 About Authors:
About Authors:
Sahu Deepak*
Ass. Professor, Geetanjali Institute of Pharmacy,
Dabok, Udaipur [Rajasthan] – 313022
deepak.sahu.bhl@gmail.com
{ DOWNLOAD AS PDF }
Abstract:
A toxin produced by mold that can damage the liver and may lead to liver cancer. Aflatoxins cause cancer in some animals. The fungi that produce aflatoxin grow on crops such as peanuts (especially) and wheat, corn, beans and rice. Aflatoxin is a problem particularly in undeveloped and developing countries.
Aflatoxin is a naturally occurring mycotoxin produced by two types of mold: Aspergillus flavus and Aspergillus parasiticus. Aspergillus flavus is common and widespread in nature and is most often found when certain grains are grown under stressful conditions such as drought. The mold occurs in soil, decaying vegetation, hay, and grains undergoing microbiological deterioration and invades all types of organic substrates whenever and wherever the conditions are favorable for its growth. Favorable conditions include high moisture content and high temperature. At least 13 different types of aflatoxin are produced in nature with aflatoxin B1 considered as the most toxic. While the presence of Aspergillus flavus does not always indicate harmful levels of aflatoxin it does mean that the potential for aflatoxin production is present.1,2,3
Reference Id: PHARMATUTOR-ART-1540
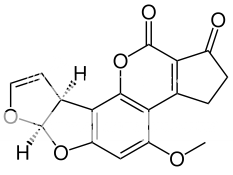
Chemical structure of aflatoxin B1
Introduction
In the 1960 more than 100,000 young turkeys on poultry farms in England died in the course of a few months from an apparently new disease that was termed "Turkey X disease" . It was soon found that the difficulty was not limited to turkeys. Ducklings and young pheasants were also affected and heavy mortality was experienced.1
Aspergillus flavus seen under an electron microscope.

Speculations made during 1960 regarding the nature of the toxin suggested that it might be of fungal origin. In fact, the toxin-producing fungus was identified as Aspergillus flavus (1961) and the toxin was given the name Aflatoxin by virtue of its origin (A.flavis--> Afla).4-7

Another microscopic view of Aspergillus flavus.6
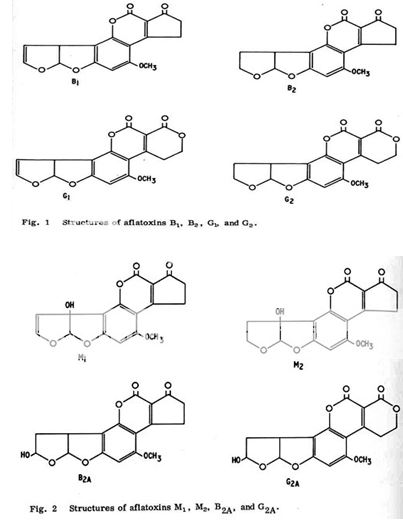
This discovery has led to a growing awareness of the potential hazards of these substances as contaminants of food and feed causing illness and even death in humans and other mammals. Studies that are summarized in the following sections revealed that aflatoxins are produced primarily by some strains of A. Flavus and by most, if not all, strains of A. parasiticus , plus related species, A. nomius and A. niger. Moreover, these studies also revealed that there are four major aflatoxins: B1, B2, G1, G2 plus two additional metabolic products, M1 and M2, that are of significance as direct contaminants of foods and feeds. The aflatoxins M1 and M2 were first isolated from milk of lactating animals fed aflatoxin preparations; hence, the M designation. Whereas the B designation of aflatoxins B1 and B2 resulted from the exhibition of blue fluorescence under UV-light, while the G designation refers to the yellow-green fluorescence of the relevant structures under UV-light. These toxins have closely similar structures and form a unique group of highly oxygenated, naturally occuring heterocyclic compounds.5,8,10 Their molecular formulas as established from elementary analyses and mass spectrometric determinations are:
* B1 : C17 H12 O6
* B2 : C17 H14 O6
* G1 : C17 H12 O7
* G2 : C17 H14 O7
Aflatoxins B2 and G2 were established as the dihydroxy derivatives of B1 and G1, respectively. Whereas, aflatoxin M1 is 4-hydroxy aflatoxin B1 and aflatoxin M2 is 4-dihydroxy aflatoxin B2.10,11,12
Occurrence
In Raw Agricultural Products:
Aflatoxins often occur in crops in the field prior to harvest. Postharvest contamination can occur if crop drying is delayed and during storage of the crop if water is allowed to exceed critical values for the mold growth. Insect or rodent infestations facilitate mold invasion of some stored commodities.10
