About Authors:
Rawat Pinki1*, Rawat Preeti2, Kumar Piyush3 Kanoujia Jovita3, Singh Sangeeta 1
1. Institute of Pharmaceutical Science and Research, Unnao, U.P., India.
2. L.T.R. College Of Technology, Meerut, U.P., India.
3. Curadev Pharmaceuticals Ltd., Kanpur, U.P., India.
*pnkrawat@gmail.com
Abstract:
Selective estrogen receptor modulators, called SERMs for short, blocks the naturally circulating estrogen in breast tissues and other estrogen-sensitive tissues in the body. Each estrogen receptor has a slightly different structure, depending on the kind of cell it is in. If a SERM binds to a estrogen receptor, there is no site available for estrogen to bind and it can't attach to the cell. SERMs are called "selective" because they bind to particular estrogen receptors. This selective binding action is sometimes called estrogen inhibition, or estrogen suppression.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1327
Introduction
Selective estrogen receptor modulators (SERMs) are structurally different compounds that interact with intracellular estrogen receptors (ERα and ERβ) in target organs as estrogen receptor agonists and antagonists. These drugs have been intensively studied over the past decade and have proven to be a highly versatile group for the treatment of different conditions associated with aging, including hormone responsive cancer and osteoporosis1.
This term appropriately indicates that these compounds are not uniformly estrogen antagonists. Rather, they display an unusual tissue-selective pharmacology. They are agonists in some tissues (bone, liver, and the cardiovascular system), antagonists in other tissues (brain and breast), and mixed agonists/antagonists [HN9] in the uterus.2
Coactivators and corepressors are recruited by the estrogen receptor to several target genes in breast and uterine cancer cells, in the presence of either the estrogen estradiol [HN13] or the SERMs tamoxifen and raloxifene. In both uterine and breast cancer cells, at genes where the estrogen receptor interacts directly with DNA, estradiol recruited coactivators, and both SERMs recruited corepressors.2
In the broadest sense, the term SERM has been used to describe compounds that bind to the estrogen receptor but that evoke tissue-specific responses.3
[adsense:468x15:2204050025]
MECHNISM OF ACTION
In general, once the SERM crosses the cell membrane, it binds to the estrogen receptor (eithera orb). The estrogen receptor next undergoes dimerization and further conformational change. In the dimerization process, the receptor may join either with a similar type of receptor or a completely different one. This receptor complex is subsequently acted upon by cellular proteins, which either enhance or inhibit the estrogen-ligand complex’s activity upon deoxyribonucleic acid transcription (Figure 1)3.
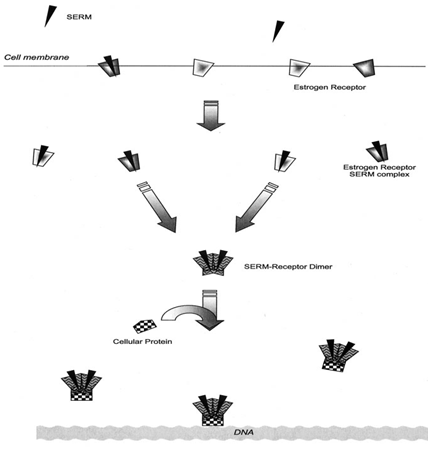
Figure 1. Mechanism of action of SERMS
A SERM can bind to either estrogen receptor (ER)-α or ER-β, and the complexes can then recruit co-activators or co-repressors.
