About Authors:
Vishwanadham Yerragunta*, T.Kumara swamy, D.Suman, V.Anusha, Prathima Patil, T. Samhitha
Department of Pharmaceutical Chemistry,
Vishnu Institute of Pharmaceutical Education and Research,
Narsapur, Medak – 502313, A.P.
Vishwanadham.y@gmail.com
{ DOWNLOAD AS PDF }
Abstract:
Chalcones are precursor compounds for flavonoids biosynthesis in plants, and they can also be synthesized in laboratory. Chalcones possess a broad spectrum of biological activities including antioxidative, antibacterial, antihelmintic, amoebicidal, antiulcer, antiviral, insecticidal, antiprotozoal, anticancer, cytotoxic and immunosuppressive. Changes in their structure have offered a high degree of diversity that has proven useful for the development of new medicinal agents having improved potency and lesser toxicity and good pharmacological actions. Chalcones became an object of continued interest in both academia and industry. Nowadays, several chalcones are used for treatment of viral disorders, cardiovascular diseases, parasitic infections, pain, gastritis, and stomach cancer, as well as like food additives and cosmetic formulation ingredients. However, much of the pharmacological potential of chalcones is still not utilized. The purpose of this review is to describe the recent efforts of scientists in pharmacological screening of synthetic chalcones, studying importance of chalcones, and synthesis of pharmacologically active chalcones and their biological activities.
REFERENCE ID: PHARMATUTOR-ART-2061
Introduction:
The chemistry of chalcones has generated intensive scientific studies throughout the world. The name “Chalcones” was given by Kostanecki and Tambor [1]. Chalcones are also known as benzyl acetophenone or benzylidene acetophenone. In chalcones, two aromatic rings are linked by an aliphatic three carbon chain. Chalcones (trans-1, 3-diaryl-2-propen-1-ones) are α, β-unsaturated ketones consisting of two aromatic rings (ring A and B) having diverse array of substituents. Rings are interconnected by a highly electrophonic three carbon α, β-unsaturated carbonyl system that assumes linear or nearly planar structure [2-4]. They contain the ketoethylenic group (–CO–CH=CH-). Chalcones possess conjugated double bonds and a completely delocalized π-electron system on both benzene rings. Chalcones have been used as intermediate for the preparations of compounds having therapeutic value [5-7]. Chalcones have been identified as interesting compounds that are associated with several biological activities. The most common chalcones found in foods are phloretin and its glucoside phloridzin (phloretin 2′-0-β-glucopyranoside), and chalconaringenin.
Chalcone bears a very good synthon so that variety of novel heterocycles with good pharmaceutical profile can be designed.
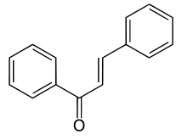
Structure of chalcone
Chemical Reaction:
Synthetic method of preparing chalcones
The most convenient method is the Claisen-Schimdt condensation of equimolar quantities of aryl methyl ketone with aryl aldehyde in the presence of alcoholic alkali [8].
Claisen-Schmidt Reaction:
A variety of methods are available for the synthesis of chalcones, the most convenient method is the one that involves the Claisen-Schmidt condensation of equimolar quantities of a substituted acetophenone with substituted aldehydes in the presence of aqueous alcoholic alkali [9]. In the Claisen-Schmidt reaction, the concentration of alkali used, usually ranges between 10 and 60 %[10].The reaction is carried out at about 50 oC for 12-15 hours or at room temperature for one week. Under these conditions, the Cannizaro reaction [11]. also takes place and thereby decreases the yield of the desired product. To avoid the disproportionation of aldehyde in the above reaction, the use of benzylidene-diacetate in place of aldehyde has been recommended. [12]
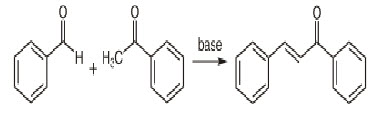
This reaction has been found to work without any solvent at all - a solid-state reaction. In a study investigating green chemistry synthesis, chalcones were also synthesized from the same starting materials in high temperature water (200 to 350 °C).
