About Authors:
Richa Thakur*, Gaurav Swami, M.S. Rathore, A.K. Sharma
CT Institute of Pharmaceutical Sciences,
Jalandhar, Punjab, India
*thakur.richa89@gmail.com
ABSTRACT
The anatomy, physiology and biochemistry of the eye render this organ exquisitely impervious to foreign substances. The main challenge to the formulator is to circumvent the protective barriers of the eye without causing permanent tissue damage. The newly developed particulate and vesicular systems like liposomes, pharmacosomes and discosomes are useful in delivering the drug for a longer extent and helpful in reaching the systemic circulation. The most recent advancements of the ocular delivery systems provide the delivery of the genes and proteins to the internal structures which were once inaccessible and thus are of great importance in treating the diseases which are caused due to genetic mutation, failure in normal homeostasis, malignancy but also maintaining the physiological function of eye. The review focuses on the developments achieved in this mode of delivery of the drugs along with the pros and cons associated with greater focus on the advanced delivery systems.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1309
INTRODUCTION
Ocular drug delivery is one of the most fascinating and challenging errands facing the Pharmaceutical researchers. One of the major barriers of ocular medication is to obtain and maintain a therapeutic level at the site of action for prolonged period of time. The anatomy, physiology and biochemistry of the eye render this organ exquisitely impervious to foreign substances. The challenging to the formulator is to circumvent the protective barriers of the eye without causing permanent tissue damage. The development of newer, more sensitive diagnostic techniques and therapeutic agents renders urgency to the development of maximum successful and advanced ocular drug delivery systems1.
In the earlier period, drug delivery to the eye has been limited to topical application, redistribution into the eye following systemic administration or directs intraocular/periocular injections. Conventional drug delivery systems; which include solutions, suspensions, gels, ointments and inserts, suffer with the problems such as poor drainage of instilled solutions, tear turnover, poor corneal permeability, nasolacrimal drainage, systemic absorption and blurred vision2.
Nanocarrier based approaches seem to be most attracting and are extensively investigated presently. it has been reported that particulate delivery system such as microspheres and nanoparticles; vesicular carriers like liposomes, niosomes, pharmacosomes and discomes improved the pharmacokinetic and pharmacodynamic properties of various types of drug molecules3.
Emerging new controlled drug delivery systems such as dendrimers, ocusert, micro emulsions, muco-adhesive polymers, hydro gels, iontophoresis, collagen shield, prodrug approaches have been developed for this purpose. These novel systems offer manifold advantages over conventional systems as they increase the efficiency of drug delivery by improving the release profile and also reduce drug toxicity.
The rapid progress of the biosciences opens new possibilities to meet the needs of the posterior segment treatments. The examples include the antisense and aptamer drugs for the treatment of cytomegalovirus (CMV) retinitis and age-related macular degeneration, respectively, and the monoclonal antibodies for the treatment of the age-related macular degeneration. Other new approaches for the treatment of macular degeneration include intravitreal small interfering RNA (siRNA) and inherited retinal degenerations involve gene therapy. This review article briefly covers general outline with examples of various conventional and recent past time formulations for ophthalmic drug delivery. It also provides the limitations of conventional delivery with a view to find modern approaches like vesicular systems, nanotechnology, stem cell therapy as well as gene therapy, oligonucleotide and aptamer therapy, protein and peptide delivery, ribozyme therapy for treatment of various ocular diseases.
[adsense:468x15:2204050025]
DISORDERS OF EYE
Human eye, from anterior to posterior segment, consists of vitreous humor, ciliary body, lens, cornea, conjunctiva, aqueous humor, iris, choroid, retina and sclera. The shape of human eye is spherical with a diameter of nearly 23mm. It has complicated arrays of delicate mechanisms behind its visible portions which work in union to transmit an image of the seen object to the brain. The extent and quality of light entering into the eye is regulated and filtered by pupil, which dilates and contracts as per requirement. Functionally structural components of the eyeball can be divided into three layers: (i) outer most coat comprises of the clear, transparent cornea and white, opaque sclera; (ii) middle layer hold the iris anteriorly, choroid posteriorly, and ciliary body as intermediate part; (iii) inner layer possess retina, which is an extension of the central nervous system
The fluid systems viz the aqueous humor and vitreous humor plays an important and decisive role in maintenance of homeostasis of the eye. Cornea, an optically transparent tissue having diameter of 11.7 mm and thickness 0.5-0.7 mm, performs as the principal refractive element of the eye. The common eye disorders include age-related macular degeneration, diabetic macular edema, cataract, proliferative vitreoretinopathy, uveitis, cytomegalovirus and glaucoma4-6. The detailed discussion on ocular disorders is beyond the scope of this manuscript. However a brief overview has been presented in Fig. (1).