Aflatoxins are detected occasionally in milk, cheese, corn, peanuts, cottonseed, nuts, almonds, figs, spices, and a variety of other foods and feeds. Milk, eggs, and meat products are sometimes contaminated because of the animal consumption of aflatoxin-contaminated feed. However, the commodities with the highest risk of aflatoxin contamination are corn, peanuts, and cottonseed.8,10,13
In Processed Foods:
Corn is probably the commodity of greatest worldwide concern, because it is grown in climates that are likely to have perennial contamination with aflatoxins and corn is the staple food of many countries. However, procedures used in the processing of corn help to reduce contamination of the resulting food product. This is because although aflatoxins are stable to moderately stable in most food processes, they are unstable in processes such as those used in making tortillas that employ alkaline conditions or oxidizing steps. Aflatoxin-contaminated corn and cottonseed meal in dairy rations have resulted in aflatoxin M1 contaminated milk and milk products, including non-fat dry milk, cheese, and yogurt.14,15
Aflatoxin Symptoms
How badly a person is affected by aflatoxin mycotoxins depends on things like the person's age, gender, level of exposure, duration of exposure, health, strength of their immune system, diet and environmental factors.9
There are two main ways people are usually exposed to aflatoxins. The first is when someone takes in a high amount of aflatoxins in a very short time. This can cause:14
· Liver damage
· Liver cancer
· Mental impairment
· Abdominal Pain
· Vomiting
· Convulsions
· Edema
· Pulmonary Edema
· Hemorrhaging
· Disruption of food digestion, absorption or metabolism
· Coma
· Death
The other way people suffer aflatoxin poisoning is by taking in small amounts of aflatoxins at a time, but over a long period. This might happen if a person's diet has a small amount of aflatoxins, for example. When this happens it can cause:11
· Growth and development impairment
· Liver cancer due to DNA mutation caused by aflatoxins
Aflatoxin Types
At least 14 different types of aflatoxin are produced in nature.15-17 Aflatoxin B1 is considered the most toxic and is produced by both Aspergillus flavus and Aspergillus parasiticus. Aflatoxin G1 and G2 are produced exclusively by A. parasiticus. While the presence of Aspergillus in food products does not always indicate harmful levels of aflatoxin are also present, it does imply a significant risk in consumption. Aflatoxins M1, M2 were originally discovered in the milk of cows that fed on moldy grain. These compounds are products of a conversion process in the animal's liver. However, aflatoxin M1 is present in the fermentation broth of Aspergillus parasiticus.
- Aflatoxin B1 & B2, produced by Aspergillus flavus and A. parasiticus
- Aflatoxin G1 & G2, produced by Aspergillus parasiticus
- Aflatoxin M1, metabolite of aflatoxin B1 in humans and animals (exposure in ng levels can come from a mother's milk)
- Aflatoxin M2, metabolite of aflatoxin B2 in milk of cattle fed on contaminated foods18
- Aflatoxicol
- Aflatoxin Q1 (AFQ1), major metabolite of AFB1 in in vitro liver preparations of other higher vertebrates19
Biosynthetic pathway of Aflatoxin B1
Aflatoxin B1 is derived from both a dedicated fatty acid synthase (FAS) and a polyketide synthase (PKS), together known as norsolorinic acid synthase. The biosynthesis begins with the synthesis of hexanoate by the FAS, which then becomes the starter unit for the iterative type I PKS.20,21,22 The PKS adds seven malonyl-CoA extenders to the hexanoate to form the C20 polyketide compound. The PKS folds the polyketide in a particular way to induce cyclization to form the anthraquinone norsolorinic acid. A reductase(E1) then catalyzes the reduction of the ketone on the norsolorinic acid side-chain to yield averantin.20,21,22 Averantin is converted to averufin via a two different enzymes, a hydroxylase (E2) and an alcohol dehydrogenase (E3). This will oxygenate and cyclize averantin's side chain to form the ketal in averufin.