Alternatively, the substituted chalcones were synthesised by piperidine mediated condensation to avoid side reactions such as multiple condensations, polymerizations, and rearrangements.
The presence of enone functionality in chalcone moiety confers biological activity upon it, like anti-inflammatory [13], anti-fungal [14], anti-oxidant [15], anti-malarial [16], anti-tuberculosis [17], analgesic [18], anti- HIV[19] and anti-tumor[20] activities.
Importance of chalcones:
(1) They have close relationship with flavones, aurones, tetralones and aziridines.
(2) Chalcones and their derivatives find application as artificial sweeteners [21], scintillator, polymerization catalyst, fluorescent whitening agent, organic brightening agent, stabilizer against heat, visible light, ultraviolet light and aging [22].
(3) 3, 2’, 4’, 6’-tetrahydroxy-4-propoxy-dihydrochalcone-4-β'-neohesperdoside [23] has been used as synthetic sweetener and is 2200 times sweeter than glucose.
(4) They contain a keto-ethylenic group and are therefore reactive towards several reagents e.g. (a) phenyl hydrazine, (b) 2-amino thiophenol etc.
(5) The chalcones have been found useful in elucidating structure of natural products like hemlock tannin, cyanomaclurin, ploretin, eriodictyol and homo eriodictyol, naringenin [24] etc.
Biological Activities of chalcones:
- Xia and co-workers were the first to demonstrate improved anti-proliferative activity of chalcones with substituted amino groups [25].
- LeBlanc et al. have shown that methoxylated chalcones with a 3'-amino group had sub-micromolar IC50 values against murine melanoma B16 cells [26].
- Dimmock and co-workers proposed that the presence of amino function increases the reactivity of chalcones as Michael acceptors and subsequently their anti-proliferative activity. They postulated that the amino function would be protonated at low pH environment normally encountered in tumors. The electron withdrawing effect of the protonated ammonium function would enhance the electrophilicity of the β-carbon in the enone linkage, hence increasing its reactivity as a Michael acceptor.
- Liquorice has been used in China for the treatment of gastric and duodenal ulcers, bronchial asthma, Addison's disease, poisoning by food and drugs and skin disease such as eczema and urticaria. It still finds medicinal application because of its wide-ranging therapeutic properties, including relief from rheumatic and other types of pain and healing effect on ulcers. The crude extract of Liquorice has also found commercial use as a food additive in Japan since it contains the sweetening principle glycyrrhizin.
- The Liquorice extracts contains a chalcone, viz. Isoliquritigenin, which is currently in use as a phosphodiesterase III inhibitor for the treatment of cardiovascular diseases [27]. In the Far East countries such as Korea, Japan, and China, another chalcone compound called 'Butein' has also been traditionally used for treatment of pain, thrombotic disease, stomach cancer, and parasitic infection as well as a food additive.
- Anti-angiogenic effect of xanthochymol and Isoxanthochymol, the chalcones isolated.
- 2'-hydroxychalcones, 4'hydroxychalcones and 2', 4'-dihydroxychalcones inhibit 12-Lipoxygenase and cyclooxygenase enzymes in the mouse epidermis and two synthetic 2'-hydroxychalcones that exert topical anti-inflammatory effects in mice have also been reported. The good selective inhibitory effects of 2', 5' dihydroxychalcones on arachidonic acid induced platelet aggregation have been suggested and these reports, taken together, suggest that some hydroxy chalcones might be promising antithrombotic or anti-inflammatory agents.