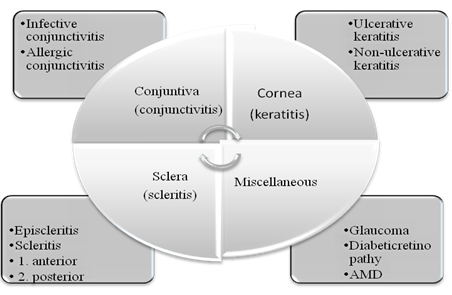
Figure 1Common ocular disorders associated with various tissues of eyes57
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
LIMITATIONS OF VARIOUS ROUTES OF OCULAR DRUG DELIVERY
Common routes of ocular drug delivery are topical, subconjunctival, intra-vitreal, retrobulbur and intracameral (Table 1). In topical route, drug is delivered with the help of eye-drops either in the form of solutions or suspensions. The major limitation is that systems have a short contact time with ocular mucosa; however it can be prolonged by modified formulations such as gels, inserts and implants7. Subconjunctival route is used to introduce drugs that do not penetrate into the anterior segment. Intravitreal route delivers the drugs directly to the vitreous and retina. Small molecules, usually below 500 Da, are diffused more rapidly than large ones.
The administered drug is eliminated by two main routes after intravitreal injection either anterior and/ or posterior (via blood flow and aqueous turnover). In retrobulbar route, drug delivered may enter the globe as the orbit is not well vascularized and hence effect by this route is very less. Retrobulbar injection involves inserting a needle through the eyelid and orbital fascia and depositing a drug behind the globe into the retrobulbar space. This route is used to provide medication to the posterior segment, to effect the structure of nerve in that space. Retrobulbar injections commonly used for regional anesthesia of the orbit or face, but can also be used for local. Physical or chemical damage to intraocular structures such as cornel endothelium, iris and less may be associated during intracameral injections. Eventually, mode and mechanism of drug action and diseased conditions are vital issues that restrict the formulator for selection of routes. Fig. (2), provides important features associated with means of drug delivery and suitable considerations required during drug designing.
Table 1
Ocular Routes for Delivery of Bioactive(s)57
|
Route of administration |
Advantages |
Limitations |
|
Topical |
Convenient to deliver drugs |
Inefficient delivery to the posterior segment, nasolacrimal drainage, short contact time of drug on the ocular surface |
|
Systematic |
Convenient to deliver large amount as compared to eye drops |
Poor bioavailability of drug in the retina and systemic absorption |
|
Intravitreal |
Drug delivered directly to the vitreous and retina in the form of injections and implants |
Problems such as cataract, endophthalmitis, retina detachment and hemorrhage occurs as a side effect |
|
Subconjunctival (S. C.) |
Both anterior and vitreous level of the drug can be achieved and act as common route of administration |
Difficult to deliver drugs to the retina due to the presence of retinal pigment epithelium |
|
Retrobulbar |
Provide medication to the posterior segments for the treatment of posterior diseases |
Effect provides by this route is very less as drug may enter the globe of eye. |
|
Intracameral |
Deliver drugs directly to the anterior and vitreous chamber |
Difficult to deliver drugs to the posterior segment |
|
Sub-retinal |
Deliver drugs to the retina |
Retinal detachment occurs as a result of sub-retinal delivery |
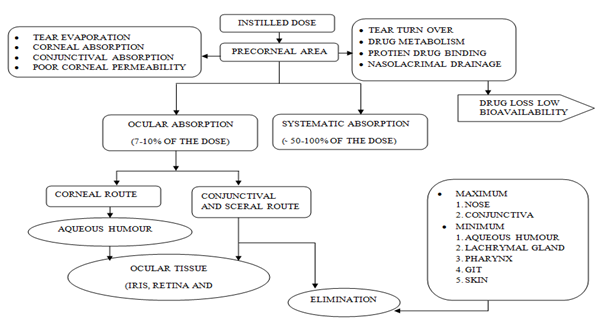
Figure 2 Flow chart of movement and distribution of drug within ocular tissues after ocular drug delivery and factors affecting absorption of drug in different ocular compartments57.

Figure 3 Different delivery systems which are currently used for ocular therapy57
LIMITATIONS OF DIFFERENT OCULAR FORMULATIONS
Conventional drug delivery systems used for the instillation of dosage form are very convenient and are easy to use by all age groups even though bear several challenges. Drugs which are active at eye or eye surface are widely administered in the form of Solutions, Emulsion and Suspension8.
Since conventional drugs upon topically administration remain in the anterior segment and do not provide drug delivery to posterior segment of eye. Therefore, there is need for safe, effective and convenient delivery systems which deliver the drug at the target sites9. Such restrictions put a ceiling on the formulator to develop efficient drug delivery conventional dosage form for above said purposes. Fig. (3) shows options and opportunities available with regard to ocular delivery systems used for ocular therapy. Table 2 briefly covers up the advantages and limitations of different drug delivery systems (conventional and novel both) which are usually employed for ocular drug delivery.
