 About Authors:
About Authors:
Kapil Sharma*, Priyanka sharma**
*Yaresun Pharmaceutical Pvt Ltd,India
**M.sc. Student, Rajasthan, India
*pharma_kapil@rediffmail.com
1. INTRODUCTION
The mammalian intestinal tract contains a complex and diverse society of both pathogenic and non pathogenic bacteria. Most research to date has focused on the mechanism by which pathogenic bacteria achieve their detrimental effect. However, more recent research has unveiled a glimpse into the mechanism of action and potential therapeutic role of indigenious non pathtogenic microorganisms (probiotic).
Probiotic, prebiotic, and synbiotic are moving from snake oil into the mainstream of medical therapy. This evolution has been facilitated by our ever increasing understanding of the mechanism of action of these agents and by the development of molecular method for analyzing and identifying complex bacterial community within the mammalian intestine.1, 2
The clearly emerging probiotic and prebiotic era over the past number of years is a subject of current debate and intense research. The emergence the probiotics concept can be attributed to the observation at the turn of the centuary that the long and generally healthy life of some peasants in Bulgaria was due to their consuming fermented milk products. The use of fermented milk product, in the form of yoghurt for example, is of course not new. The concept that is new is the apparent importance of some specific microorganism (e.g. lactobacilli, bifidobacteria) used in the fermentation of such products in relation to the potential beneficial effects they confer on health in general.2
We know that this organism- probiotic- have prophylatic and therapeutic benefits in various gastrointestinal (GI) disorder such as diarrhoeas, lactose intolerance, irritable bowel syndrome, inflammatory bowel diseases, and in other non-gastrointestinal conditions. The benefits documented thus far are of varying degree and are most likely dependent on the bacterial strain in the formulation, the bacterial counts (number of active cells), the dosing pattern, and the characteristics of the host and its underlying luminal microbial environment. Supplementing probiotic with prebiotics, which are agent that stimulate the growth and/or activity of nonpathogenic bacteria, is more beneficial than administering only prebiotics. This combination of probiotic and prebiotics, called synbiotics, is gaining high interest and widespread use in the medical fraternity. However, understanding the importance of key characteristics that make a good combination of prebiotic and probiotic is a vital use.1, 2
Probiotics represents an expanding research area. A Medline search of the term probiotics illustrates the significant increase in research undertaken in this area during the past 5 years: over 1,000 publications cited, compared to 85 for the previous 25 years. While this demonstrates the potential significance of this emerging field, much still remains to be done to standardize the meaning of the term probiotic and which strains actually fulfill the criteria of true probiotic microorganisms. In addition, although clinical evidence of the tangible benefits of probiotics is mounting, this does not yet reflect the commercial front. Unfortunately, many so-called probiotic products have not been properly identified, documented, manufactured under good manufacturing practices, or proven clinically, yet various companies make claims that lead consumers and caregivers to believe that they are using reliable products. Thus, the establishment of standards and guidelines represents a necessary first step in making sure that probiotic products are indeed legitimate and effective. Such standards and guidelines have recently been generated and will be presented later.2, 3
Reference ID: PHARMATUTOR-ART-1270
2.1 PROBIOTICS
2.1.1 Introduction and Definition
Lilly and Stillwell introduced the term probiotics in 1965 for growth promoting factors produced by microorganisms. The word probiotic, derived from Greek, means ‘pro life’. In 1974, Parker used the term for ‘organisms and substances’ that influence the intestinal microflora and had beneficial effects on animals. The term ‘substances’ is imprecise and would include even antibiotics. Therefore, in 1989 Fuller defined probiotics as ‘a live microbial feed supplement, which beneficially affects the host animal by improving its intestinal microbial balance’. However, according to this definition probiotics were restricted to feed supplements, animals and the intestinal tract, and the term probiotic thus could not used for living microorganisms administeredin any other way than in food or feed, or for locations other than the GI tract. Consequently, in 1992 Havenaar and Huis Veld proposed to Broaden Fuller’s defination into ‘a probiotic is a mono-or mixed culture of live microorganisms which, applied to animal or man, affect beneficially the host by improving the properties of the indigenious microflora’.2,3,4
Probiotic bacteria are commonly defined as viable bacteria, in single or mixed culture, that have a beneficial effect on the health of the host. Commons descriptive for probiotic include ‘friendly’, ‘beneficial’ or ‘healthy’ bacteria. These are used to treat disturbed intestinal microflora and altered gut permeability which are characteristic to many intestinal disorders. Examples include children with acute rotavirus diarrhea, subjects with food allergy and patients undergoing pelvic radiotherapy. Altered intestinal microflora has been treated by oral intake of probiotic bacteria, which are able to survive gastric conditions, colonise the intestine, at least temporarily, by adhering to the intestinal epithelium. Such probiotic microorganisms appear to be promising candidates for the treatment of clinical conditions with abnormal gut microflora and altered gut mucosal barrier functions.4
Criteria that aid the selection of efficacious probiotic includes:-
· Those isolated from the same species as the intended use ought to have an enhanced chance of survival.