From this point on the biosynthetic pathway of aflatoxin B1 becomes much more complicated, with several major skeletal changes. Most of the enzymes have not been characterized and there may be several more intermediates that are still unknown.20 However, what is known is that averufin is oxidized by a P450-oxidase, AvfA (E4), in a Baeyer-Villiger oxidation. This opens the ether rings and upon rearrangement versiconal acetate is formed. Now an esterase, EstA (E5), catalyzes the hydrolysis of the acetyl, forming the primary alcohol in versiconal.20,22 The acetal in versicolorin A is formed from the cyclization of the side-chain in versiconal, which is catalyzed by VERB synthase (E6), and then VerB, a desaturase (E7), reduces versicolorin B to form the dihydrobisfuran.20,22
There are two more enzymes that catalyze the conversion of versicolorin A to demethylsterigmatocystin: AflN, an oxidase (E8) and AflM, a reductase (E9). These enzymes utilize both molecular oxygen and two NADPH's to dehydrate one of the hydroxyl groups on the anthraquinone and open the quinine with the molecular oxygen.20,22 Upon forming the aldehyde in the ring opening step, it is oxidized to form the carboxylic acid and subsequently a decarboxylation event occurs to close the ring, forming the six-member ether ring system seen in demethylsterigmatocystin. The next two steps in the biosynthetic pathway is the methylation by s-adenosylmethionine (SAM) of the two hydroxyl groups on the xanthone part of demethysterigmatocystin by two different methyltransferases, OmtB (E10) and OmtA (E11).20,22 This yields 'O'-methylsterigmatocystin. In the final steps there is an oxidative cleavage of the aromatic ring and loss of one carbon in O-methylsterigmatocystin, which is catalyzed by OrdA, an oxidoreductase (E12).20,22 Then a final recyclization occurs to form aflatoxin B1.
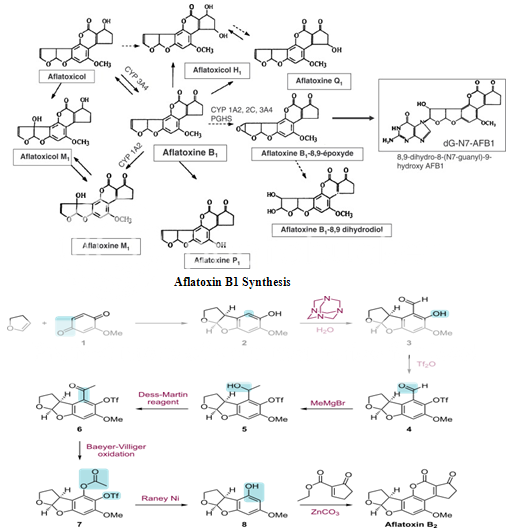
Aflatoxin B2 Synthesis
Contamination conditions
Aflatoxin-producing members of Aspergillus are common and widespread in nature. They can colonize and contaminate grain before harvest or during storage. Host crops are particularly susceptible to infection by Aspergillus following prolonged exposure to a high-humidity environment, or damage from stressful conditions such as drought, a condition that lowers the barrier to entry.15
The native habitat of Aspergillus is in soil, decaying vegetation, hay, and grains undergoing microbiological deterioration, and it invades all types of organic substrates whenever conditions are favorable for its growth. Favorable conditions include high moisture content (at least 7%) and high temperature.
The toxin can also be found in the milk of animals that are fed contaminated feed.
International sources of commercial peanut butter, cooking oils (i.e. olive oil, etc.), and cosmetics have been identified as contaminated with aflatoxin. In some instances, liquid chromatography-tandem mass spectrometry (LC-MS/MS), and other analytical methods, revealed anywhere from 48–80% of selected product samples as containing detectable quantities of aflatoxin. In many of these contaminated food products, the aflatoxin exceeded FDA, or other regulatory agency, safe limits.2,3,14,18
The United States Food and Drug Administration (FDA) has established action levels for aflatoxin present in food or feed to protect human and animal health.
Levels must not exceed:
|
PPB |
Criterion |
|
20 |
For all food for human consumption and for corn and other grains intended for immature animals (including immature poultry) and for dairy animals, or when its destination is not known, and for animal feeds, other than corn or cottonseed meal |
|
100 |
For corn and other grains intended for breeding beef cattle, breeding swine, or mature poultry |
|
200 |
For corn and other grains intended for finishing swine of 100 pounds or greater |
|
300 |
For corn and other grains intended for finishing (i.e., feedlot) beef cattle, and for cottonseed meal intended for beef cattle, swine or poultry. |
Aflatoxin Toxicity
Aflatoxin mycotoxins are toxic to humans and even more toxic to animals. They also cause cancer in humans and animals.
It is believed that eating vegetables like carrots and celery reduces the carcinogenic effects of aflatoxins. The aflatoxin LD50 rate (the dosage level that causes 50% of a group to die) for animals is between 0.5 and 10 mg/kg of the animal's weight.14,18,19
Aflatoxicosis - Aflatoxin Poisoning12
The technical term for poisoning by aflatoxin mycotoxins is aflatoxicosis. This usually occurs from eating food contaminated with aflatoxin mycotoxins.
Aflatoxicosis is not contagious and drugs and antibiotics do little to help. Aflatoxicosis damages the liver more than any other organ. Aflatoxin mycotoxins also suppress the immune system.