Table 2
Advantages and Limitations of Different Ocular Formulations57
|
S.No |
Carrier |
Advantages |
Limitations |
|
1 |
Eye Solution |
· Easy to instilled · Convenient · Economic |
· Drainage · Drug loss by tear fluid · Low bioavailability · Regular instillation |
|
2 |
Suspension |
· Prolonged contact time · Patient compliance |
· Irritation ( particle size) · Loss of drug solution |
|
3 |
Ointments |
· Prolonged contact time · No tear dilution · Improved stability · Improved bioavailability |
· Blurring vision · Poor patient compliance |
|
4 |
Ocular Inserts |
· Comfortable · Prolonged delivery · Reduced dose frequency |
· Difficult to insert & remove · Requires skill for insertion |
|
5 |
Liposomes |
· Stable · Control drug release · Reduced dosing frequency · Improved bioavailability |
· Stability problems · Not reproducible · Rapid clearance · Uptake by conjunctival cells |
|
6 |
Niosomes |
· Stable · Controlled drug release · Reduced dosing frequency · High entrapment efficiency |
· Less bioavailability when uptake by conjunctival cells |
|
7 |
Nanoparticles |
· Small size · Long shelf life, · Highly stable · Improved bioavailability · Reduced dosing frequency |
· Particle contamination |
|
8 |
Microparticles |
· Stable · Improved bioavailability · Reduced dosing frequency |
· Irritation occurs · Large particle size |
|
9 |
Implants |
· Biodegradable · Non-toxic |
· Surgical application |
|
10 |
Penetration Enhancers |
· Promote penetration of drugs · Improved bioavailability |
· Toxicity & irritation · Large concentration |
|
11 |
Prodrugs |
· Prolonged residence time · Reduced dosing |
· Metabolism problems |
|
12 |
Hydrogels |
· Prolonged residence time · Increase bioavailability |
· Triggered by temperature, pH and Ionic strength |
|
13 |
Dendrimers |
· Small size · Decreased dosing frequency · Prolonged residence time · Improved bioavailability |
· Blurring vision |
|
14 |
Micro-Emulsions |
· Stable · Improved solubility · Improved bioavailability · Reduced dosing frequency |
· Toxicity (higher concentration), · Selection of surfactant/co-surfactant and · aqueous/organic phase affects its stability |
|
15 |
Nano-Suspensions |
· Stable · Prolonged contact time · Improved bioavailability · Enhance solubility |
· Used for poorly soluble drugs |
|
16 |
Cyclodextrins |
· Improved solubility · Improved bioavailability · Improved permeation · Prolonged residence time |
· Used to enhance solubility of poorly soluble drugs |
|
17 |
Gene Therapy |
· Viral and Nonviral vectors · High transfection efficiency |
· Ethics and immunologic problems |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
NOVEL OCULAR DRUG DELIVERY SYSTEMS
1. Nanotechnology system
Nanotechnology based treatment strategies have now been routinely applied in the treatment of ocular disorders affecting the anterior and the posterior segment of the human eye. The noteworthy impact of nanotechnology on this issue is that limitations of conventional delivery systems in ocular drug delivery and different barriers present inside the eye can be successfully overcome. Nanocarriers, which are now widely accepted for controlled and targeted drug delivery, seems the next hope in this area. Nanocarriers offer selective targeting along with sustained release of molecules at the desired site. Liquid dosage form like microemulsion, nanosuspensions; particulate carriers like microparticles, polymeric and lipid - nanoparticles, vesicular carriers like liposome, niososme, pharmacosomes and discomes along with many others such as dendrimers, hydrogel systems and prodrug approaches etc., have been developed. In the following sections, we will discuss these nanocarriers and their potential in ocular drug delivery with comprehensive description of each. The overall advantageous features of nanocarriers and shortcoming of other possible means of ocular drug delivery57.
2. Vesicular ocular system
2.1 Liposomes
Liposomes are biocompatible and biodegradable lipid vesicles made up of natural lipids and about 25–10,000 nm in diameter10. They are having an intimate contact with the corneal and conjunctival surfaces which is desirable for drugs that are poorly absorbed, the drugs with low partition coefficient, poor solubility or those with medium to high molecular weights and thus increases the probability of ocular drug absorption11. The corneal epithelium is thinly coated with negatively charged mucin to which the positive charged surface of the liposomes may bind12. Although liposomes are versatile carriers but they exhibit some disadvantages such as poor stability and reproducibility, low drug entrapment efficiency, uptake by phagocytosis and intraocular clouding while administered through intravitreal injection13.
2.2 Niosomes and Discomes
The major limitations of liposomes are chemical instability, oxidative degradation of phospholipids, cost and purity of natural phospholipids. To avoid this niosomes are developed as they are chemically stable as compared to liposomes and can entrap both hydrophobic and hydrophilic drugs. They are non toxic and do not require special handling techniques. Niosomes are nonionic surfactant vesicles that have potential applications in the delivery of hydrophobic or amphiphilic drugs. Vyas et al reported that there was about 2.49 times increase in the ocular bioavailability of timolol maleate encapsulated in niosome as compared to timolol maleate solution14. Non-ionic surface active agents based discoidal vesicles known as (discomes) loaded with timolol maleate were formulated and characterized for their in vivo parameters. In vivo studies showed that discomes released the contents in a biphasic profile if the drug was loaded using a pH gradient technique. Discomes may act as potential drug delivery carriers as they released drug in a sustained manner at the ocular site15.