· Strain count- The supplement should have adequate number of colony forming units to confer a health benefit.
· Strain taxonomy- The probiotic strain should be identified by an accurate taxonomy.
· Inhabitation – Should be a normal inhabitant of the human species.
· Safety- Probiotic should be generally recognized as safe (GRAS) with minimal possibilities for the transfer of antibiotic resistance. It should be nontoxic and nonpathogenic.
· Survivability- Both in the product and after ingestion. Strains that have improved resistance to acid, bile secretions and able to attach gut epithelium would have better survival characteristics.
· Production characteristics- Able to be grown in bulk culture, without genetic variation.
· Processing- Robust enough to withstand the rigors associated with incorporation into oral delivery systems. Amenable to production processing: adequate growth, recovery, concentration, freezing, dehydration, storage, and distribution.
· Microbiological properties – Required to survive in the gastrointestinal microbial ecosystem.
· Effects on the consumer – No adverse side effect such as bloating and effect on gut transit should occur. Should be able to exert one or more clinically documented health benefits.
· Adherence – To enhance survival in the gut.
· Effect on pathogens – Many probiotic is able to inhibit adverse microorganisms by the production of acid, bacteriocins or competitive exclusion.
· Modulation of metabolic activities – Such as the inactivation of pro-carcinogens.
· Immunomodulation – Probiotic may affect the immune system such that improved pathogen resistance occurs, as well as positive aspects with to food allergy.
· Genetic stability – Should be genetically stable.
· Viability – Should be viable at high populations.
· Produce antimicrobial substances – Should be able to produce antimicrobial substances, including bacteriocins, H2O2, and organic acids.
· Antagonistic activity – Antagonistic toward pathogenic/cariogenic bacteria.
· Organoleptic properties – Should have desirable organoleptic qualities (or no undesirable qualities) when included in fermented products.
2.1.2 The microorganisms used as probiotic
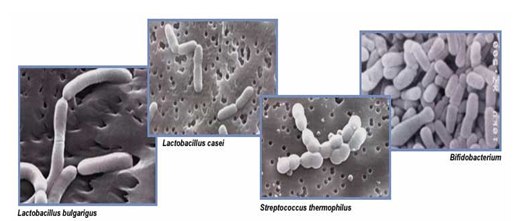
Lactobacilli:
Lactobacilli are found primarily in the small intestine and play an important role in the initial stages of digestion and assimilation of food. Lactobacilli provide a number of important benefits that affect the health of the intestinal mucosa as well as the general overall health of the individual.2,5
L. acidophilus: L.acidophilus is microaerophilic, and hence can colonize the ends of the small intestine and the colon. It enhances phagocytosis, helps in inhibition of uropathogens in human vagina (especially C. albicans), aids in developing natural defenses against intestinal bacteria and viral infections as well as decreasing the duration of diarrhoea in children, reduces faecal putrefactive bacteria and appears to decrease H. pylori density in the human stomach.2,5
L. rhamnosus: This probiotic is also one of the normal microfloras of the intestinal and vaginal tract. It is resistant to gastric acidity and is able to grow well in the presence of bile. It transits the intestinal tract relatively rapidly. However, it is capable of adhering to intestinal mucosa, and cells are capable of persistence within the gut.2,5
Bifidobacteria:
While Lactobacilli are resident of the small intestine, Bifidobacteria are abundant in the colon, the lower portion of the intestine and the vaginal tract. The health of the large intestine is dependent upon adequate colonisation of these organisms. Bifidobacteria produce short-chain fatty acids(SCFAs), including acetic, propionic, butyric, lactic and formic acids, with acetic acids being most plentifully produced. Acetic acid is important because GI health exerts a wide range of antimicrobial activity against yeasts, moulds, and bacteria. Thus, a healthy intestinal environment allows for active production of organic acids and antibiotics, which function as an integral part of our immune system.2,5
· B. longum: this probiotic microorganism reduces erythromycin induced GI distress, ulcerative colitis and display anticarcinogenic activity in the colon. B. longum has high affinity for intestinal colonisation, improves intestinal environment, defaecation frequency and faecal characteristics.