Aflatoxicosis is primarily a hepatic disease. The susceptibility of individual animals to aflatoxins varies considerably depending on species, age, sex, and nutrition. In fact, aflatoxins cause liver damage, decreased milk and egg production, recurrent infection as a result of immunity suppression (eg. salmonellosis), in addition to embryo toxicity in animals consuming low dietary concentrations. While the young of a species are most susceptible, all ages are affected but in different degrees for different species. Clinical signs of aflatoxicosis in animals include gastrointestinal dysfunction, reduced reproductivity, reduced feed utilization and efficiency, anemia, and jaundice. Nursing animals may be affected as a result of the conversion of aflatoxin B1 to the metabolite aflatoxin M1 excreted in milk of dairy cattle.16
The induction of cancer by aflatoxins has been extensively studied. Aflatoxin B1, aflatoxin M1, and aflatoxin G1 have been shown to cause various types of cancer in different animal species. However, only aflatoxin B1 is considered by the International Agency for Research on Cancer (IARC)as having produced sufficient evidence of carcinogenicity in experimental animals to be identified as a carcinogen.
Aflatoxins and Human Health
Humans are exposed to aflatoxins by consuming foods contaminated with products of fungal growth. Such exposure is difficult to avoid because fungal growth in foods is not easy to prevent. Even though heavily contaminated food supplies are not permitted in the market place in developed countries, concern still remains for the possible adverse effects resulting from long-term exposure to low levels of aflatoxins in the food supply.4,6,11
Evidence of acute aflatoxicosis in humans has been reported from many parts of the world, namely the Third World Countries, like Taiwan, Ouganda, India, and many others. The syndrome is characterized by vomiting, abdominal pain, pulmonary edema, convulsions, coma, and death with cerebral edema and fatty involvment of the liver, kidneys, and heart.15
Conditions increasing the likelihood of acute aflatoxicosis in humans include limited availability of food, environmental conditions that favor fungal development in crops and commodities, and lack of regulatory systems for aflatoxin monitoring and control.20
Because aflatoxins, especially aflatoxin B1, are potent carcinogens in some animals, there is interest in the effects of long-term exposure to low levels of these important mycotoxins on humans . In 1988, the IARC placed aflatoxin B1 on the list of human carcinogens. This is supported by a number of epidemiological studies done in Asia and Africa that have demonstrated a positive association between dietary aflatoxins and Liver Cell Cancer (LCC) . Additionally, the expression of aflatoxin-related diseases in humans may be influenced by factors such as age, sex, nutritional status, and/or concurrent exposure to other causative agents such as viral hepatitis (HBV) or parasite infestation.18
Factors Favorizing Aflatoxin Production
Fungal growth and aflatoxin contamination are the consequence of interactions among the fungus, the host and the environment. The appropriate combination of these factors determine the infestation and colonization of the substrate, and the type and amount of aflatoxin produced. However, a suitable substrate is required for fungal growth and subsequent toxin production, although the precise factor(s) that initiates toxin formation is not well understood. Water stress, high-temperature stress, and insect damage of the host plant are major determinig factors in mold infestation and toxin production . Similarly, specific crop growth stages, poor fertility, high crop densities, and weed competition have been associated with increased mold growth and toxin production. Aflatoxin formation is also affected by associated growth of other molds or microbes . For example, preharvest aflatoxin contamination of peanuts and corn is favored by high temperatures, prolonged drought conditions, and high insect activity; while postharvest production of aflatoxins on corn and peanuts is favored by warm temperatures and high humidity.17,19
Aspergillus & Aflatoxin Production
Aflatoxin mycotoxins are produced by the Aspergillus species of molds. Aspergillus molds grow mostly on crops, such as grains and nuts. Under the right conditions, Aspergillus often grows on grain before it is harvested. But it can also grow on harvested grain if the grain is stored damp. Aspergillus also grows on substances like soil, hay and decaying vegetation. The best conditions for Aspergillus to grow on organic materials is when the temperature is warm and when the material has a high level of moisture (7% or more).12
Aflatoxin in Food
The American Food and Agriculture Organization estimates that 25% of the food crops in the world are affected by mycotoxins. Of these mycotoxins, aflatoxins are the biggest problem.
Corn, cottonseed and peanuts are the crops most at risk of being contaminated by aflatoxins. Aspergillus also commonly grows on beans, rice, tree nuts and wheat. It grows less often on other grains and nuts.18
If animals are given feed contaminated with aflatoxins then aflatoxin mycotoxins can end up in milk, eggs and meat. Aflatoxin M1 and M, which are often found in cow's milk, are metabolites produced by animals which have eaten aflatoxins.14
Aflatoxin Levels in Food
Aflatoxins are found all over the world. However they are much more of a problem in undeveloped or developing nations than they are in developed countries.