2.3 Pharmacosomes
This term is used for pure drug vesicles formed by the amphiphilic drugs. Any drug possessing a free carboxyl group or an active hydrogen atom can be esterified (with or without a spacer group) to the hydroxyl group of a lipid molecule, thus generating an amphiphilic prodrug. The amphiphilic prodrug is converted to pharmacosomes on dilution with water. The pharmacosomes show greater shelf stability, facilitated transport across the cornea, and a controlled release profile16.
3. Control delivery systems
The first controlled drug delivery device for ocular purpose was developed in the form of Ocusert in 1975 which was capable enough to deliver the pilocarpine at constant rate for a week in the ocular conjunctival sac. Afterward journey of sustained and controlled delivery of drugs to the ocular tissues started and still unremitting to be a major objective for formulation scientists in the emergence of more potent drugs/biomolecules. Sahoo et al. 2008 discussed application of nanotechnology in ocular drug delivery. They elaborated how the various upcoming of nanotechnology like nanodiagnostics, nanoimaging and nanomedicine can be utilized to explore the frontiers of ocular drug delivery and therapy17. Mainardes RM18reviewed progress in development of colloidal carriers for ophthalmic drug delivery.
3.1. Implants
For chronic ocular diseases like cytomegalovirus (CMV) retinitis, implants are effective drug delivery system. Earlier non biodegradable polymers were used but they needed surgical procedures for insertion and removal. Presently biodegradable polymers such as Poly Lactic Acid (PLA) are safe and effective to deliver drugs in the vitreous cavity and show no toxic signs19. Intravitreal implants of fluocinolone acetonide were developed for the treatment of posterior segment and reported to control the ocular inflammation of retina20.
3.2.Iontophoresis
In Iontophoresis direct current drives ions into cells or tissues. For iontophoresis the ions of importance should be charged molecules of the drug21. Positively charged of drug are driven into the tissues at the anode and vice versa. Ocular iontophoresis delivery is not only fast, painless and safe but it can also deliver high concentration of the drug to a specific site. Iontophoresis application of antibiotics in eye not only increases their bactericidal activity but also reduce the severity of disease. Similarly application of anti-inflammatory agents can reduce vision threatening side effects22-23
3.3. Dendrimers
Dendrimers can successfully used for different routes of drug administration and have better water-solubility, bioavailability and biocompatibility. Vandamme24 developed and evaluated poly (amidoamine) dendrimers containing fluorescein for controlled ocular drug delivery and determined the influence of size, molecular weight and number of amine, carboxylate and hydroxyl surface groups in several series of dendrimers. The residence time was longer for the solutions containing dendrimers with carboxylic and hydroxyl surface groups.
3.4.Cyclodextrin
Cyclodextrins (CDs) are cyclic oligosaccharides capable of forming inclusion complexes with many guest molecules24. CD complexes are reported to increase corneal permeation of drugs like dexamethasone, dexamethasone acetate, cyclosporine and pilocarpine resulted in higher bioavailability than the conventional eye drops25-26. This complexation of CD does not interrupt the biological membrane compared to conventional permeation enhancer like benzalkonium chloride. Due to inclusion, the free drug is not available, so drugs with inherent irritant properties can be successfully delivered by this approach. CD molecules are inert in nature and were found to be non irritant to the human and animal eye24.
3.5.Contact lenses
Water soluble drugs soaked in drug solutions can be absorbed through Contact lenses. The drug saturated contact lenses are placed in the eye which releases the drug in eye for a long period of time. For prolongation of ocular residence time of the drugs, hydrophilic contact lenses can be used. Greater penetration of fluorescein has been reported by Bionite lens made from hydrophilic polymer (2-hydroxy ethyl methacrylate) in human27.
3.6.Ocuserts and Lacrisert
Ocular insert (Ocusert) are sterile preparation that prolong residence time of drug with a controlled release manner and negligible or less affected by nasolacrimal damage28 . Inserts are available in different varieties depending upon their composition and applications. Lacrisert is a sterile rod shaped device for the treatment of dry eye syndrome and keratitis sicca and was introduced by Merck, Sharp and Dohme in 1981. They act by imbibing water from the cornea and conjunctiva and form a hydrophilic film which lubricates the cornea29 .
3.7.Collagen Shield
Collagen shield basically consist of cross linked collagen, fabricated with foetal calf skin tissue and developed as a corneal bandage to promote wound healing. Topically applied antibiotic conjugated with the shield is used to promote healing of corneal ulcers. Tear fluid makes these devices soft and form a thin pliable film which is having dissolution rate up to 10, 24 or 72 hours. Because of its structural stability, good biocompatibility and biological inertness, collagen film proved as a potential carrier for ophthalmic drug delivery system. Collagen ophthalmic inserts are available for delivery of pilocarpine to the eye30.
3.8.Microemulsion
Microemulsion is dispersion of water and oil stabilized using surfactant and cosurfactant to reduce interfacial tension and usually characterized by small droplet size (100 nm), higher thermodynamic stability and clear appearance31. Selection of aqueous phase, organic phase and surfactant/cosurfactant systems are critical parameters which can affect stability of the system. Optimization of these components results in significant improvement in solubility of the drug molecule e.g. indomethacin, chloramphenicol for eye diseases32.