· B. bifidum: Abundant in the lower portion of the small intestine and vaginal tract, B. bifidum helps manufacture B vitamins and inhibits colonisation of candida. It provides enhanced immune response, inhibits harmful enzymes and lowers pH. It is antibacterial against many pathogens including E. coli, Salmonella spp and Shigella spp.
· B. infantis: This probiotic species has been shown to be the main inhabitant of every healthy infant’s GI tract. It is also found in small amount in the vaginal tract. B. infantis functions synergistically with B. bifidium.2,5
· B.breve: It enhances the immune response, provides passive protection against rotavirus-induced diarrhoea, helps in stabilization of intestinal flora, stimulates the immune system, and helps in infection control against S. aureus in neonatal ICU.2,5
Saccharomyces:
Saccharomyces belongs to the yeast family. The principal probiotic yeast is Saccharomyces boulardii. Saccharomyces boulardii is also known as Saccharomyces cerevisiae Hansen CBS 5296 and S. boulardii. S. boulardii is normally nonpathogenic yeast. S. boulardii has been used to treat diarrhea associated with antibiotic use.5
Streptococcus Thermophilis:
Streptococcus thermophilus is a gram-positive facultative anaerobe. It is a cytochrome-, oxidase- and catalase-negative organism that is nonmotile, non-spore forming and homofermentative. Streptococcus thermophilus is an alpha-hemolytic species of the viridans group. It is also classified as a lactic acid bacterium (LAB). Steptococcus thermophilus is found in milk and milk products. It is a probiotic and used in the production of yogurt. Streptococcus salivarus subspecies thermophilus type 1131 is another probiotic strain.5
Enterococcus:
Enterococciare gram-positive, facultative anaerobic cocci of the Streptococcaceae family. They are spherical to ovoid and occur in pairs or short chains. Enterococci are catalase-negative, non-spore forming and usually nonmotile. Enterococci are part of the intestinal microflora of humans and animals. Enterococcus faecium SF68 is a probiotic strain that has been used in the management of diarrheal illnesses5
2.2 PREBIOTICS
2.2.1 Introduction and Defination
Gibson and Roberfoid define prebiotic as ‘a non-digestiable food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon that can improve the host health’.
The term prebiotic generally refers to a food component that cannot be digested in the gut but has potentially beneficial effects on the host by selectively promoting the growth/activity of a number of microorganisms in the large bowel, such as Bifidobacteria and Lactobacilli, which have potential health promoting effects. Thus, to be effective, prebiotics must escape digestion in the upper gastrointestinal tract and be used by a limited number of the microorganisms comprising the colonic microflora. Prebiotics are principally oligosaccharides. They mainly stimulate growth of bifidobacteria, for which reason they are referred to as bifidogenic factors.2,5,7
For a substance to be classified as a prebiotic, it must:
· Neither be hydrolysed nor absorbed in the upper part of the Gi tract
· Be a selective substrate for one or a limited number of potentially beneficial bacteria commensal to the colon, which are metabolically activated.
· Consequently, be able to alter the colonic microflora towards a healthier composition, for example by increasing the number of saccharolytic species and reducing putrefactive microorganisms such as asaccharolytic clostridia and enterobacteriaceae.