There is important evidence that the binding of co-regulatory proteins on the surface of the SERM–ER complex can modulate estrogenic and antiestrogenic actions. Agonist-activated ERs bind to coactivators, such as steroid receptor coactivator protein 1 (SRC-1), that ultimately form a large complex of proteins linking ER to the transcription apparatus that acetylates histones and subsequently decondenses chromatin, allowing gene transcription to take place.4
Unlike estrogen, which facilitates the interaction of ER with coactivators, SERMs bind to ER and induce different conformational states that facilitate the interaction of corepressors (CoR) to the ERE-driven promoter, which inhibits the recruitment of the basal transcriptional machinery and thus inhibits transcription.5
Transcriptional co-repressors such as nuclear receptor co-repressors (NcoR) and ‘silencing mediator of retinoid and thyroid hormone’ (SMRT) interact with the antiestrogen ER complex to deacetylate histones, condense chromatin and subsequently inhibit gene transcription. Thus, SERM action appears to depend on the relative levels of different co-regulators at a specific target site.4
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
SERMS IN BREAST CANCER
Breast cancer remains the most common cancerand the second leading cause of cancer death inwomen in the US and Europe.6
Most breast cancers are believed to develop spontaneously through accumulation of mutations and perhaps epigenetic changes throughout life. Early menarche and late menopause increase the risk of breast cancer whereas pregnancy especially at young age decreases the risk.7
Prolonged exposure to high levels of estrogen may cause changes in cell proliferation from normal growth to hyperplasia to neoplasia with DNA mutations and uncontrolled cell proliferation. Women may be at increased risk for breast cancer from prolonged exposure to estrogen such as with early menarche before age 12, first full-term pregnancy after age 30, late menopause after age 55, or recent long-term use of postmenopausal hormone replacement therapy (HRT).8
Most cases of breast cancer develop from the ductal epithelium and may be classified as invasive or noninvasive. Invasive breast cancers have broken through the basement membrane of the ducts or lobules into the fatty tissue of the breast. About 70%–80% of all breast cancers are termed estrogen receptor positive (ER+) if they express the ER protein a. These ER+ breast cancers are more likely to respond to hormonal therapy such as SERMs.
Breast cancers that do not express the ER protein a are termed estrogen receptor negative (ER–) and are unlikely to respond to hormonal therapy. Noninvasive, or in situ, breast cancers develop in the ducts (ductal carcinoma in situ [DCIS]) or lobules (lobular carcinoma in situ [LCIS]) of the breast tissue. Such cancers have not spread through the basement membrane and historically have been treated with mastectomy followed by axillary node dissection.8
A substantial body of experimental, clinical and epidemiologicalevidence indicates that steroid hormones play a majorrole in the aetiology of breast cancer.
SelectiveOestrogen Receptor Modulator (SERM) was aterm coined to describe the phenomenon of apparentblocking of the ER at one site (e.g., the tumour)and stimulatory activity at another site (e.g., bone).Thus, a single drug could have both agonist and antagonistactivity depending on the cell type. SERMswere divided into SERM 1 (tamoxifen), SERM2 (raloxifene) and SERM 3 (a compound with noother common name closely related structurally to raloxifene). However nearly all estrogens may haveagonist or antagonist activity depending upon drugdose and target cell type.6
SERMs maybe classified according to their core structure, which is typically a variation of the 17 beta-estradiol template and subclassified according to the side chain at the helix 12 affector region. The best known are the triphenylethylenes such as tamoxifen, used in the management of breast cancer. However, the clinical application of this class of SERMs has been limited due to endometrial stimulation.