3.9.Nanosuspensions
Nanosuspensions have emerged as a promising strategy for the efficient delivery of hydrophobic drugs because they enhanced not only the rate and extent of ophthalmic drug absorption but also the intensity of drug action with significant extended duration of drug effect. For commercial preparation of nanosuspensions, techniques like media milling and highpressure homogenization have been used33. The higher drug level in the aqueous humour was reported using Eudragit RS100 nanosuspensions for the ophthalmic controlled delivery of ibuprofen34.
3.10. Microneedle
As an alternative to topical route Researchers have developed microneedle to deliver drug to posterior segment. The extent of lateral and transverse diffusion of sulforhodamine was reported to be similar across human cadaver sclera. Microneedle had shown prominent in vitro penetration into sclera and rapid dissolution of coating solution after insertion while in vivo drug level was found to be significantly higher than the level observed in topical drug administration like pilocarpine35.
3.11. Prodrugs
The ideal Prodrugs for ocular therapy not only have increased lipophilicity and a high partition coefficient, but it must also have high enzyme susceptibility to such an extent that after corneal penetration or within the cornea they are either chemically or enzymatically metabolized to the active parent compound. The partition coefficient of ganciclovir found to be increased using an acyl ester prodrug, with substantially increased the amount of drug penetration to the cornea which is due to increased susceptibility of the ganciclovir esters to undergo hydrolysis by esterases in the cornea36.
3.12. Penetration Enhancers
Transport of drug across the cornea is increased by increasing the permeability through corneal epithelial membranes. For such purpose Penetration enhancers can be used37. Examples of enhancers include actin filament inhibitors, surfactants, bile salts, chelators, and organic compounds. Selection of enhancer is critical due to unique characteristics and great sensitivity of the corneal conjunctival tissues. Penetration enhancers themselves can penetrate the eye and may lead to unknown toxicological complications e.g., benzalkonium chloride (BAC) was found to accumulate in the cornea for days38.
3.13. Mucoadhesive Polymers
They are basically macromolecular hydrocolloids with plentiful hydrophilic functional groups, such as hydroxyl, carboxyl, amide and sulphate having capability for establishing electrostatic interactions39. A Mucoadhesive drug formulation for the treatment of glaucoma was developed using a highly potent beta blocker drug, levobetaxolol (LB) hydrochloride and partially neutralized poly acrylic acid (PAA). Complexes were prepared with varying degrees of drug loading, such that the same PAA chain would have free -COOH groups for mucoadhesion along with ionic complexes of LB with COO- groups. Thin films of the complexes dissociated to release the drug by ion exchange with synthetic tear fluid40.
3.14. Phase Transition Systems/Insitu gel system
Phase transition of the formulation from the liquid form to the gel or solid phase occurs when these systems instilled into the cul-de-sac of eye lead to increase the viscosity of a drug formulation in the precorneal region results in increased bioavailability, due to slower drainage from the cornea. These systems can be influenced by pH, temperature or by ion activation. A sol to gel system with Mucoadhesive property to deliver the steroid fluorometholone to the eye Middleton and Robinson41 was prepared.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
4. Particulates (nanoparticles and microparticles)
The maximum size limit for microparticles for ophthalmic administration is about 5-10 mm above which a scratching feeling in the eye can result upon ocular instillation. That is why microspheres and nanoparticles are promising drug carriers for ophthalmic application42. Nanoparticles are prepared using bioadhesive polymers to provide sustained effect to the entrapped drugs. An optimal corneal penetration of the encapsulated drug was reported in presence of bioadhesive polymer chitosan43. Similarly Poly butyl cyanoacrylate nanoparticles, containing pilocarpine into collagen shields, showed greater retention and activity characteristics with respect to the controls44. Nanospheres made up of poly lactic acid (PLA) coated with Poly Ethylene Glycol (PEG) shown better efficacy compared to conventional dosage form of Acyclovir for the treatment of ocular viral infections45. Microspheres of poly lacto glycolic acid (PLGA) for topical ocular delivery of a peptide drug vancomycin were prepared by an emulsification/ spray-drying technique46.
5. Advanced delivery system
5.1. Cell Encapsulation
The entrapment of immunologically isolated cells with hollow fibres or microcapsules before their administration into the eye is called Encapsulated Cell Technology (ECT) which enables the controlled, continuous, and long-term delivery of therapeutic proteins directly to the posterior regions of the eye. The polymer implant containing genetically modified human RPE cells secretes ciliary neurotrophic factor into the vitreous humour of the patients’ eyes. ECT can potentially serve as a delivery system for chronic ophthalmic diseases like neuroprotection in glaucoma, anti-angiogenesis in choroidal neovascularization, anti-inflammatory factors for uveitis47.