Like probiotics, prebiotics belong to a more general class of colonic food, i.e. food
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
3.1 Introduction and Defination
First probiotics then prebiotics. Now the two are being combined to produce synbiotics. The principal behind probiotics is that our gastrointestinal system is populated by a large number and variety of different bacteria. In very general terms, some of the bacteria contribute to our good health, but others are responsible for disease and infections. By consuming foods that contain probiotic bacteria, it is believed that the population of beneficial bacteria can be increased – perhaps only temporarily – and good metabolism and heath will be achieved. However, finding bacteria that have probiotic properties has not been easy. In addition, since the probiotic bacteria that are consumed do not normally reside in the digestive system, they are soon washed out of the intestines if consumption of the probiotic product is stopped.5, 9
Synbiotics refer to combination nutritional supplements comprised of probiotics and prebiotics. Probiotics are live microbial food supplements that sebeneficially affect the host by improving its intestinal microflora balance. Prebiotics (are nondigestible dietary substances, typically oligosaccharides and disaccharides, that beneficially affect the host by selectively stimulating the growth and/or activity of a limited number of bacterial species already resident in the large intestine.Synbiotics could improve the survival of the probiotic organism by providing the specific substrate to the probiotic organism for its fermentation.5,9
Synbiotic supplements that are currently available include combinations of bifidobacteria and fructo-oligosaccharides (FOS), Lactobacillus GG and inulins and bifidobacteria and lactobacilli and FOS or inulins. Symbiotic is a term that is being used increasingly to denote synergistic relationships between viable beneficial bacteria and their selective substrate, i.e. when a product contains both probiotics and prebiotics. Because the word alludes to synergism, this term should be reserved for product in which prebiotic compound selectively favour the probiotics compound, eg. Fos in combination with prebiotics such as B. infants, B. longum etc. combining prebiotic with probioticcould improve the survival of bacteria crossing the upper part of the GI tract, thus enhancing their effects in the large bowel. Moreover the local and systemic beneficial effects of probiotics and prebiotics might be additive or even synergistic.5, 9
3.2 Rationale for the concept of synbiotics
The symbiotic concept combines both the prebiotic and probiotic approaches. According to this approach, a food or food supplement will include both the live cells of beneficial bacteria and a selective substrate;
The idea being that the beneficial bacteria cells that survive their transit through the stomach can grow quickly andcompetitively because of the presence of the selective substrate and establish their predominance.2,6,9
According to Crittenden, the prebiotic approach for increasing beneficial bacteria in the colon potentially provides some advantages over the probiotic strategy. Namely, consumed probiotic bacteria must survive transit through the hostile conditions in the stomach and then adapt quickly to their new environment (survivability and colonization may be a problem). On the contrary, prebiotic offer not only the potential to increase the number of beneficial bacteria, but also their metabolic activity through the supply of fermentable substrate.2,6,9
The increase in metabolic activity of autochthonous or allochthonous (probiotic) microorganisms is fundamental to many of the currently proposed mechanisms of health promotion by prebiotics. A further step in this direction is the use of synbiotics, where prebiotics and probiotics are combined. Thus, the living microbial additions would be used in conjunction with a specific substrate for growth. The results of many researches point to a synergistic effect of probiotic and prebiotic combination on faecal microflora of experimental animals. This effect was demonstrated by increased total anaerobes, aerobes, lactobacilli, and bifidobacteria counts as well as by decreased Clostridia, Enterobacteriaceae and E.coli counts.
The combination of probiotics and non-digestible carbohydrate may be a way of stabilisation and/or improvement of the probiotic effect. Such synbiotics indicate a realistic way of using biological preparations in the prevention of gastrointestinal diseases.2,6,9
MECHANISM OF ACTION PROBIOTICS & PREBIOTICS
|
Targets |
Postulated Mechanism |
|
Resistence to enteric pathogens |
Secretory immune effect · Alteration of intestinal conditions to be less favorable for pathogenicity (pH, short chain fatty acids, bacteriocins) · Alteration of toxin binding sites to enterocytes · Influence on gut flora populations. · Adherence to intestinal mucosa, interfering with pathogen adherence. |
|
Small bowel bacterial infections |
Influence on activity of overgrown flora, decreasing toxic metabolite production. |
|
Urogenital infection |
· Adhesion to urinary and vaginal tract ce |











.png)