- Saxena and co-workers grafted chalcone derivatives on estradiol framework some of which showed potent anticancer activity against some human cancer cell lines. Potent activity against estrogen receptor-positive and hormone-dependent human breast cancer cell lines, MCF-7. Active anticancer derivatives were also evaluated for osmotic hemolysis using the erythrocyte as a model system. It was observed that chalcone derivatives showing cytotoxicity against cancer cell lines did not affect the fragility of erythrocytes and hence may be considered as non-toxic to normal cells; however, Nitric oxide production by trimethoxy chalcone derivatives, with various patterns of fluorination, has also been evaluated [28]. One of this compounds, 2, 4, 6-trimethoxy-20-trifluoromethylchalcone, inhibited the production of NO and prostaglandin E2 in lipopolysaccharide stimulated RAW 264.7 macrophage cells.
- The inhibition (76.3% inhibition at 10 μM concentration) was dose-dependent without any evidence of a cytotoxic effect. It was suggested that NO reduction was a consequence of inhibition of the expression PGE2 accumulation.
- The fluorinated chalcones tested by Nakamura et al. showed 5-lipoxygenase inhibition on rat basophilic leukemia-1 (RBL-1) cells and inhibitory action on Fe3+-ADP induced NADPH-dependent lipid peroxidation in rat liver microsomes [29]. The potencies were comparable or better than those of the lead compound, viz. 3, and 4-dihydroxychalcone.
Biological importance:
The presence of α,β-unsaturated carbonyl system of chalcone makes it biologically active. They have shown antibacterial activity against S. aureus, E. coli, C. albicans, T. utilis, S. sake, W. anomala and some other organisms [30].
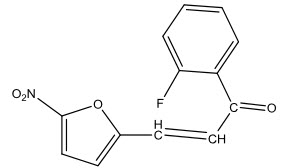
Devaux, Nuhrich and Dargelos [31] synthesized some nitrofuryl chalcones and tested for their antibacterial activity. The Chalcones most efficient was, which inhibited Staphylococcus landon at concentration 1 µg/ml.
Chalcones incorporated with benzopyran moiety were reported by Hismat, El-Diwani and Melek [32].
Some chalcones containing indole moiety were synthesized and tested for anti-bacterial and antifungal activity by Dandia, Sehgal and Singh.
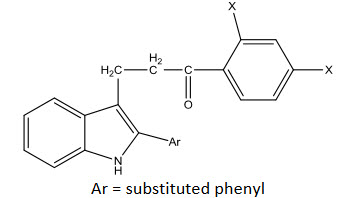
Salvie, Richard and John [33] reported α-substituted chalcones. The α- to methyl compound was found be the most active and tested for the chemotherapy of leukemias.
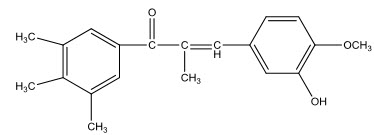
Heterocyclic substituted chalcones were prepared by Bombardeli and Valenti [34]. They reported that some of them were introduced for the treatment of breast cancer, menopausal disorders and osteoporosis.
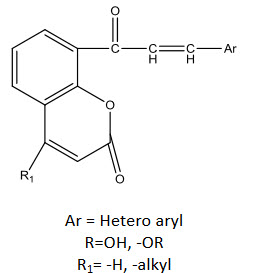
Ar = Hetero aryl
R=OH, -OR
R1= -H, -alkyl
Uenaka, Kawata, Nagai and Endoh [35] synthesized β-hydroxy chalcones. Compounds having fluoro substitution showed considerable activity against Human Immuno Virus (HIV).
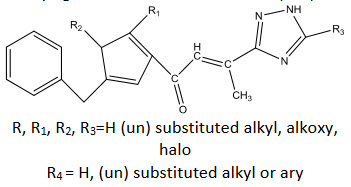
R, R1, R2, R3=H (un) substituted alkyl, alkoxy, halo
R4 = H, (un) substituted alkyl or ary
Seele [36] reported chalcone having heterocyclic moiety and reported their insecticidal activity.