5.2.Gene Therapy
Along with tissue engineering, gene therapy approaches stand on the front line of advanced biomedical research to treat blindness arising from corneal diseases, which are second only to cataract as the leading cause of vision loss48. Several kinds of viruses including adenovirus, retrovirus, adeno-associated virus, and herpes simplex virus, have been manipulated for use in gene transfer and gene therapy applications49. Topical delivery to the eye is the most expedient way of ocular gene delivery. However, the challenge of obtaining substantial gene expression following topical administration has led to the prevalence of invasive ocular administration48. Retroviral vectors have been widely used due to their high efficacy; however, they do not have the ability to transduce non-dividing cells, leads to restrict their clinical use49. The advanced delivery systems that prolong the contact time of the vector with the surface of the eye may enhance transgene expression, thereby facilitate non-invasive administration48.
5.3.Stem cell Therapy
Emerging cell therapies for the restoration of sight have focused on two areas of the eye that are critical for visual function, the cornea and the retina50. Current strategy for management of ocular conditions consists of eliminating the injurious agent or attempting to minimize its effects. The most successful ocular application has been the use of limbal stem cells, transplanted from a source other than the patient for the renewal of corneal epithelium. The sources of limbal cells include donors, autografts, cadaver eyes, and (recently) cells grown in culture. Stem-cell Therapy has demonstrated great success for certain maladies of the anterior segment50.
5.4.Protein and Peptide therapy
Delivery of therapeutic proteins/peptides has received a great attention over the last few years51. The intravitreous injection of ranibizumab is one such example. The designing of optimized methods for the sustained delivery of proteins and to predict the clinical effects of new compounds to be administered in the eye, the basic knowledge of Protein and Peptide is required52. However, several limitations such as membrane permeability, large size, metabolism and solubility restrict their efficient delivery. A number of approaches have been used to overcome these limitations. Poor membrane permeability of hydrophilic peptides may be improved by structurally modifying the compound, thus increasing their membrane permeability. Ocular route is not preferred route for systemic delivery of such large molecules. Immunoglobulin G has been effectively delivered to retina by trans scleral route with insignificant systemic absorption52.
5.5.Scleral Plug therapy
Scleral plug can be implanted using a simple procedure at the pars plana region of eye, made of biodegradable polymers and drugs, and it gradually releases effective doses of drugs for several months upon biodegradation. The release profiles vary with the kind of polymers used, their molecular weights, and the amount of drug in the plug. The plugs are effective for treating vitreoretinal diseases such as proliferative vitreoretinopathy, cytomegalovirus retinitis responds to repeated intravitreal injections and for vitreoretinal disorders that require vitrectomy53.
5.6.siRNA therapy
For various angiogenesis-related diseases, the use of siRNA is considered as a promising approach54. Feasibility of using siRNA for treatment of choroidal neovascularization has been demonstrated using siRNA directed against vascular endothelial growth factor (VEGF) or VEGF receptor 1 (VEGFR1), and both of these approaches are being tested in clinical trials. Topical delivery of siRNAs directed against VEGF or its receptors has also been shown to suppress corneal neovascularisation. siRNA has become a valuable tool to explore the potential role of various genes in ocular disease processes. It appears that siRNAs may be valuable in the pathogenesis and development of new treatments for several ocular diseases, based on in vivo and in vitro studies55. However, its use in vivo remains problematic, largely due to unresolved difficulties in targeting delivery of the siRNA to the tumor cells. Viral gene delivery is very efficient however it currently lacks adequate selectivity for the target cell type. New encapsulated siRNA have been developed using liposomes, coupled-antibodies or others polymer vesicles. Therapeutic approach using siRNA provides a major new class of drugs that will shed light the gap in modern medicine56.
5.7.Oligonucliotide therapy
Oligonucleotide (ON) therapy is based on the principle of blocking the synthesis of cellular proteins by interfering with either the transcription of DNA to mRNA or the translation of mRNA to proteins. Among several mechanisms by which antisense molecules disrupt gene expression and inhibit protein synthesis, the ribonuclease H mechanisms is the most important. A number of factors have been determined to contribute to the efficacy of antisense ON. One primary consideration is the length of the ON species. Lengths of 17– 25 bases have been shown to be optimal, as longer ONs have the potential to partially hybridize with nontarget RNA species. Biological stability is the major barrier to consider when delivering both DNA and RNA oligonucleotides to cells. Protection from nuclease action has been achieved by modification of phosphate backbones, sugar moiety, and bases56.
5.8.Aptamer
Aptamers are oligonucleotide ligands that are used for high-affinity binding to molecular targets[61]. They are isolated from complex libraries of synthetic nucleic acid by an iterative process of adsorption, recovery, and reamplification. They bind with the target molecules at a very low level with high specificity. One of the earliest aptamers studied structurally was the 15 mer DNA aptamer against thrombin, d(GGTTGGTGTGGTTGG)57. Pegaptanib sodium (Macugen; Eyetech Pharmaceuticals/Pfizer) is an RNA aptamer directed against VEGFb165, where VEGF isoform primarily responsible for pathological ocular neovascularization and vascular permeability56.