Developed countries prohibit high levels of aflatoxin mycotoxins in foods. For example, the United States limits the level of aflatoxins to under 20 parts per billion in food and specifies that the aflatoxins M must be below 0.5 parts per billion in milk.17
Chemical processes are used to remove aflatoxins in foods such as nuts, corn, grains and milk. Most foods do still contain very small amounts of aflatoxins though. Although the aflatoxin levels are usually far below the safety limits, this has raised concern about the effects on humans of the long term intake of small amounts of aflatoxins.21
Aflatoxin in Pet Food
Pets have died from eating pet foods contaminated with aflatoxin mycotoxins. Between late 2005 and early 2006, 23 or more dogs died from eating Diamond Pet Foods dog food contaminated with aflatoxins.21
Pathology
High-level aflatoxin exposure produces an acute hepatic necrosis, resulting later in cirrhosis, and/or carcinoma of the liver. Acute hepatic failure is made manifest by hemorrhage, edema, alteration in digestion, changes to the absorption and/or metabolism of nutrients, and mental changes and/orcoma.
No animal species is immune to the acute toxic effects of aflatoxins; however, adult humans have a high tolerance for aflatoxin exposure and rarely succumb to acute aflatoxicosis.6,14,21
Chronic, subclinical exposure does not lead to symptoms as dramatic as acute aflatoxicosis. Children, however, are particularly affected by aflatoxin exposure, which leads to stunted growth and delayed development.17 Chronic exposure also leads to a high risk of developing liver cancer, as aflatoxin metabolites can intercalate into DNA and alkylate the bases through its epoxide moiety. This is thought to cause mutations in the p53 gene, an important gene in preventing cell cycle progression when there are DNA mutations, or signaling apoptosis. These mutations seem to affect some base pair locations more than others — for example, the third base of codon 249 of the p53 gene appears to be more susceptible to aflatoxin-mediated mutations than nearby bases.14
Medical research indicates that a regular diet including apiaceous vegetables such as carrots, parsnips, celery and parsley, reduces the carcinogenic effects of aflatoxin. Moreover, aflatoxin B1 can permeate through the skin. Dermal exposure to this aflatoxin in particular environmental conditions can lead to serious health risks.18,20
Microbiology
Aflatoxins are still recognized as the most important mycotoxins. They are synthesized by only a few Aspergillus species of which A. flavus and A. parasiticus are the most problematic. The expression of aflatoxin-related diseases is influenced by factors such as species, age, nutrition, sex, and the possibility of concurrent exposure to other toxins. The main target organ in mammals is the liver, so aflatoxicosis is primarily a hepatic disease. Conditions increasing the likelihood of aflatoxicosis in humans include limited availability of food, environmental conditions that favour mould growth on foodstuffs, and lack of regulatory systems for aflatoxin monitoring and control.11
A. flavus and A. parasiticus are weedy molds that grow on a large number of substrates, in particular under high moisture conditions. Aflatoxins have been isolated from all major cereal crops, and from sources as diverse as peanut butter and marijuana. The staple commodities regularly contaminated with aflatoxins include cassava, chillies, corn, cotton seed, millet, peanuts, rice, sorghum, sunflower seeds, tree nuts, wheat, and a variety of spices intended for human or animal consumption. When processed, aflatoxins get into the general food supply where they have been found in both pet and human foods, as well as in feedstocks for agricultural animals. Aflatoxin transformation products are sometimes found in eggs, milk products and meat when animals are fed contaminated grains.8,12,22
Detection in humans
There are two principal techniques that have been used most often to detect levels of aflatoxin in humans.
The first method is measuring the AFB1-guanine adduct in the urine of subjects. The presence of this breakdown product indicates exposure to aflatoxin B1 in the past 24 hours. However, this technique measures only recent exposure, and, due to the half-life of this metabolite, the level of AFB1-guanine measured can vary from day to day, based on diet, and thus is not ideal for assessing long-term exposure.
Another technique that has been used is a measurement of the AFB1-albumin adduct level in the blood serum. This approach provides a more integrated measure of exposure over several weeks/months.22
Detection in Animals
Aflatoxin has potential to lead to liver disease in dogs; however, not all dogs exposed to aflatoxin will develop liver disease. As with any toxic exposure, development of aflatoxicosis is a dose-related occurrence. Some dogs that develop liver disease will recover; those exposed to large doses for extended periods may not.