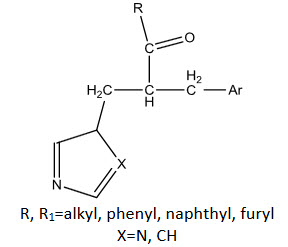
R, R1=alkyl, phenyl, naphthyl, furyl
X=N, CH
Some other biological activity of chalcone such as antiviral, anti-inflammatory, prostaglandin binding, antiulcer, anti-tumor, cardiovascularand anti-cancer [37] were also reported.
Metal Complexation:
The synthesis and structural studies of complexes of Co (II), Ni (II), Cu (II), Zn (II) and Cd (II) with substituted chalcones has been reported [38] In general, for metal Complexation reactions, the Schiff derivatives of the chalcones are preferred which not only offer selectivity in metal Complexation reactions but also an enhancement in biological activities.
Conclusion:
From the above review it can be said that chalcones and their derivatives display a wide range of Pharmacological activities, such as antimalarial, anticancer, antiprotozoal (antileishmanial and antitrypanosomal), anti-inflammatory, antibacterial, antifilarial, antifungal, antimicrobial, anticonvulsant and anti oxidant activities.because of this, chalcones and their derivatives have attracted increasing attention of the scientists for the search of new potent pharmacological activity in it.
References:
1.S. V. Kostanecki and Tambor, J. Chem Ber., (1899), 32, 1921.
2. Awasthi SK, MishraN, KumarB, SharmaM, BhattacharyaA, MishraLC, BhasinVK, Potent antimalarial activity of newly synthesized substituted chalcone analogs in vitro. MedicinalChemistryResearch. (2009), 18:407-420.
3.ChengMS, ShiliR, KenyonG. A solid phase synthesis of chalconesby Claisen-Schmidt condensations.Chinese Chemical Letters, (2000), 11: 851-854.
4. LimSS, KimHS, LeeDU. In-vitro antimalarial activity of flavonoids and chalcones. Bulletin of theKorean Chemical Society, (2007), 28:2495-2497.
5.Straub, T. S. Tetrahedron Lett (1995), 36, 663.
6.Sandler, S., Karo, W. In Organic Functional Group Preparations. (1972), 3, 372.
7.Bergman, E. D., Ginsibm, L., Pappo, R. Org. React. (1959), 10, 179.
8.E. C. Taylor and R. W. Morrison, J. Org. Chem., (1967), 32, 2379 .
9. E. P. Kohler and H. M. Chandwell, Org. Synth. Coll., (1922), 2, 1,
10.Falcao de Fonseca L and Bol Fac Farm, Univ. Coimbra, Ed. Cient, 28, 49, (1968), C. A., (1970), 72, 121124p.
11.. N. Dhar and J. B. Lal, J. Org. Chem., (1958), 23, 1159.
12. (a) W. Davey and J. R. Gwilt, J. Chem. Soc., (1957), 1008, (b) R. E. Lyle and L. P. Paradis, J. Am. Chem. Soc., (1955),77, 6667 (c) M. G. Marathey, J. Uni. Poona, (1952), 1-19 .
13.Ballesteros, J. F., Sanz, M.J., Ubeda, A., Miranda, M. A., Iborra, S., Paya, M., Alcaraz, M. J. Med. Chem. (1995), 38, 2794.
14.Go, M. L., Wu, X., Liu, X.L. Curr. Med. Chem. (2005), 12, 483.
15.Mukerjee, V. K., Prased, A. K., Raj, A. G., Brakhe, M. E., Olsen, C. E., Jain, S. C., Parmer, V. P. Bioorg. Med. Chem. (2001), 9,337.
16.Liu, U. M., Wilairat, P., Croft, S. L., Tan, A. L., Go, M. Bioorg. Med .Chem. (2003), 11, 2729.
17.Sivakumar, P. M., Geetha Babu, S. K., Mukesh, DChem. Pharm. Bull. (2007) 55, 44.
18.Viana, G. S., Bandeira, M. A., Mantos, F. J. Phytomedicine. (2003) 10, 189.