5.9.Ribozyme therapy
RNA enzymes or ribozymes are a relatively new class of single-stranded RNA molecules capable of assuming three dimensional conformations and exhibiting catalytic activity that induces site-specific cleavage, ligation, and polymerization of nucleotides involving RNA or DNA. They function by binding to the target RNA moiety through Watson-Crick base pairing and inactivate it by cleaving the phosphodiester backbone at a specific cutting site. A disease named, Autosomal dominated retinitis pigmentosa (ADRP) is caused by mutations in genes that produce mutated proteins, leading to the apoptotic death of photoreceptor cells. Lewin and Hauswirth have worked on in the delivery of ribozymes in ADRP in rats shows promise for ribozyme therapy in many other autosomal dominant eye diseases, including glaucoma56.
CONCLUSION
The novel ocular delivery systems offer more protective and effective means of the therapy for the nearly inaccessible diseases or syndromes of eyes. The latest available advance targeted/controlled drug delivery systems focus on the localized delivery of the drugs as well as certain macromolecular substances like proteins, genes like DNA, aptamers, siRNA to the internal parts of the eye.These delivery methods should provide prolonged action, less invasive administration, higher efficacy, and improved safety. Especially the advances in nanotechnology and biomaterials science may provide new smart technologies to augment ophthalmic drug delivery. Further developments are preferable which will eliminate the cons of these available advanced delivery systems and readily acceptable with the regulatory authorities as well.
References
1. Katz, I. M., (1982) Shaped ophthalmic inserts for treating dry eyes syndrome. U.S. Patent. 4,343,787.
2. Bourlais CL, Ophthalmic drug delivery systems- recent advances, Prog Retin Eye Res 17, 33-58,(1988).
3. Kaur IP, Vesicular systems in ocular drug delivery: an overview, Int J Pharm 269, 1-14, (2004).
4. Urtti A, Challenges and obstacles of ocular pharmacokinetics and drug delivery, Adv Drug Deliv Rev 58, 1131-1135, (2006).
5. Gaudana R, Recent perspectives in ocular drug delivery, Pharm Res 26(5), 1197-1216, (2009).
6. Vandervoort J, Ocular drug delivery: nanomedicine applications, Nanomedicine 2, 11-21, (2007).
7. Hornof M, Cell culture models of the ocular barriers, Eur J Pharm Biopharm 60, 207-225, (2005).
8. Mueller WH, Ophthalmic vehicles: The effect of methyl cellulose on the penetration of Homatropine hydro bromide through the cornea, J Am Pharma Assoc 45, 334-341, (1956).
9. Amo EM, Current and future ophthalmic drug delivery systems: a shift to the posterior segment, Drug Discovery Today 13, 135-143, (2008).
10. Ebrahim S, Applications of liposomes in ophthalmology, Surv. Ophthalmol, 50, 167–182, (2005).
11. Kaur IP, Vesicular systems in ocular drug delivery: An overview, Int J Pharm 269, 1-14, (2004).
12. Shek PN, Liposomes are effective carriers for the ocular delivery of prophylactics, Biochim Biophys Acta 902, 229–236, (1987).
13. Amo EM, Current and future ophthalmic drug delivery systems: a shift to the posterior segment. Drug Discovery Today 13, 135-143, (2008).
14. Vyas SP, Discoidal niosome based controlled ocular delivery of timolol maleate, Pharmazie, 53(7), 466-469, (1998).
15. Guinedi AS, Preparation and evaluation of reverse-phase evaporation and multilamellar niosomes as ophthalmic carriers of acetazolamide, Int J Pharm, 306, 71-82, (2005).
16. Kaur IP, Ocular preparations: The formulation approach, drug development and industrial pharmacy, 28(5), 473-493, (2002).
17. Sahoo SK, Nanotechnology in ocular drug delivery, Drug Discov Today 13, 144-151, (2008).
18. Mainardes RM, Colloidal carriers for ophthalmic drug delivery. Curr Drug Targets 6, 363-371, (2005).
19. Kimura H, A new vitreal drug delivery system using an implantable biodegradable polymeric device, Invest Ophthalmol Vis Sci, 35, 2815-2819, (1994).
20. Taban M, Outcome of fluocinolone acetonide implant (retisert trade mark) reimplantation for chronic non-infectious posterior uveitis, Retina, 28(9), 1280-1288, (2008).
21. Rootman DS, Pharmacokinetics and safety of transcorneal iontophoresis of tobramycin in the rabbit, Invest Ophthalmol Vis Sci, 29, 1397-1401, (1988).
22. Callegan MC, Ocular drug delivery: A comparison of transcorneal iontophoresis to corneal collagen shields, Int J Pharma, 123, 173- 179, (1995).
23. Vandamme TF, Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide, J Control Release 102, 23- 38, (2005).
24. Loftssonaand T, Cyclodextrins in ophthalmic drug delivery, Adv. Drug Deliv. Rev 36, 59–79, (1999).
25. Freedman KA, Betacyclodextrins enhance bioavailability of pilocarpine, Curr. Eye Res 12, 641–647, (1993).
26. Usayapant A, Effect of 2-hydroxypropyl-beta cyclodextrin on the ocular absorption of dexamethasone and dexamethasone acetate, Pharm. Res 8, 1495–1499, (1991).
27. Vadnere M, Thermodynamic studies on the gelsol transition of some pluronic polyols, Int J Pharma 22, 207-218, (1984).