A second class is the benzothiophenes such as raloxifene and arzoxifene, which have skeletal benefit with little, if any, uterine stimulation. Indole-based SERMs such as bazedoxifene have a 2-phenyl ring system that serves as a core binding unit. Other classes include benzopyrans and naphthalenes (eg, lasofoxifene).9
TAMOXIFEN
Tamoxifen, a non-steroidal, triphenylethylene-based anti-oestrogen, with tissue specific estrogenic (agonist) and anti-estrogenic (antagonist) activity, has been the anti-estrogen of choice for over 25 years. The anti-estrogenic activity of tamoxifen in the breast has established it as the 'gold standard' for the treatment of all stages of breast cancer. Tamoxifen given for different durations in an adjuvant setting has been associated with a reduction in the risk of both contralateral breast cancer andmetastatic cancer.10
Tamoxifen produces its antiestrogen effect on breast tissue by competing with estradiol for binding to estrogen receptors, thus preventing cell replication. The usual dosage for tamoxifen is 20 mg daily for five years.11
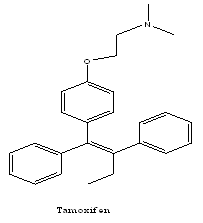
In order to improve its efficacy and reduce the toxicity of tamoxifen, researchers tried to change the chemical structure of the drug. One possibility was to alter the side chains in order to produce new ‘tamoxifen-like’ analogues (toremifene, droloxifene, idoxifene), which became known as triphenylethylene derivatives.12
TOREMIFENE
Toremifene is a chlorinated analogue of tamoxifen, with similar site-specific estrogenic and anti-estrogenic activity. It has been shown in pre-clinical studies to have similar ER binding and anti-tumour activity to tamoxifen, but less DNA adduct formation in the endometrium.10
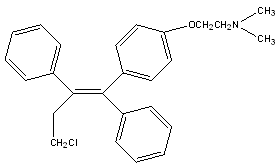
Toremifene
FULVESTRANT
Fulvestrant is a steroidal 7α-alkylsulfinyl analogue of estradiol. It has a higher affinity for the ER compared with tamoxifen (8.9% vs 2.5% of the binding affinity of estradiol, respectively) and acts as pure antiestrogen.1 It is a novel ER antagonist that destroys the ER and its signaling pathway.13
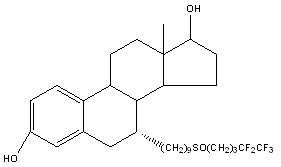
Fulvestrant
RALOXIFENE
Raloxifene is the best known member of the benzothiophene family that has previously been referred to as keoxifene, LY139481, or LY156758. Raloxifene had, at one point, been considered a candidate for the treatment of breast cancer but was never developed for this indication. However, recently raloxifene has been shown to be efficacious in preventing bone loss in postmenopausal women.14
It has been shown in pre-clinical studies to have anti-estrogenic effects on both the breast and the uterus and estrogenic effects on the bone, cholesterol levels and vascular smooth muscle cells.10
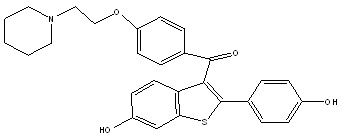
NAFOXIDENE
The best characterized naphthalene derivative is nafoxidine which had also been considered at one point for the treatment of breast cancer. It was shown to significantly lower serum cholesterol levels but had complex bone effects in ovariectomized rats.14
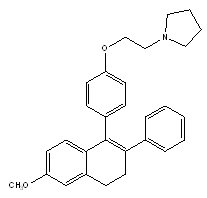
Nafoxidene
ADVERSE EFFECTS
Although the beneficial effects of SERMs are very intriguing, the clinician must be acutely aware of the potentially harmful side effects of tamoxifen and raloxifene (most commonly used SERMS). Endometrial hyperplasia, endometrial polyps, and uterine carcinoma have been associated with tamoxifen, especially in postmenopausal patients. Tamoxifen has been reported to cause hot flashes, vaginal discharge, menstrual irregularities, and transient bone pain.
The most common side effects of raloxifene are hot flashes and leg cramps, most commonly reported during the first 6 months of treatment. Both tamoxifen and raloxifene are contraindicated in women with either an active or past history of venous thromboembolism, including deep venous thrombosis, pulmonary embolism, and retinal vein thrombosis.15
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
STATISTICS
In India, the average age of developing a breast cancer has undergone a significant shift over last few decades.
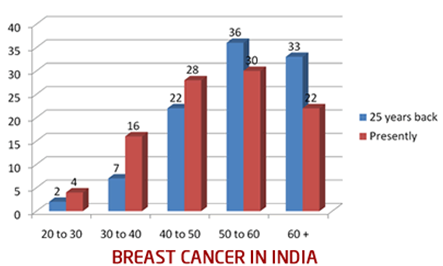
Figure 2: Representation of % of cases in different age groups
The horizontal line lower down represents the age groups: 20 to 30 years, 30 to 40 yrs and so on. And the vertical line represents the percentage of cases. The blue colour represents the incidence 25 years back, and maroon colour represents the situation today.
25 years back, out of every 100 breast cancer patients, 2% were in 20 to 30 years age group, 7% were in 30 to 40 and so on. 69% of the patients were above 50 years of age. Presently, 4% are in 20 to 30 yrs age group, 16% are in 30 to 40, 28% are in 40 to 50 age group. So, almost 48% patients are below 50. An increasing numbers of patients are in the 25 to 40 years of age, and this definitely is a very disturbing trend.16
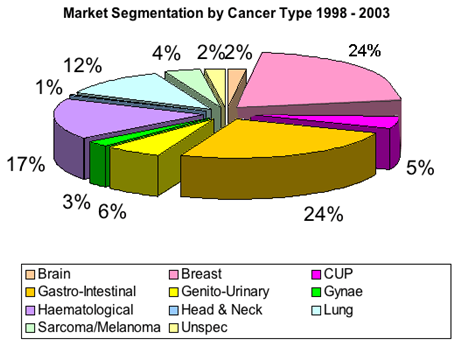
Figure 3: Market Segnentation of Cancers
Breast cancer is now the most common cancer in most cities in India, and 2nd most common in the rural areas. Please have a look at the following bar graph about percentage distribution of top ten cancers in females in Mumbai.17
Relative proportion %
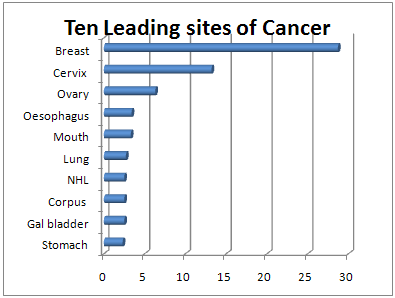
Figure 4: Ten Leading sites of Cancer in Females
After going through all the graphs, the point worth noting is that, breast cancer accounts for 25% to 32% of all female cancers in all these cities. This implies, practically, one fourth (or even approaching one thirds) of all female cancer cases are breast cancers.18
FUTURE OF SERMS
The early termination of both arms of Women’s Health Initiative Trial, due to increased risk of breast cancer, increased risk of stroke, and lack of effectiveness on incidence of coronary heart disease in postmenopausal women, has spurred the interest for novel SERMs with improved therapeutic index.
Furthermore, although the risk of breast cancer and endometrial cancer is set aside by the SERMs they have not shown the same efficacy as estrogen to prevent for example bone fractures of the hip. Furthermore, existing SERMs increase the incidence of or aggravate hot flushes in postmenopausal women, and the incidence of venous thromboembolism is equal to existing hormone replacement therapy.19
CONCLUSION
Today, one of the most effective strategies for the treatment and prevention of breast cancer involves the use of drugs that block estrogen action in the breast. The success of the first clinically relevant SERM, tamoxifen, opened the door for the testing of this drug to reduce breast cancer incidence in high-risk women.5
As the molecular mechanisms of the action of SERMs become more completely understood, rational drug design will replace the current empirical method for the discovery of new drugs that will selectively express desirable actions and suppress undesirable actions of the various steroid hormones. A tissue-selective drug would have all the beneficial effects of estrogen, none of its side effects, and might therefore offer protection against numerous conditions including breast cancer. Current research has identified ligands with selective potency, efficacy and antagonist properties at ER subtypes. As our understanding grows there is the potential for selective ER modulators to be developed as useful clinical medicines.20
In the treatment of advanced breast cancer, different, non cross-resistant endocrine therapies are generally required in a sequential manner as patients relapsing or progressing under the first endocrine therapy generally remain hormone sensitive. More and more aromatase inhibitors are being used as first-line therapy of advanced breast cancer because of their superiority in terms of outcome.12
REFERENCES
1. Gennari Luigi, Merlotti Daniela, Valleggi Fabrizio, Martini Giuseppe, Nuti Ranuccio, “Selective Estrogen Receptor Modulators for Postmenopausal Osteoporosis”; Drugs Aging, 2007, 24 (5), pp 361-379.