Low levels of aflatoxin exposure require continuous consumption for several weeks to months in order for signs of liver dysfunction to appear.23 Some articles have suggested the toxic level in dog food is 100–300 ppb and requires continuous exposure/consumption for a few weeks to months to develop aflatoxicosis.24 No information is available to suggest that recovered dogs will later succumb to an aflatoxin-induced disease.
There is no specific antidote for aflatoxicosis. Symptomatic and supportive care tailored to the severity of the liver disease may include intravenous fluids with dextrose, active vitamin K, B vitamins, and a restricted but high-quality protein diet with adequate carbohydrate content.
As a precautionary measure, both human and pet food recalls have occurred, casting a wide safety net to prevent exposure to potentially unsafe food. Recalled food products are subsequently sampled and tested for aflatoxin.
Recent Methods of Analysis for Aflatoxins in Foods and Feeds20-26
Sampling and Sample Preparation:
Sampling and sample preparation remain a considerable source of error in the analytical identification of aflatoxins. Thus, systematic approaches to sampling, sample preparation, and analysis are absolutely necessary to determine aflatoxins at the parts-per-billion level. In this regard, specific plans have been developed and tested rigorously for some commodities such as corn, peanuts, and tree nuts; sampling plans for some other commodities have been modeled after them. A common feature of all sampling plans is that the entire primary sample must be ground and mixed so that the analytical test portion has the same concentration of toxin as the original sample.22
Solid-Phase Extraction:
All analytical procedures include three steps: extraction, purification, and determination. The most significant recent improvement in the purification step is the use of solid-phase extraction. Test extracts are cleaned up before instrumental analysis (thin layer or liquid chromatography) to remove coextracted materials that often interfere with the determination of target analytes.21,22
Thin-Layer Chromatography:
Thin layer chromatography (TLC), also known as flat bed chromatography or planar chromatography is one of the most widely used separation techniques in aflatoxin analysis. Since 1990, it has been considered the AOAC official method and the method of choice to identify and quantitate aflatoxins at levels as low as 1 ng/g. The TLC method is also used to verify findings by newer, more rapid techniques.22
Liquid Chromatography :
Liquid chromatography (LC) is similar to TLC in many respects, including analyte application, stationary phase, and mobile phase. Liguid chromatography and TLC complement each other. For an analyst to use TLC for preliminary work to optimize LC separation conditions is not unusual.
Liquid chromatography methods for the determination of aflatoxins in foods include normal-phase LC (NPLC), reversed-phase LC (RPLC) with pre- or before-column derivatization (BCD), RPLC followed by postcolumn derivatization (PCD), and RPLC with electrochemical detection.24
Immunochemical Methods:
Thin layer chromatography and LC methods for determining aflatoxins in food are laborious and time consuming. Often, these techniques require knowledge and experience of chromatographic techniques to solve sepatation and and interference problems. Through advances in biotechnology, highly specific antibody-based tests are now commercially available that can identify and measure aflatoxins in food in less than 10 minutes. These tests are based on the affinities of the monoclonal or polyclonal antibodies for aflatoxins. The three types of immunochemical methods are radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), and immunoaffinity column assay (ICA).24
Confirmation of Identities of the Aflatoxins :
Although analytical methods might consist of different extraction, clean-up, and quantitation steps, the results of the analyses by such methods should be similar when the methods are applied properly. Since the reliability of the quantitative data is not in question, the problem still to be solved is the confirmation of identity of the aflatoxins. The confirmation techniques used involve either chemical derivatization or mass spectrometry (MS).21,22,26
Monitoring Techniques for Assessing Human Exposure to Aflatoxins
In the last few years, new technologies have been developed that more accurately monitor individual exposures to aflatoxins. Particular attention has been paid to the analysis of aflatoxin DNA adducts and albumin adducts as surrogates for genotoxicity in people. Autrup et al.(1983) pioneered the use of synchronous fluorescence spectroscopy for the measurement of aflatoxin DNA adducts in urine. Urine samples collected after exposure to alfatoxins were found to contain 2,3-dihydroxy-2-(N7-guanyl)-3-hydroxyaflatoxin B1, trivially known as AFB-Gual . Wild et al.(1986) used highly sensitive immunoassays to quantitate aflatoxins in human body fluids. An enzyme linked immunosorbent assay (ELISA) was used to quantitate aflatoxin B1 over the range of 0.01 ng /ml to 10 ng/ml, and was validated in human urine samples. Using this method, aflatoxin-DNA adduct excretion into urine was found to be positively correlated with dietary intake, and the major aflatoxin B1-DNA adduct excreted in urine was shown to be an appropriate dosimeter for monitoring aflatoxin dietary exposure.27
A- Regulatory Control:
Aflatoxins are considered unavoidable contaminants of food and feed, even where good manufacturing practices have been followed. The FDA has established specific guidelines on acceptable levels of aflatoxins in human food and animal feed by establishing action levels that allow for the removal of violative lots from commerce. The action level for human food is 20 ppb total aflatoxins, with the exception of milk which has an action level of 0.5 ppb for aflatoxin M1. The action level for most feeds is also 20 ppb. However, it is very difficult to accurately estimate aflatoxins concentration in a large quantity of material because of the variability associated with testing procedures; hence, the true aflatoxin concentration in a lot cannot be determined with 100% certainty.25

B- Detoxification Strategies :
Because aflatoxin contamination is unavoidable, numerous strategies for their detoxification have been proposed. These include physical methods of separation, thermal inactivation, irradiation, solvent extraction, adsorption from solution, microbial inactivation, and fermentation. Chemical methods of detoxification are also practiced as a major strategy for effective detoxification.25
Structural Degradation Following Chemical Treatment :
A diverse group of chemicals has been tested for the ability to degrade and inactivate aflatoxins. A number of these chemicals can react to destroy (or degrade) aflatoxins effectively but most are impractical or potentially unsafe because of the formation of toxic residues or the perturbation of nutrient content and the organoleptic properties of the product. Two chemical approaches to the detoxification of aflatoxins that have received considerable attention are ammoniation and reaction with sodium bisulfite.
Many studies provide evidence that chemical treatment via ammoniation may provide an effective method to detoxify aflatoxin-contaminated corn and other commodities. The mechanism for this action appears to involve hydrolysis of the lactone ring and chemical conversion of the parent compound aflatoxin B1 to numerous products that exhibit greatly decreased toxicity.21,25
On the other hand, sodium bisulfite has been shown to react with aflatoxins (B1, G1 , and M1) under various conditions of temperature, concentration, and time to form water-soluble products.13
Modification of Toxicity by Dietary Chemicals :
The toxicity of mycotoxins may be strongly influenced by dietary chemicals that alter the normal responses of mammalian systems to these substances. A variable array of chemical factors, including nutritional components (e.g. dietary protein and fat, vitamins, and trace elements), food and feed additives (e.g. antibiotics and preservatives), as well as other chemical factors may interact with the effects of aflatoxins in animals.17
Alteration of Bioavailability by Aflatoxin chemisorbents :
A new approach to the detoxification of aflatoxins is the addition of inorganic sorbent materials, known as chemisorbents, such as hydrated sodium calcium aluminosilicate (HSCAS) to the diet of animals. HSCAS possesses the ability to tightly bind and immobilize aflatoxins in the gastrointestinal tract of animals, resulting in a major reduction in aflatoxin bioavailability.21,28
Economic Impact of Aflatoxins
The economic impact of aflatoxins derive directly from crop and livestock losses as well as indirectly from the cost of regulatory programs designed to reduce risks to animal and human health. The Food and Agriculture Organization (FAO) estimates that 25% of the world's food crops are affected by mycotoxins, of which the most notorious are aflatoxins. Aflatoxin losses to livestock and poultry producers from aflatoxin-contaminated feeds include death and the more subtle effects of immune system suppression, reduced growth rates, and losses in feed efficiency. Other adverse economic effects of aflatoxins include lower yields for food and fiber crops.21,24,26,28
In addition, the abilitiy of aflatoxins to cause cancer and related diseases in humans given their seemingly unavoidable occurrence in foods and feeds make the prevention and detoxification of these mycotoxins one of the most challenging toxicology issues of present time.
References
1. Anon. 1989. Mycotoxins , Economic and Health Risks. Council for Agricultural science and Technology, Report No.116 pp91.
2. Eaton, D.L. and Groopman, J.D. 1994. The Toxicology of Aflatoxins. Academic Press, New York. pp383-426.
3. Finley, J.W.,Robinson, S.F. and Armstrong, D.J. 1992. Food Safety Assessment. American Chemical Society, Washington, D.C. pp261-275.
4. Goldbatt, L.A. 1969. Aflatoxin. Academic Press,New York. pp1-40.
5. Heathcote, J.G. and Hibbert, J.R. 1978. Aflatoxins : Chemical and biological aspect. Elsevier, New York. pp.173-186.
6. Liener, I.E. 1969. Toxin constituents of plant foodstuffs. Academic Press , New york. pp392-394.
7. Wyllie, T.D. and Morchause,L.G. 1978. Mycotoxin Fungi, Mycotoxins, Mycotoxicoses-An Encyclopedic Handbook.Vols.1,2, and 3.Marcel Dekker, Inc. New york.