19.Tiwari, N., Dwivedi, B., Nizamuddin, K. F., Nakanshi, Y., Lee, K. H. Bioorg .Med. Chem. (2000), 10, 699.
20.Ducki, S., Forrest, R., Hadfield, J. A., Kendall, A., Lawrence, N. J., Mc'Gown, A.T., Rennison, D. Bioorg. Med. Chem. (1998), 8, 1051.
21. A. B. Linke and D. E. Eveleigh, Z. Naturorsch, (1975), 30B, 740
22.D. . A. Akhmedzade, V. D. Yasnopol'skii and Y. I. Golovanova, Azerb KhimZh. 117 (1968), Chem. Abstr., (1968), 69, 107333n
23. International Minerals and Chemical Corpn.; British Patent 1, 189, 573 (1970), Chem. Abstr., 73, 45798q
24.E. Schraufstater and S. Deutch, Chem. Abstr., (1950), 44, 3563
25. Xia Y, Yang ZY, Xia P, Bastow KF, Nakanishi Y, Lee KH: Antitumor agents. Part 202: novel 2'-amino chalcones: design, synthesis and biological evaluation. Bioorg Med Chem Lett (2000), 10:699-701.
26.LeBlanc R, Dickson J, Brown T, Stewart M, Pati HN, VanDerveer D, Arman H, Harris J, Pennington W, Holt HL Jr, Lee M: Synthesis and cytotoxicity of epoxide and pyrazole analogs of the combretastatins. Bioorg Med Chem (2005), 13:6025-6034.
27.Wegener JW, Nawrath H: Cardiac effects of isoliquiritigenin. Eur J Pharmacol (1997), 326:37-44.
28.Rojas J, Paya M, Dominguez JN, Luisa FM: The synthesis and effect of fluorinated chalcone derivatives on nitric oxide production. Bioorg Med Chem Lett (2002), 12:1951-1954.
29. Nakamura C, Kawasaki N, Miyataka H, Jayachandran E, Kim IH, Kirk KL, Taguchi T, Takeuchi Y, Hori H, Satoh T: Synthesis and biological activities of fluorinated chalcone derivatives. Bioorg Med Chem (2002), 10:699-706.
30.S. Ishida, A. Matsuda and A. Kawamura, Chemotherapy, (1960), 8, 146.
31.G. Devaux, A. Nuhrich and V. Dargelos, Fr. Demande 2, 357, 247 (1978); Chem. Abstr., 89, 193384e.
32. H. Hismat, H. I. El-Diwani and F. R. Melek, Indian J. Chem., (1996), 35 (B), 30.
33. D. Salvie, F. Richard and A. John, Bio Org. Med. Chem. Lett., (1998), 9, 8.
34. E. Bombardeli and P. Valenti, Chem. Abstr., (2001), 134, 222628k
35.M. Uenaka, K. Kawata, M. Nagai and T. Endoh, Chem. Abstr., (2001), 134, 29421.
36.R. Seele, Eur. Patent 337198 (1989); Chem. Abstr., (1990), 113, 211-828.
37.M. Gschwendt, W. Kittstein, G. Fuerstenberger and F. Mark, Cancer Lett., 25, 177 (1984); Chem. Abstr., (1985) , 102, 216605
38.Prabhakar B, Laxma Reddy K, Lingaiah K: Synthesis, thermal, spectral and magnetic studies of complexes of Co(II), Ni(II), Cu(II), Ru(II), Pd(II) and Pt(II) with 2,3-disubstituted quinazolin-(3H)-4-ones. J Chem Sci (1989), 101:121-132.
|
PharmaTutor (ISSN: 2347 - 7881) Volume 1, Issue 2 Received On: 19/11/2013; Accepted On: 28/11/2013; Published On: 20/12/2013 How to cite this article: Yerragunta V, Kumaraswamy T, Suman D, Anusha V, Patil P, Samhitha T, A review on Chalcones and its importance, PharmaTutor, 2013, 1(2), 54-59 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE









