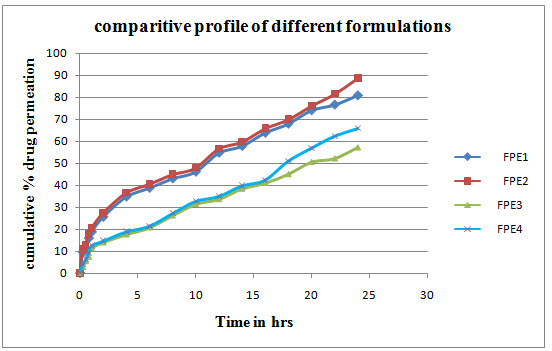
In-vitro skin permeation of various formulations (With permeation enhancers)
THE IN VITRO SKIN PERMEATION KINETIC DATA FOR TRANSDERMAL PATCHES (WITHOUT PERMEATION ENHANCER)
|
S.No |
Formulation code |
Slope of zero order plot |
Permeation coefficient(p) |
Flux(J) |
|
1 2 3 4 |
F1W F2W F3W F4W |
1.7135 2.1916 2.1792 1.8520 |
2.7285 3.4898 3.4701 2.9490 |
17 17.45 13.2 14.6 |
THE IN VITRO SKIN PERMEATION KINETIC DATA FOR TRANSDERMAL PATCHES (WITH PERMEATION ENHANCER)
|
S.No |
Formulation code |
Slope of zero order plot |
Permeation coefficient(p) |
Flux(J) |
Enhancement ratio(Er) |
|
1 2 3 4 |
FPE1 FPE2 FPE3 FPE4 |
3.005 3.143 2.196 2.494 |
4.78 5.00 3.49 3.97 |
23.9 25 17.45 19.85 |
1.405 1.432 1.321 1.359 |
INVIVO DRUG ABSORPTION STUDIES
|
S. No |
Time (in hrs) |
Mean Area Under Curve (AUC) |
Concentration ( μg/ml) |
% drug absorption (%) |
|
1 |
Control |
172950.73 |
10 |
- |
|
2 |
0.5 |
1429283.25 |
8.265 |
16.54 |
|
3 |
2 |
2914583.07 |
16.85 |
33.71 |
|
4 |
4 |
3787204.87 |
21.90 |
43.80 |
|
5 |
8 |
5039821.69 |
29.14 |
58.52 |
|
6 |
16 |
5785842.70 |
33.45 |
66.90 |
|
7 |
24 |
6874342.48 |
39.75 |
79.50 |
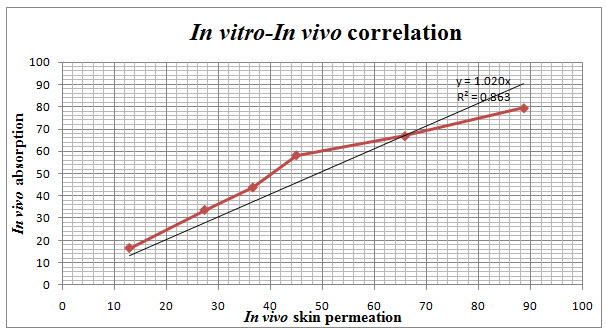
IN VITRO- IN VIVO CORRELATION
|
In vitro skin permeation (%) |
In vivo absorption (%) |
|
12.88 |
16.54 |
|
27.36 |
33.71 |
|
36.61 |
43.80 |
|
44.96 |
58.28 |
|
65.83 |
66.90 |
|
88.70 |
79.50 |
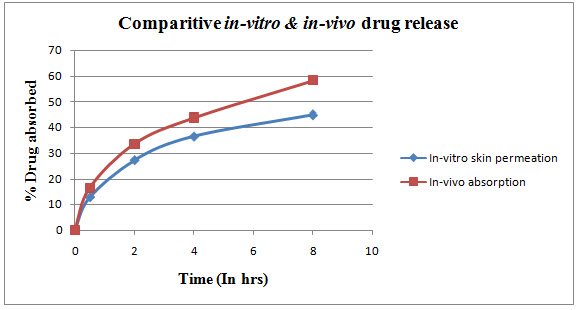
Comparison graph of in-vitro skin permeation and in-vivo absorption studies
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
DISCUSSION:
THE IN VITRO SKIN PERMEATION STUDIES
The results indicated that span 80 greatly influenced the permeation of drug through the skin. Thus, suggesting the use of permeation enhancer in transdermal drug delivery system. We concluded that the mechanism of drug release as zero order and non-fickian release. The permeability parameters like J, P, Er were significantly increased in formulations containing permeation enhancers.
THE IN VIVO DRUG ABSORPTION STUDIES
The in vivo drug absorption studies reveal that there is a sustained release of drug into the rat systemic circulation of 79.5% of drug absorption was after 24 hrs.
IN-VITRO/IN-VIVO CORRELATION
The relationship between in-vitro and in-vivo studies becomes linear with a slope of 1, then the curves are super imposable and there is a 1:1 relationship which is defined as point to point or level A correlation. In this study the slope between the in-vitro and in-vivo studies was 0.863. So, it is said to be near correlated and the curves are not super imposable and are not linear.
CONCLUSION
It was concluded that prepared transdermal patch showed Lamivudine and HPMC in the ratio 1:5 (FPE2) formulation showed a good sustained release characteristics with the 1% Span 80 as a permeation enhancer, thus providing a better way of preventing first pass metabolism. The in-vitro skin permeation studies (FPE2) showed a best sustained release effect over the formulations. The comparative statically analysis done by ANOVA method proved that all the formulations is extremely significant for the transdermal route. The in-vivo studies (FPE2) also showed a sustained release effect of the drug into the systemic circulation. The in-vitro/in-vivo correlations are found to be not super imposable and said to be near correlated and linear.
REFERENCES
1. Ratan Mehta. “Topical and transdermal drug delivery”: What a Pharmacist need to know. ACEP; 1-10.
2. Joint united nations programme on HIV/AIDS (ON AIDS) and WHO” AIDS epidemic update 2005”, Geneva http://www.Unaids.org.
3. Roopa pai S. and Kusum Devi V. “Lamivudine liposomes for transdermal delivery formulation, Characterization, Stability and Invitro Evaluation”. International journal of pharmaceutical sciences and nanotechnology Volume 1. Issue 4. January – March 2009 p317-326.
4. Gupta Ritu, Bajpai Meenakshi, Bhattaracharya Arundhati. “Formulation and invitro evaluation of transdermal drug delivery system of Tizanidine hydrochloride” Journal of pharmaceutical research.2008;7(4):p208-213.
5. G.S. Sanap,G.Y.Dama, “Preparation of transdermal monolithic systems of indapamide by solvent casting method and the use of vegetable oils as permeation enhancer” International journal of green pharmacy, April – June,2008,p129- 133.
6. Rathore RPS, Chauhan C S, Naruka P S, Tanwar Y S and Chauhan L S. “Transdermal formulation of Terbutaline sulphate”. Pharmacy On-Line, April 2006. www.priory.com.
7. Yuveraj Singh Tanwar, Chetan Singh Chauhn, Anshu Sharma. “Development and Evaluation of Carvedilol Transdermal patches”. Acta Pharm. 57(2007)p151-59.
8. Janardhanan Bagyalakshmi, Ramachandra Purapu Vamsikrishna, Rajappan Manavalan, Thengungal Kochupappy Ravi and Probal Kumar Manna. “Formulation Development and in vitro and in vivo Evaluation of Membrane Moderated Transdermal Systems of Ampicillin Sodium in Ethanol: ph 4.7 Buffer Solvent System”. AAPS PharmSciTech.2007; 8(1) Article 7. http://www.aapspharmscitech.org.
9. Jianping Wang, Jinlan Ruan,Changgog Zhang, Yujie Ye, Yaling Cai and Yanxia Wu. “Development and Evaluation of the Sinomenin Transdermal patch”. Pak. J. Pharm. Sci., Vol.21, No.4, October 2008,p407-410.
10. V kasum Devi, S saisivam G R maria “Design and evaluation of matrix controlled transdermal patch of verpamil Hcl” Drug development and industrial pharmacy 2003.vol-29 NO5 page-503.
11. Talasila Eswara Gopala Krishna Murthy, Vankayalapati Sai Kishore. Efect of Casting solvent and polymer on permeability of propranol hydrochloride through membrane-controlled transdermal drug delivery system”. Asian Journal of Pharmaceutics - April- June 2008.p86-90.
12. Kulkarni Raghavendra, H. Doddayya, Dr.S.C. Marihal, C.C.Patil, and P.V. Habbu. “Comparitive Evaluation of Polymeric Films for Transdermal Application”. The Eastern Pharmacist.2000; 43;p109-111.
13. G. Ramesh, Vamshi Vishnu Y, Kishan V and Madhusudan Rao Y. “Studies on the influence of penetration enhancers on in vitro permeation of carvedilol across rat abdominal skin”. Current Trends in Biotehnology and Pharmacy, Vol.1 (1)p62-69(2007).
14. Dr. Shyam S. Agrawal , Dr. Manoj P. Jadhav.“In-vitro, in-vivo, transdermal permeation of Carvedilol from Chitosan based matrix-type transdermal patches” AAPS National Biotechnology Conference , 2006.
15. Roop K Khar, S P Vyas, “Controlled drug delivery concept and advances” Ist Edition , 2002 Page No-442-443.
16. Mohamed aqili, Yasmis sultana, Asgar Ali, “Matrix type transdermal drug delivery system of Metaprolol tatarate: In vitro characterization” Acta pharm, 53 (2003).p 120.
17. Gupta Ritu, Bajpai Meenakshi, Bhattacharya Arundhati, “Formulation and In-vitro evaluation of Transdermal drug delivery system of Tizanidine Hydrochloride”, Journal of Pharmaceutical Research, Volume:7, No:4, October 2008, Page:208 to 213.
18. Faiyaz Shakeel, Sanjula Baoota, Alka Ahuja, Javed Ali, Mohammed Aqil and Sheikh Shafiq. “Nanoemulions a vehicles for Transdermal Delivery of Aceclofenac”. AAPS PharmSciTech.2007; 8(4) Article 104. http://www.aapspharmscitech.org.
19. Srinivas Mutalik, nyanabhirama Udapa, “Formulation, Development ,invitro and invivo evaluation of membrane controlled transdermal system system of Glibenclamide”, J Pharm Pharmaceutical Sci, 2005;8(1): p26-38.
20. Chanshun Ren, Liang Fang, “Design and in vivo evaluation of an Indapamide transdermal patch”, International Journal of Pharmaceutics, 2009;p129-135.
21. Mr.C.Kumaresan, “In -Vitro In -Vivo Studies in Pharmaceutical Development, pharmainfo.net” , vol 6, Issue 2, 2008.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