28. Mishra DN, Design and characterization of bioadhesive in-situ gelling ocular insert of gatifloxacin sesquihydrate, DARU 16, 1-8, (2008).
29. Lawrenson JG, Comparison of the efficacy and duration of action of topically applied proxymetacaine using a novel ophthalamic delivery system versus eye drops in healthy young volunteers, Br J Opthalmol 77, 713-715, (1993) .
30. Vasantha R, Collagen ophthalmic inserts for Pilocarpine drug delivery system, Int J Pharma 47, 95-102, (1988).
31. Ansari MJ, Microemulsions as potential drug delivery systems: A review, PDA J. Pharm. Sci. Technol 62, 66–79, (2008).
32. Vandamme, T.F. Microemulsions as ocular drug delivery systems: recent developments and future challenges, Prog. Retin. Eye Res 21, 15–34, (2002).
33. Patravale VB, Nanosuspensions: a promising drug delivery strategy, J Pharm Pharmacol, 56, 827-840, (2004).
34. Pignatello R, Flurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic application, Biomaterials 23, 3247-3255, (2002).
35. Jiang J, Coated microneedles for drug delivery to the eye, Invest. Ophthalmol. Vis. Sci. 48, 4038– 4043, (2007).
36. Tirucherai GS, Corneal permeation of ganciclovir: Mechanism of ganciclovir permeation enhancement by acyl ester prodrug design, J Ocul Pharmacol Ther. 18(6), 535-48, (2002).
37. Lee TW, Ocular penetration enhancer. In: Mitra AK. Ophthalmic Drug Delivery Systems. 2nd ed. New York: M. Dekker Inc, 281-307, (1993).
38. Green K, Detergent penetration into young and adult rabbit eyes: comparative pharmacokinetics, J Toxicol Cut Ocul Toxicol 6, 89–107, (1987).
39. Ch’ng HS, For oral controlled delivery II: Synthesis and evaluation of some swelling, water-insoluble bioadhesive polymers, J Pharm Sci 74, 399-405, (1985).
40. Lele BS, Insoluble ionic complexes of polyacrylic acid with a cationic drug for use as a mucoadhesive ophthalmic drug delivery system, J Biomater Sci Polym Ed 11(12), 1319-1131, (2000).
41. Middleton DL, Design and evaluation of an ocular bioadhesive delivery system, S.T.P. Pharma Sci, 1, 200-206, (1991).
42. Kreuter, J. Nanoparticles and nanocapsules: New dosage forms in the nanometer size range, Pharm Acta Helv 53, 33–39, (1978).
43. Calvo D, Evaluation of cationic polymer coated nanocapsules as ocular drug carriers, Int J Pharm 153, 41 – 50, (1997).
44. Jacob L, Investigation of pilocarpine-loaded polybutyl cyanoacrylate nanocapsules in collagen shields as a drug delivery system,Invest Opthalmol Vis Sci 31, 485, (1990).
45. Giannavola C, Influence of preparation conditions on acyclovir-loaded poly-d,l-lactic acid nanospheres and effect of PEG coating on ocular drug bioavailability, Pharm Res 20(4), 584-590, (2003).
46. Gavini E, PGLA microspheres for the ocular delivery of a peptide drug, vancomycin using emulsification/spray-drying as the preparation method: In vitro/in vivo studies, Eur J Pharm Biopharm, 57, 207–212, (2004).
47. Tao W, Application of encapsulated cell technology for retinal degenerative diseases, Expert Opin Biol Ther 6, 717–726, (2006).
48. Klausner EA, Review Corneal gene therapy, Journal of Controlled Release 124, 107–133, (2007).
49. Selvam S, Current status of gene delivery and gene therapy in lacrimal gland using viral vectors, Advanced Drug Delivery Reviews 58, 1243–1257, (2006).
50. Pellegrinia G, Review towards therapeutic application of ocular stem cells, Seminars in Cell & Developmental Biology 18, 805–818, (2007).
51. Levin LA, Stem- cell therapy for ocular disorders, Arch Ophthalmol 122(4), 621-627, (2004).
52. Ambati J, Transscleral delivery of bioactive protein to the choroid and retina, Invest Ophthalmol Vis Sci. 41(5), 1186-1191, (2000).
53. Yasukawa T, Biodegradable scleral plugs for vitreoretinal drug delivery, Advanced Drug Delivery Reviews 52(1), 25-36, (2001).
54. Hadj-Slimane R, Short interfering RNA (siRNA), a novel therapeutic tool acting on angiogenesis, Biochimie, 89(10), 1234- 1244, (2007).
55. Campochiaro PA, Potential applications for RNAi to probe pathogenesis and develop new treatments for ocular disorders, Gene Therapy 13, 559–562, (2006).
56. Das SK, Gene, oligonucleotide, and ribozyme therapy in the eye,In: Mitra AK. Ophthalmic Drug Delivery Systems. 2nd ed. New York: M. Dekker Inc, 609-657, (1993).
57. Wadhwa S, Nanocarriers in Ocular Drug Delivery: An Update Review, Current Pharmaceutical Design 15, 2724-2750, (2009)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