2. Batista J.M. Jr, Lopes A.A., Ambrosio D.L., Regasini L.O., Kato M.J., Bolzani Vda S., Cicarelli R.M., Furlan M.; “Natural chromenes and chromene derivatives as potential anti-trypanosomal agents”; Biol Pharm Bull., 2008, 31(3), 538-40.
3. Leosvaldo S.M. Velozo a, Marcelo J.P. Ferreira b, Maria Isabel S. Santos c, Davyson L. Moreira a, Vicente P. Emerenciano b, Marai Auxiliadora C. Kaplan; “Unusual chromenes from peperomia blanda”; Phytochemistry, 2006, 67, 492-496.
4. Data obtained from wikipedia.org (wikipedia.org/ wiki/Chromone)
5. Rona Thompson, Sheila Doggrell, John O. Hoberg; “Potassium Channel activators based on the benzopyran substructure: synthesis and activity of the C-8 subsituent”; Bioorganic & Medicinal Chemistry, 2003, 11(8), 1663-1668.
6. Vijay K. Agrawal, Jyoti Singh, Madhu Gupta, Yusuf Ali Jaliwala, Padmakar V. Khadikar, Claudiu T. Supuran; “QSAR studies on benzopyran potassium channel activators”; European Journal Of Medicinal Chemistry, 2006, 41(3), pp 360-366.
7. Gerike R., Harting J., Leus I., Schittenhelm C.; “3-Methyl-2H-1-benzopyran potassium channel activators”; Journal of Medicinal Chemistry, 1991, 34(10), pp 3074-3085.
8. Data obtained from wikipedia.org (en.wikipedia.org/ wiki/Benzopyrone)
9. Silverman L. Stuart, “New Selective Estrogen Receptor Modulators (SERMs) in Development”; Current Osteoporos Reports, 2010, 8(3), pp 151–153.
10. Howell Anthony, “Tamoxifen versus the newer SERMs: what is the evidence?”; Ann Oncol. 2000,11 Suppl 3, pp 255-65.
11. Johansen M. Ann, RN, BSN, OCN, “Breast Cancer Chemoprevention: A Review of Selective Estrogen Receptor Modulators”; Clinical Journal Of Oncology Nursing , 9(3), pp 317-320.
12. Baumann K. Christa, Gertsch-Castiglione Monica; “Estrogen Receptor Modulators and Down Regulators”; Drugs 2007, 67 (16), pp 2335-2353.
13. S.J. Wambi-Lewis, Jordan Craig V; “Treatment of Postmenopausal Breast Cancer with Selective Estrogen Receptor Modulators (SERMs)”; Breast Dis. 2005-2006, 24(93), pp105.
14. Sato Masahiko, Grese A. Timothy, Dodge A. Jeffrey, Bryant U. Henry, Turner H. Charles; “Emerging Therapies for the Prevention or Treatment of Postmenopausal Osteoporosis”; J. Med. Chem., 1999, 42 (1), pp 1-24.
15. Sexton J. Michael, Gherman B. Robert, “Selective Estrogen Receptor Modulators: The Ideal Estrogen Replacement?”; Elsevier Science , 2001, 8(1), pp 25-30.
16. Data obtained from breastcancerindia.net (breastcancerindia.net/ …/trends.htm)
17. Data obtained from breastcancerindia.net (breastcanceraustralia.org/ statistics.html)
18. Data obtained from nsmfoundation.com (nsmfoundation.com/ten-leading-sites-of-cancer)
19. Nilsson Stefan, Koehler F. Konrad; “Oestrogen Receptors and Selective Oestrogen Receptor Modulators:Molecular and Cellular Pharmacology”; Basic & Clinical Pharmacology & Toxicology, 2005, 96, pp 15–25.
20. Kustrin Agatonovic S, Tutner V.J; “Molecular Structural Characteristics of Estrogen Receptor Modulators as Determinants of Estrogen Receptor Selectivity”; Mini-Reviews in Medicinal Chemistry, 2008, 8, pp 943-951.
Received on 24th Jun, 2012 | Published on 16th Jul, 2012
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