8. Mahoney, Noreen; Russell J. Molyneux (14 April 2010). "A Rapid Analytical Method for Determination of Aflatoxins in Plant-Derived Dietary Supplement and Cosmetic Oils". J Agric Food Chem. 58(7): 4065–4070. PMC 2858461.
9. Leong, Y. -H.; Ismail, N.; Latiff, A. A.; Manaf, N. A.; Rosma, A. (1 January 2011). "Determination of aflatoxins in commercial nuts and nut products using liquid chromatography tandem mass spectrometry". World Mycotoxin Journal 4 (2): 119–127. doi:10.3920/WMJ2010.1229.
10. Li, Feng-Qin; Li, Yu-Wei; Wang, Ye-Ru; Luo, Xue-Yun (13 May 2009). "Natural Occurrence of Aflatoxins in Chinese Peanut Butter and Sesame Paste". Journal of Agricultural and Food Chemistry 57(9): 3519–3524. doi:10.1021/jf804055n.
11. McDaniel, A. (1 January 2011). "Effect of Matrix Clean-Up for Aflatoxin Analysis in Corn and Dried Distillers Grains". Natural Resources 02 (04): 250–257. doi:10.4236/nr.2011.24032.
12. "Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions". American Journal of Clinical Nutrition.
13. Abbas, Hamed K. (2005). Aflatoxin and Food Safety. CRC Press. ISBN 0-8247-2303-1.
14. F Aguilar, S P Hussain and P Cerutti (September 1993). "Aflatoxin B1 induces the transversion of G-->T in codon 249 of the p53 tumor suppressor gene in human hepatocytes". PNAS 90 (18): 8586–90.
15. Peterson S, Lampe JW, Bammler TK, Gross-Steinmeyer K, Eaton DL (September 2006). "Apiaceous vegetable constituents inhibit human cytochrome P-450 1A2 (hCYP1A2) activity and hCYP1A2-mediated mutagenicity of aflatoxin B1". Food Chem. Toxicol. 44 (9): 1474–84. doi:10.1016/j.fct.2006.04.010. PMID 16762476.
16. Boonen, Jente; Malysheva, Svetlana V.; Taevernier, Lien; Diana Di Mavungu, José; De Saeger, Sarah; De Spiegeleer, Bart (2012). "Human skin penetration of selected model mycotoxins". Toxicology301 (1–3):21–32. doi:10.1016/j.tox.2012.06.012. PMID 22749975.
17. Machida, M; Gomi, K (editors) (2010). Aspergillus: Molecular Biology and Genomics. Caister Academic Press. ISBN 978-1-904455-53-0.
18. Fratamico, PM et al. (editors) (2008). Foodborne Pathogens: Microbiology and Molecular Biology. Horizon Scientific Press. ISBN 978-1-898486-52-7.
19. Bingham AK, Phillips TD, Bauer JE (March 2003). "Potential for dietary protection against the effects of aflatoxins in animals". J. Am. Vet. Med. Assoc. 222 (5): 591–6. PMID 12619837.
20. Bastianello SS, Nesbit JW, Williams MC, Lange AL (December 1987). "Pathological findings in a natural outbreak of aflatoxicosis in dogs". Onderstepoort J. Vet. Res. 54 (4): 635–40.PMID 3444619.
21. FDA Inspection Report-Diamond Gaston SC Plant 12/21/2005-1/19/2006.
22. AKC Standard Article Contaminated Diamond Pet Food Products and 'Best By' Dates Narrowed Akcstandard.com.
23. E. Boutrif, Prevention of aflatoxin in pistachios
24. Aflatoxin M2 product page from Fermentek
25. Smith, John E., Sivewright-Henderson, Rachel; Mycotoxins and animal foods, CRC Press, 1991 p.614 ISBN 978-0-8493-4904-1 (retrieved 19 February 2012 via
26. Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach (2009), 3rd edition, John Wiley and Sons Ltd., 122–124.
27. Singh, R.; Hsieh, D.P.H. Archives of Biochemistry and Biophysics (1977), 178, 285–292.
28. Yu, J.; Chang, P.K.; Ehrlich, K.C.; Cary, J.W.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A.; Linz, J.E.; Woloshuk, C.P.; Bennett, J.W. Applied and Environmental Microbiology (2004), 70(3), 1253–1262.
|
PharmaTutor (ISSN: 2347 - 7881) Volume 1, Issue 1 Received On: 05/10/2013; Accepted On: 28/10/2013; Published On: 25/11/2013 How to cite this article: Sahu D, Naturally Occurring Mycotoxin -Aflatoxin, PharmaTutor, 2013, 1(1), 39-52 |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









